Thalidomide was administered to 83 patients with myelodysplastic syndrome (MDS), starting at 100 mg by mouth daily and increasing to 400 mg as tolerated. Thirty-two patients stopped therapy before 12 weeks (minimum period for response evaluation), and 51 completed 12 weeks of therapy. International Working Group response criteria for MDS were used to evaluate responses. Intent-to-treat (ITT) analysis classified all off-study patients as nonresponders. Off-study patients belonged to a higher risk category (P = .002) and had a higher percentage of blasts in their pretherapy bone marrow than patients who completed 12 weeks of therapy (P = .003). No cytogenetic or complete responses were seen, but 16 patients showed hematologic improvement, with 10 previously transfusion-dependent patients becoming transfusion independent. Responders had lower pretherapy blasts (P = .016), a lower duration of pretherapy platelet transfusions (P = .013), and higher pretherapy platelets (P = .003). Among responders, 9 had refractory anemia (RA); 5 had RA with ringed sideroblasts; and 2 had RA with excess blasts. By ITT analysis, 19% of patients (16 of 83) responded, and when only evaluable patients were analyzed, 31% (16 of 51) responded. It was concluded that thalidomide, as a single agent, is effective in improving cytopenias of some MDS patients, especially those who present without excess blasts.
Introduction
Myelodysplastic syndromes (MDSs) have few therapeutic options that are even palliative, and none that are curative,1 especially if the affected patients are not suitable candidates for hematopoietic stem cell transplantation.2-4 Despite intense interest in growth factors,5-12 differentiating agents,13-15 and cytotoxic drugs,16-19 supportive care continues to be the standard therapy for the majority of patients. The clinical and biological heterogeneity of MDS suggests the possibility of multiple therapeutic targets, especially in light of the recent novel insights into the pathogenesis of cytopenias. It appears that large numbers of hematopoietic cells are not only rapidly proliferating in the bone marrow, but also simultaneously undergoing programmed cell death.20-28 Furthermore, apoptosis in the hematopoietic cells is mediated by cytokines, such as tumor necrosis factor alpha (TNF-α), transforming growth factor beta, interleukin 1 beta (IL-1β), and IL-6.29-33 Attempts to suppress this excessive cytokine-mediated apoptosis in MDS with cytoprotective34 and/or anticytokine therapies35-37 have resulted in substantial improvements in the cytopenias of some MDS patients.
The bone marrows of MDS patients also demonstrate markedly increased neo-angiogenesis and higher-than-normal levels of vascular endothelial growth factor.38,39 Finally, in addition to the frequent involvement of B-cells in the MDS process itself,40 a more intrinsic immune defect may also exist in these patients.41 T-cell lymphopenia, especially that affecting the CD4+ cells, has been described,42 and suppression of T-cell function by means of antithymocyte globulin (ATG) and cyclosporin is associated with improvement in the cytopenias.43,44 Thalidomide was considered a potentially useful drug for MDS patients. It is an immune-modulatory agent with anticytokine activities45-51 and has antiangiogenic effects.52-54 Thalidomide has meaningful activity in multiple myeloma,55 even though the precise mechanism of its action remains unclear. On the basis of this rationale, a pilot study was conducted to test the efficacy of thalidomide in improving the ineffective hematopoiesis seen in patients with myelodysplastic syndromes. The results of this pilot study are encouraging enough to warrant larger prospective trials in the future.
Patients, materials, and methods
All patients, after signing an informed consent form approved by the Institutional Review Board of Rush-Presbyterian–St Luke's Medical Center, Chicago, IL, participated in the study, MDS 98-21, titled “A pilot study of thalidomide (thalidomide) in patients with myelodysplastic syndromes.” Every patient had a pretherapy bone marrow aspirate and biopsy examination performed at the Rush Cancer Institute (RCI). All samples were reviewed at the central facility by a hematopathologist at RCI/Rush-Presbyterian–St Luke's Medical Center to confirm the diagnosis of MDS. Each patient started by taking 100 mg thalidomide by mouth at bedtime and increased the dose as tolerated to 400 mg by mouth at bedtime over the next several weeks. The drug was provided free of charge for the patients by Celgene (Warren, NJ). In the present study, no premenopausal woman of childbearing age was included. Newly diagnosed, as well as previously diagnosed, patients were eligible as were patients with both primary de novo and secondary MDS cases. Patients belonging to all subtypes of MDS as per the French-American-British (FAB) classification56 and to all risk categories according to the International Prognostic Scoring System (IPSS)57 were eligible. Patients were required not to have received any therapy for MDS for at least 4 weeks prior to starting thalidomide except for supportive care with transfusions. No other treatment for the primary disease such as growth factors could be administered to study patients while they were on thalidomide. Pyridoxine at 100 mg by mouth daily was prescribed for every patient as prophylaxis against peripheral neuropathy. Weekly complete blood counts (CBCs) with differentials were obtained, and upon completion of 12 weeks of therapy, the patients returned to RCI for a response evaluation, at which time all the pretherapy studies were repeated. In case of any evidence of a partial or complete response or stable disease as judged by the principal investigator, thalidomide was continued at the maximum tolerated dose for up to 1 year. Therapy was stopped in nonresponding patients at this time and they were taken off the study.
The clinical end point of the study was to determine the efficacy of thalidomide in those patients who were able to complete at least 12 weeks of therapy at the maximally tolerated dose.
Response criteria
Response criteria outlined in the report of an International Working Group (IWG) to standardize response criteria for MDS58 were applied by an independent team (Global Therapeutic Development, Seattle, WA) to assess responses. Minor modifications had to be made to these criteria because this analysis was retrospective. The subsections below on “Pretherapy assessments” and “Assessments during therapy” describe modified criteria.
Complete remission (CR).
Bone marrow evaluation: CR is defined as repeat bone marrow evaluation showing fewer than 5% myeloblasts with normal maturation of all cell lines and no evidence for dysplasia. When erythroid precursors constitute fewer than 50% of bone marrow nucleated cells, the percentage of blasts is based on all nucleated cells; when there are 50% or more erythroid cells, the percentage of blasts should be based on the nonerythroid cells.
Peripheral blood evaluation.
(Absolute values must last at least 2 months.) (1) hemoglobin levels greater than 11 g/dL (untransfused patient not on erythropoietin); (2) neutrophil levels of 100/μL or higher (for patients not on a myeloid growth factor); (3) platelet levels of 100 000/μL or higher (for patients not on a thrombopoietic agent); (4) 0% blasts; (5) no dysplasia.
Partial remission (PR) criteria.
(Absolute values must last at least 2 months.) PR is defined as a patient's meeting all the CR criteria (if abnormal before treatment) except for bone marrow evaluation. The bone marrow criteria for PR are either that blasts decrease by at least 50% from pretreatment levels or that the MDS FAB classification be a less advanced category than at pretreatment. Cellularity or morphology is not relevant.
Other criteria.
(1) Stable disease is defined as failure to achieve at least a PR, but with no evidence of progression for at least 2 months. (2) Failure is defined as death during treatment or as disease progression characterized by worsening of cytopenias, increase in the percentage of BM blasts, or progression to an MDS FAB subtype more advanced than at pretreatment. (3) Disease transformation is defined as transformation to acute myelogenous leukemia (AML), with at least 5% blasts. (4) A cytogenetic response requires 20 analyzable metaphases by conventional cytogenetic techniques. (5) A major cytogenetic response is defined as no detectable cytogenetic abnormality if pre-existing abnormality was present. (6) A minor cytogenetic response is defined as 50% or more reduction in abnormal metaphases.
Pretherapy assessments.
(These incorporate minor modifications from the IWG response criteria.) For all patients, the baseline CBC to which improvements were compared was standardized with the use of a mean value for the 4 weeks prior to the start of therapy.
Assessments during therapy.
(These incorporate minor modifications from the IWG response criteria.) Responses were assessed at 12, 16, and 20 weeks of therapy. Absolute values closest to the 12- and 16-week assessments were used, and responses had to be sustained for at least the subsequent 8 weeks. With regard to packed red blood cell transfusions (PRBCs) and transfusion independence, the same 4-week time period prior to treatment was used to determine transfusion dependence and to obtain a baseline monthly requirement. Subsequent transfusions were reviewed at 12, 16, 20, 24, and 28 weeks. Patients who received a transfusion from day 0 to week 12 were not considered transfusion independent; however, a patient who did not receive any transfusion at week 16 and sustained that independence for another 8 weeks was then considered transfusion independent. Patients were called late responders if they showed hematologic improvement (HI) after 20 weeks of therapy.
Hematologic improvement.
All improvements must last at least 8 weeks. For a designated response (CR, PR, HI), all relevant response criteria must be noted on at least 2 successive determinations at least 1 week apart after an appropriate period following therapy.
Erythroid response (HI-E).
(1) Major response is defined as an increase in hemoglobin (Hb) greater than 2 g/dL for patients with pretreatment Hb below 11 g/dL, and as transfusion independence for transfusion-dependent patients. (2) Minor response is defined as an increase of 1 to 2 g/dL in Hb for patients with pretreatment Hb less than 11 g/dL, and as a 50% decrease in PRBC requirements for transfusion-dependent patients.
Platelet response (HI-P).
(1) Major response is defined as an absolute increase of 30 000/μL or higher for patients with a pretreatment platelet count lower than 100 000/μL, and as stabilization of platelet counts and platelet transfusion independence for platelet transfusion–dependent patients. (2) Minor response is defined as a 50% or greater increase in platelet count with a net increase greater than 10 000/μL, but less than 30 000/μL, for patients with a pretreatment platelet count less than 100 000/μL.
Absolute neutrophil response (HI-ANC).
(1) Major response is defined as at least a 100% increase or an absolute increase of 500/μL, whichever is greater, for patients with an ANC (absolute neutrophil count) lower than 1500/μL before therapy. (2) Minor response is defined as an ANC increase of at least 100% but an absolute increase less than 500/μL for an ANC that was lower than 1500/μL before therapy.
Cytogenetic studies
Standard karyotypic analysis by means of GTG banding was performed on every patient before therapy was started and each time a marrow was performed thereafter.
Statistical analysis
Distributions of numerical values were described as means and standard deviations or as medians and percentiles as appropriate. Comparisons of numerical values over time or across groups were made by means of Wilcoxon signed rank or paired tests as appropriate. Categorical variables were summarized with the use of percentages, and comparisons were performed by means of continuity-corrected chi-square tests or Fisher exact tests as appropriate. All tests were 2-sided, andP values of less than 5% were considered statistically significant. Analyses were performed by means of SPSS for Windows (SPSS, Chicago, IL) 10.0 and Splus 2000 (Insightful, Seattle, WA).
Results
There were 83 patients with a confirmed diagnosis of myelodysplastic syndromes registered on this protocol. Thirty-two patients discontinued the protocol before completing 12 weeks of therapy; 51 completed 12 weeks. We will present 2 separate sets of analysis: (1) an intent-to-treat (ITT) analysis of all 83 patients who either completed the 12 weeks on the study (51 patients) or prematurely discontinued therapy for various reasons (32 patients) and (2) an efficacy-analyzable analysis including only those subjects who completed the required minimum of 12 weeks on therapy.
Baseline descriptive values
The median age of the 83 patients was 67 years (Table1). There were 55 males and 28 females; 77 had primary de novo MDS, whereas 6 had secondary MDS. Thirty-six patients had refractory anemia (RA); 13 had RA with ringed sideroblasts (RARS); 24 had RA with excess blasts (RAEB); 6 had RAEB in transformation (RAEB-t); and 4 had chronic myelomonocytic leukemia (CMMoL). These patients were also characterized into prognostic categories by means of the IPSS.57 Briefly, 21 patients belonged to low-risk, 37 to intermediate-1 (INT-1), 12 to INT-2, and 13 to the high-risk category. Biopsy cellularity was available in 80 cases; of these, 54 were hypercellular, 14 normocellular, and 12 hypocellular. The median duration of MDS for the group was 426 days (range, 9-3351 days). Sixty-three patients were PRBC dependent, and 21 were dependent on platelet transfusions at the time treatment started.
Baseline descriptive values for all subjects in study
| Total no. patients enrolled in MDS 98-21 | |
| On study | 51 |
| Off study | 32 |
| Median age of patients, y | 67 |
| Sex | |
| Male | 55 |
| Female | 28 |
| FAB | |
| RA | 36 |
| RAEB | 24 |
| RAEB-t | 6 |
| RARS | 13 |
| CMMoL | 4 |
| MDS duration, d | |
| Median | 426 |
| Range | 9-3351 |
| Transfusion history | |
| PRBC dependent | |
| Yes | 63 |
| No | 20 |
| Platelet dependent | |
| Yes | 21 |
| No | 62 |
| Primary/secondary MDS | |
| Primary | 77 |
| Secondary | 6 |
| IPSS | |
| Low | 21 |
| INT-1 | 37 |
| INT-2 | 12 |
| High | 13 |
| Biopsy cellularity | |
| Hyper | 54 |
| Normal | 14 |
| Hypo | 12 |
| Thalidomide dose by duration, wk | |
| 400 mg for at least 8 weeks | 8 |
| 400 mg for less than 8 weeks | 36 |
| 200 mg to 350 mg | 28 |
| Less than 200 mg | 10 |
| Total no. patients enrolled in MDS 98-21 | |
| On study | 51 |
| Off study | 32 |
| Median age of patients, y | 67 |
| Sex | |
| Male | 55 |
| Female | 28 |
| FAB | |
| RA | 36 |
| RAEB | 24 |
| RAEB-t | 6 |
| RARS | 13 |
| CMMoL | 4 |
| MDS duration, d | |
| Median | 426 |
| Range | 9-3351 |
| Transfusion history | |
| PRBC dependent | |
| Yes | 63 |
| No | 20 |
| Platelet dependent | |
| Yes | 21 |
| No | 62 |
| Primary/secondary MDS | |
| Primary | 77 |
| Secondary | 6 |
| IPSS | |
| Low | 21 |
| INT-1 | 37 |
| INT-2 | 12 |
| High | 13 |
| Biopsy cellularity | |
| Hyper | 54 |
| Normal | 14 |
| Hypo | 12 |
| Thalidomide dose by duration, wk | |
| 400 mg for at least 8 weeks | 8 |
| 400 mg for less than 8 weeks | 36 |
| 200 mg to 350 mg | 28 |
| Less than 200 mg | 10 |
MDS, myelodysplastic syndrome; FAB, French-American-British classification; RA, refractory anemia; RAEB, RA with excess blasts; RAEB-t, RAEB in transformation; RARS, RA with ringed sideroblasts; CMMoL, chronic myelomonocytic leukemia; PRBC, packed red blood cell transfusion; platelet, platelet transfusion; IPSS, International Prognostic Scoring System; low, low-risk group; INT-1, intermediate-I risk group; INT-2, intermediate-II risk group; high, high-risk group.
Comparison of clinical characteristics of patients who stopped therapy before 12 weeks versus those who continued for at least 12 weeks
There were 2 significant differences between patients who stopped therapy before 12 weeks and those who continued. First, 17 of 32 off-study patients belonged to the high-rish or INT-2 category, whereas only 8 of 51 patients who continued had a high or INT-2 IPSS score (P = .002). Second, off-study patients had a higher percentage of blasts in their pretherapy bone marrow (7% versus 2%,P = .003). It appears therefore that patients with more advanced disease had decreased tolerance for the side effects of thalidomide or were taken off thalidomide owing to disease progression as described in the following section.
Drug tolerance and toxicity
Of the 83 patients entered in the study, 32 could not complete the 12 weeks of therapy. Among these 32, the median duration of therapy was 4 weeks (range, 0-8 weeks). One patient never started the drug; 6 were discontinued for disease progression; 11 discontinued the study for other medical problems (fever, bleeding, Sweet syndrome, worsening of renal amyloidosis, spinal chloroma, gum hyperplasia, transfusion reaction); and 14 discontinued the treatment because of side effects from the drug. The most common side effects were fatigue (79%), constipation (71%), shortness of breath (54%), fluid retention (48%), dizziness (40%), rash (31%), numbness and tingling in fingers and/or toes (29%), fever and headache (27% each), and nausea (25%). Fewer than 5% of patients had grade IV toxicity.
Among the 51 evaluable cases, 34 increased the dose of thalidomide as tolerated from 100 mg by mouth at bedtime to 400 mg by mouth at bedtime within 4 weeks of starting therapy, but only 8 were able to continue at that dose for a full 8 weeks (Table 1). The median duration of 400 mg therapy for the rest was 14 days (range, 1-42 days). Thus, although some of the patients increased the dose levels successfully, the higher doses were maintained for only a short period, with the majority taking between 150 to 200 mg by mouth at bedtime.
Response evaluation
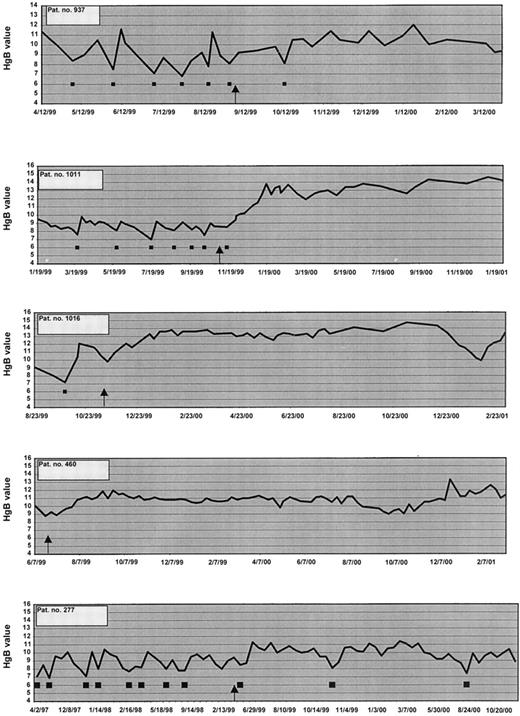
Of the 83 patients registered in this study, there were no complete responders. Sixteen patients showed a hematologic improvement as described in Table 2, with 15 responding in the erythroid series and 1 showing a minor platelet response. There were no ANC responders. Among the 15 showing HI in the erythroid series, 11 had a major erythroid response and 4 had a minor erythroid response. Of the 11 major responders, 6 responded by reducing their PRBC transfusion requirements by 100%; 4 showed both a decrease in transfusions by 100% and an increase in Hb; and 1 non–PRBC-dependent patient showed an increase in Hb of more than 2 g/dL. One of these patients also showed a minor platelet response. Figure 1 shows 5 of the erythroid responders. In addition, 4 patients also had a minor erythroid response, as shown in Table 2. All of these 4 patients showed a 50% reduction in PRBC transfusions. The 16th patient had a minor platelet response.
Hematological responses
| Patient no.* . | FAB . | BM cellularity . | PRBC dependent . | Responses . | Response type . | Response duration, d† . | Comment . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before . | After . | Before . | After . | Before . | After . | Reduction in PRBC, % . | Increase in Hb . | Platelets . | ANC . | ||||
| 937 | RA | RA | 20 | 30 | Y | N | 100 | Yes | No | No | HI-E major, 100% PRBC Tx reduction at wk 16; minor Hb response at wk 16 | 200 | Died of lymphoma |
| 1011 | RA | RARS | 99 | 70 | Y | N | 100 | Yes | No | No | HI-E major (PRBC), Tx independent at wk 16; major (Hb) | 422+ | Continues in remission |
| 277 | RA | RA | 90 | N/A | Y | N | 100 | No | No | No | HI-E major, 100% PRBC Tx reduction | 454+ | Continues in remission |
| 963 | RA | RARS | 80 | 90 | Y | N | 100 | No | No | No | HI-E major | 227 | Stopped responding |
| 400 | RARS | RARS | 70 | 70 | Y | Y | 50 | No | No | No | HI-E minor, 50% PRBC Tx reduction | 306 | Stopped after 1 year owing to side effects |
| 460 | RARS | RARS | 50 | 50 | N | N | Yes | No | No | HI-E major (Hb) | 620+ | Continues in remission | |
| 1016 | RA | RA | 30 | 30 | Y | N | 100 | Yes | No | No | HI-E major | 527+ | Continues in remission |
| 340 | RARS | RA | 80 | 90 | Y | Y | 50 | No | No | No | HI-E minor, at 16 wks and post-50% reduction in PRBC Tx | 168 | Stopped responding |
| 333 | RARS | N/A | 50 | N/A | Y | Y | 50 | No | No | No | 50% PRBC reduction from wk 20 to wk 24 & 28 | 239 | Stopped owing to side effects |
| 585 | RARS | RA | 80 | 80 | Y | Y | No | No | Yes | No | H1-P minor | 90 | Stopped owing to side effects |
| 1023 | RAEB | RAEB | 60 | 70 | Y | N | 100 | No | No | No | HI-E major (PRBC), Tx independent at wk 16 | 177 | Stopped responding |
| 1007 | RA | RA | 20 | 30 | Y | Y | 50 | No | No | No | HI-E minor, 50% PRBC Tx reduction at wk 20, sustained for 8 wks | 365 | Stopped owing to side effects |
| 1010 | RAEB | RAEB | 40 | 10 | Y | N | 100 | Yes | No | No | HI-E major (PRBC) major (Hb) | 381 | Stopped responding |
| 988 | RA | RA | 80 | 70 | Y | N | 100 | No | Yes | No | HI-E major, 100% PRBC Tx reduction at wk 16, minor Plt response at wk 16 (not wk 12 owing to plt Tx) | 359 | Stopped responding |
| 551 | RA | RA | 30 | N/A | Y | N | 100 | No | No | No | HI-E major, Tx dependent until wk 20, then independent through wk 28 | 188 | Stopped responding |
| 274 | RA | RA | 10 | 30 | Y | N | 100 | No | No | No | HI-E major, patient received 2 U PRBC day 1 of treatment, then was Tx independent until wk 28 with 2 U, then independent again | 365 | Stopped after 1 year owing to side effects |
| Patient no.* . | FAB . | BM cellularity . | PRBC dependent . | Responses . | Response type . | Response duration, d† . | Comment . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before . | After . | Before . | After . | Before . | After . | Reduction in PRBC, % . | Increase in Hb . | Platelets . | ANC . | ||||
| 937 | RA | RA | 20 | 30 | Y | N | 100 | Yes | No | No | HI-E major, 100% PRBC Tx reduction at wk 16; minor Hb response at wk 16 | 200 | Died of lymphoma |
| 1011 | RA | RARS | 99 | 70 | Y | N | 100 | Yes | No | No | HI-E major (PRBC), Tx independent at wk 16; major (Hb) | 422+ | Continues in remission |
| 277 | RA | RA | 90 | N/A | Y | N | 100 | No | No | No | HI-E major, 100% PRBC Tx reduction | 454+ | Continues in remission |
| 963 | RA | RARS | 80 | 90 | Y | N | 100 | No | No | No | HI-E major | 227 | Stopped responding |
| 400 | RARS | RARS | 70 | 70 | Y | Y | 50 | No | No | No | HI-E minor, 50% PRBC Tx reduction | 306 | Stopped after 1 year owing to side effects |
| 460 | RARS | RARS | 50 | 50 | N | N | Yes | No | No | HI-E major (Hb) | 620+ | Continues in remission | |
| 1016 | RA | RA | 30 | 30 | Y | N | 100 | Yes | No | No | HI-E major | 527+ | Continues in remission |
| 340 | RARS | RA | 80 | 90 | Y | Y | 50 | No | No | No | HI-E minor, at 16 wks and post-50% reduction in PRBC Tx | 168 | Stopped responding |
| 333 | RARS | N/A | 50 | N/A | Y | Y | 50 | No | No | No | 50% PRBC reduction from wk 20 to wk 24 & 28 | 239 | Stopped owing to side effects |
| 585 | RARS | RA | 80 | 80 | Y | Y | No | No | Yes | No | H1-P minor | 90 | Stopped owing to side effects |
| 1023 | RAEB | RAEB | 60 | 70 | Y | N | 100 | No | No | No | HI-E major (PRBC), Tx independent at wk 16 | 177 | Stopped responding |
| 1007 | RA | RA | 20 | 30 | Y | Y | 50 | No | No | No | HI-E minor, 50% PRBC Tx reduction at wk 20, sustained for 8 wks | 365 | Stopped owing to side effects |
| 1010 | RAEB | RAEB | 40 | 10 | Y | N | 100 | Yes | No | No | HI-E major (PRBC) major (Hb) | 381 | Stopped responding |
| 988 | RA | RA | 80 | 70 | Y | N | 100 | No | Yes | No | HI-E major, 100% PRBC Tx reduction at wk 16, minor Plt response at wk 16 (not wk 12 owing to plt Tx) | 359 | Stopped responding |
| 551 | RA | RA | 30 | N/A | Y | N | 100 | No | No | No | HI-E major, Tx dependent until wk 20, then independent through wk 28 | 188 | Stopped responding |
| 274 | RA | RA | 10 | 30 | Y | N | 100 | No | No | No | HI-E major, patient received 2 U PRBC day 1 of treatment, then was Tx independent until wk 28 with 2 U, then independent again | 365 | Stopped after 1 year owing to side effects |
BM indicates bone marrow; Hb, hemoglobin; ANC, absolute neutrophil count; HI-E, hematological improvement in erythroid series; Tx, transfusion; HI-P, hematological improvement in platelets; Plt, platelets; for other abbreviations, see Table1.
Unique Rush Cancer Institute patient numbers.
Plus sign indicates a continuing response.
Hemoglobin and PRBC transfusions in 7 patients.
These patients became transfusion independent on thalidomide. ▪, 2 units of PRBC transfused; ↑, start of thalidomide.
Hemoglobin and PRBC transfusions in 7 patients.
These patients became transfusion independent on thalidomide. ▪, 2 units of PRBC transfused; ↑, start of thalidomide.
Time to response
Five erythroid responders showed HI within 12 weeks of starting thalidomide, 6 within 16 weeks, and 4 at week 20. The platelet responder improved his count within 12 weeks of therapy.
Response duration
Patient no. 937 died of a reactivated low-grade lymphoma at day 260. Of the other 15 responding patients, the median duration of response was 306 days (range, 90-620 days). Four patients are still responding and continuing to take thalidomide. Six patients stopped responding and are listed in Table 2. Of the remaining 5 patients, 3 stopped owing to intolerable side effects, and 2 opted to stop after 1 year of therapy was completed and they were given the option of continuing or stopping.
Comparison of clinical characteristics in responders and nonresponders
A comparison of pretherapy characteristics in responders and nonresponders among the 51 patients who completed at least 12 weeks of therapy showed 3 significant findings (Table3). Responders had a lower median percentage of blasts to begin with (2% versus 3%, respectively,P = .016), a shorter median duration of platelet transfusions (median 0 for both, P = .013), and a higher median pretherapy platelet count (142 000 versus 57 000/μL,P = .003).
Clinical characteristics of responders and nonresponders
| Characteristics . | Median levels . | P . | |
|---|---|---|---|
| NR (n = 35) . | R (n = 16) . | ||
| Age, y | 67 | 69 | .428 |
| MDS duration, d | 670 | 321 | .951 |
| BM biopsy cellularity, % | 70 | 55 | .152 |
| BM blasts, % | 3 | 2 | .0163-150 |
| PRBC duration, d | 91 | 132 | .79 |
| Platelet transfusion duration, d | 0 | 0 | .0133-150 |
| Maximum thalidomide dose, mg/d | 400 | 400 | .902 |
| Duration of maximum dose, d | 14 | 12 | .532 |
| Hemoglobin, g/dL | 8.9 | 8.7 | .59 |
| ANC, per μL | 1350 | 1050 | .136 |
| Platelets, per μL | 57 000 | 142 000 | .0033-150 |
| Initial IPSS scores | INT-1 | INT-1 | .468 |
| Characteristics . | Median levels . | P . | |
|---|---|---|---|
| NR (n = 35) . | R (n = 16) . | ||
| Age, y | 67 | 69 | .428 |
| MDS duration, d | 670 | 321 | .951 |
| BM biopsy cellularity, % | 70 | 55 | .152 |
| BM blasts, % | 3 | 2 | .0163-150 |
| PRBC duration, d | 91 | 132 | .79 |
| Platelet transfusion duration, d | 0 | 0 | .0133-150 |
| Maximum thalidomide dose, mg/d | 400 | 400 | .902 |
| Duration of maximum dose, d | 14 | 12 | .532 |
| Hemoglobin, g/dL | 8.9 | 8.7 | .59 |
| ANC, per μL | 1350 | 1050 | .136 |
| Platelets, per μL | 57 000 | 142 000 | .0033-150 |
| Initial IPSS scores | INT-1 | INT-1 | .468 |
Significant at .05 level.
Delayed response to thalidomide
A curious observation, which became apparent in a striking manner, was related to the matter of delayed response seen in one of the patients. This is described in greater detail below because it may throw some light on the possible mechanism by which thalidomide improves the cytopenias in MDS patients. This patient is considered a nonresponder by the criteria specified in this paper and is not included in Table 2.
Patient no. 123
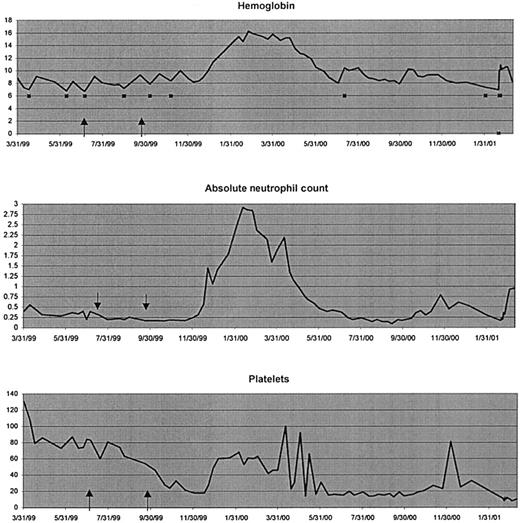
This 65-year-old white male was diagnosed with MDS, RA, approximately 2 years before starting therapy with thalidomide (Figure2). Cytogenetics revealed a complex karyotype including a 20q deletion with further evolution in the clone. The patient received therapy for his primary MDS with the cytoprotective agent amifostine and a combination of pentoxifylline, ciprofloxacin, and dexamethasone for 3 months as per protocol MDS 96-0236 with no response, and with the TNF-soluble receptor Enbrel (Immunex, Seattle, WA)37 for 3 months, again with no appreciable hematopoietic response but with clearing of rather painful aphthous ulcers in his mouth. After a hiatus of several months during which he received only supportive care with frequent PRBC transfusions, he began taking thalidomide at 100 mg by mouth at bedtime on July 1, 1999. His pretherapy CBC revealed a hemoglobin of 7.1 g/dL, an ANC of 200/μL, and platelets of 84 000/μL. He could not tolerate a dose of thalidomide greater than 200 mg by mouth at bedtime. At the end of 12 weeks, he was evaluated for response and was found to have no evidence of improvement in any of the cytopenias. The post-treatment CBC revealed a hemoglobin of 8.1 g/dL, an ANC of 90/μL, and a platelet count of 38 000/μL. The platelets and ANC were found to be in a continuous decline. He was taken off the thalidomide on September 29, 1999, and entered a 3-month washout period before being considered for further experimental therapy. He continued to require red cell transfusions, the last one being on November 12, 1999. On December 1, 1999, his hemoglobin was 8.7 g/dL, his ANC was less than 200/μL, and his platelets had fallen to 18 000/μL. On December 16, 1999, his hemoglobin was still 8.4 g/dL, but he had not required a PRBC transfusion in over a month; his ANC was below 200/μL; and his platelets were 18 000/μL. Interestingly, from the next week on, a steady improvement was noted in all 3 cell lines. The patient did not require another transfusion for approximately 9 months.
Delayed response.
Peripheral blood indices in an MDS patient (no. 123) with RA showing a delayed response to therapy with thalidomide. ↑, duration of thalidomide therapy (indicated between 2 arrows); ▪, 2 units of PRBC transfused.
Delayed response.
Peripheral blood indices in an MDS patient (no. 123) with RA showing a delayed response to therapy with thalidomide. ↑, duration of thalidomide therapy (indicated between 2 arrows); ▪, 2 units of PRBC transfused.
Cytogenetic responses
Cytogenetic results were available in 15 of 16 responders; 7 had a normal karyotype, 1 had −Y, 1 had 8+, 1 had del(20q), 1 had t(2;8), 1 had der(7), and 3 had del(5q). No cytogenetic responses were observed.
Discussion
We report upon the success of thalidomide in alleviating the cytopenias of some patients with MDS. By the most conservative estimation, assuming that all patients who went off the study before being evaluable for a response were in fact nonresponders, the response rate is approximately 19% (16 of 83). In an analysis of only those patients who finished 12 weeks of therapy, approximately 31% (16 of 51) of evaluable individuals responded, with 15 of 16 showing an erythroid response; some of the responses were quite striking. Ten transfusion-dependent patients acquired transfusion independence. The current study using thalidomide is the latest in a series of clinical trials conducted over the last 6 years using anticytokine and cytoprotective agents34-37 aimed at improving the ineffective hematopoiesis of MDS. An advantage of using thalidomide is that in addition to neutralizing the effects of some proinflammatory, proapoptotic cytokines,47-51 this drug is also a known modulator of the immune system45,46 as well as an antiangiogenic agent.52-54
This pilot study was designed to test the efficacy of thalidomide in producing complete or partial remissions in either low- or high-risk patients with MDS. The response criteria specified in the protocol were similar to those that we and others have used in previous trials.34-37 As the study was reaching completion, however, the report of an IWG to standardize response criteria for myelodysplastic syndromes was published,58 and it was considered more appropriate to use the standardized criteria to assess response. Given the complexity of the disease and the sensitivity of the proposed criteria, an independent team of investigators was asked to apply the IWG criteria for response evaluation. In this way, both investigator bias and confusion about precisely what is meant by a response were eliminated. One potentially confusing aspect of these recommendations is that the IWG did not specify the method for determining baseline values of ANCs, Hb counts, and platelet counts against which response is to be measured. For example, given the frequently fluctuating nature of cytopenias in MDS patients, and the fact that patients being considered for therapy are most likely to be transfusion dependent, these values can vary widely from week to week. It is therefore important to decide whether an average of several values or one pretherapy absolute CBC result will be used as the baseline. The same applies to the number of pretherapy transfusions. Finally, the IWG did not specify how the CBC values during therapy are to be handled for response evaluation. For example, an assessment can be made every 3 months or every 6 months, but should the average CBC results be used for each cycle? The IWG itself anticipated that future modifications would be made to its standardized criteria as independent investigators began to apply these recommendations to assess the results of their clinical trials. Therefore, modifications to the criteria were made related to the calculation of pretherapy baseline CBC values and values during therapy; these modifications are described in detail in “Patients, materials, and methods.” We present the results of this analysis, showing that thalidomide is capable of producing major hematologic improvements in a subset of MDS patients, especially those with low-risk disease.
Eighty-three patients were registered on the protocol, but only 51 completed at least 12 weeks of therapy. Of these 51 patients, 34 were able to increase the thalidomide dose to 400 mg within a month, but only 8 stayed at this dose for the remainder of their treatment duration. The rest could tolerate this dose for a median of 2 weeks before decreasing back to the 100 to 200 mg range. A dose-response effect was not appreciated, and it can be concluded that maintaining the maximally tolerated dose for a longer period of up to 6 months is more important than pushing for a higher dose for a shorter duration. The most common dose-limiting side effects were related to fatigue (79%) and constipation (71%), followed by shortness of breath (54%) and fluid retention, as manifested frequently by ankle edema (54%). More than 50% of the patients had grades 1 and 2 toxicity, and fewer than 5% had grade 4 shortness of breath and fluid retention. Of the 32 patients who were taken off the study, 6 had shown unequivocal signs of transformation to AML; 11 discontinued the drug owing to worsening medical problems, some of which could also be due to disease progression; and 14 could not tolerate the side effects. Patients who discontinued therapy belonged to a higher risk MDS category than those who continued for at least 12 weeks as shown by both their IPSS scores (P = .002) as well as their initial bone marrow blasts (P = .003). Only 12 of 24 RAEB, 1 of 6 of RAEB-t, and one fourth of CMMoL patients were able to complete 12 weeks of therapy. Of 12 RAEB patients who completed 12 weeks of therapy, 2 had a major HI, whereas 3 showed an improvement in their cytopenias, but were not considered responders because blast count in their marrows had increased. Whether this reflects natural disease progression or an increased incidence of transformation owing to thalidomide needs to be assessed in larger trials. Although caution clearly needs to be exercised in prescribing thalidomide for RAEB/RAEB-t patients, there is no evidence of accelerated disease progression in the RA/RARS group of patients, some of whom have achieved significant hematologic improvements.
There were no complete responders, but 16 patients showed hematologic improvement as defined by IWG; 15 of these had an erythroid response, and 1 patient had a platelet response. Responders had lower pretherapy blast counts, higher pretherapy platelet counts, and a shorter duration of platelet transfusions prior to starting thalidomide. Ten patients became transfusion independent, and some of these responses are so dramatic that they deserve special mention. For example, patient no. 937 (Table 2) became transfusion independent within a month of starting therapy. Patient no. 1011 in Table 2, who belonged to IPSS INT-1 category, requiring 2 to 4 units of PRBCs per month for approximately 10 months prior to starting thalidomide, was able to entirely normalize his hemogram by day 80. Interestingly, he had a previous history of cardiac disease, because of which he developed fluid retention, and was therefore unable to tolerate the higher doses of thalidomide, mostly taking 100 to 200 mg per night. Even with this low dose, not only did he have a significant hematologic response, but an ugly keloid scar on his sternal area has almost completely resolved. It can be speculated that this may be related to the drug's antiangiogenic properties and activity in treating Kaposi's sarcoma.59 He is still in remission and is being maintained on 50 to 100 mg of thalidomide daily. Curiously enough, one patient (patient no. 123) demonstrated a delayed trilineage response 12 weeks after stopping thalidomide, which implies a rather complex mechanism of thalidomide action.
The timing of the responses may shed some light on yet another possible mechanism of thalidomide action. An effect of therapy could be appreciated within the first week of starting treatment in a handful of patients, but gradual improvement in counts over an extended period of time was more common. Six responses were documented at 12 weeks, 6 at 16 weeks, and 4 at 20 weeks. From these data, it appears that the prudent approach is to continue therapy, at least in the absence of disease progression, for 6 months before terminating the trial for lack of response. The delayed effect of thalidomide described for patient no. 123 is rather reminiscent of ATG-induced responses in aplastic anemia and may imply immunomodulation as the predominant mechanism of action of thalidomide in at least some patients.
Thalidomide is definitely an exciting new agent deserving further investigation, especially in the treatment of low-risk MDS patients. The most significant effect of the drug was on the erythroid cells. No patient experienced an ANC response, and only 2 patients showed minor platelet responses. On the basis of this experience, it may be worthwhile to combine thalidomide in future trials with an agent like Enbrel, the soluble TNF-receptor whose administration to MDS patients is associated with an improvement in ANC and platelets but not in hemoglobin.37 The dose of thalidomide best tolerated in the majority of MDS patients in the present study appears to be between 150 and 200 mg per day. For future trials, we recommend starting at 100 mg thalidomide by mouth at bedtime and increase as tolerated to 200 mg by mouth at bedtime. Larger numbers of patients with RAEB, RAEB-t, and CMMoL need to be studied before the potential efficacy of thalidomide can be unequivocally determined for this group. The fact that 10 transfusion-dependent MDS patients became transfusion independent in response to thalidomide is an encouraging finding in this otherwise refractory group of patients.
Supported by a National Cancer Institute grant (PO1 CA75606), The Roy Ringo Grant for Research in MDS, and a grant from The Woman's Board TIME Center.
J.Z. is an employee of the Celgene Corporation, which supplied thalidomide free of charge to the patients in this study. A.R. and P.V. have declared a financial interest in the Celgene Corporation, and A.R. is a member of the Speakers Bureau for the Celgene Corporation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Azra Raza, Professor of Medicine, Director, Pre-Leukemia and Leukemia Program, Rush-Presbyterian–St Luke's Medical Center, 2242 West Harrison St, Suite 108, Chicago, IL 60612-3515; e-mail: araza@rush.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal