In vitro studies have indicated that the granulocyte-macrophage colony-stimulating factor (GM-CSF) gene expression is regulated at the posttranscriptional level by the AU-rich element (ARE) sequence present in its 3′ untranslated region (UTR). This study investigated the importance of the ARE in the control of GM-CSF gene expression in vivo. For this purpose, transgenic mice bearing GM-CSF gene constructs containing or lacking the ARE (GM-CSF AU+ or GM-CSF AU−, respectively) were generated. Both transgenes were under the transcriptional control of the immediate early promoter of the cytomegalovirus (CMV) to ensure their early, widespread, and constitutive expression. The regulation imposed by the ARE was revealed by comparing transgene expression at day 14 of embryonic development (E14); only the ARE-deleted but not the ARE-containing construct was expressed. Although GM-CSF AU+ embryos were phenotypically normal, overexpression of GM-CSF in E14 GM-CSF AU− embryos led to severe hematopoietic alterations such as abnormal proliferation of granulocytes and macrophages accompanied by an increased number of peroxidase-expressing cells, their putative progenitor cells. These abnormalities compromise development because no viable GM-CSF AU− transgenic pups could be obtained. Surprisingly, by E18, significant accumulation of transgene messenger RNA was also observed in GM-CSF AU+ embryos leading to similar phenotypic abnormalities. Altogether, these observations reveal that GM-CSF ARE is a developmentally controlled regulatory element and highlight the consequences of GM-CSF overexpression on myeloid cell proliferation and differentiation.
Introduction
Many messenger RNAs (mRNAs) that encode cytokines or oncogenes are transiently expressed due to multiple regulatory controls. A common feature of these mRNAs is the presence of a conserved AU-rich element (ARE) in their 3′ untranslated region (UTR).1 AREs have been classified in 3 categories based on the number and distribution of the pentanucleotide AUUUA they contain.2 Class I AREs are characterized by the presence of 1 to 3 pentamers distributed into a large part of the 3′UTR coupled with a nearby U-rich region. Class II AREs have at least 2 overlapping copies of the nonamer UUAUUU(U/A)(U/A) in a U-rich environment. Finally, class III AREs do not contain any pentamer but present U-rich stretches. All 3 categories of AREs confer mRNA instability through different mechanisms all implying mRNA deadenylation as a first step of mRNA decay (for review, see reference 2). Moreover, some class II AREs have been shown to mediate translation regulation.3-6Although AREs of most oncogene mRNAs fall into classes I and III, class II mainly includes AREs from cytokine mRNAs. In this regard, the ARE present in granulocyte-macrophage colony-stimulating factor (GM-CSF) mRNA constitutes the prototype of class II ARE and was the first of these elements shown to regulate mRNA stability.7 Indeed, insertion of GM-CSF ARE into the 3′ UTR of the otherwise stable β-globin mRNA induced rapid mRNA degradation. Later on, several in vitro studies demonstrated the crucial role of the ARE in the control of GM-CSF mRNA stability and consequently in the regulation of GM-CSF production.8-11
Granulocyte-macrophage CSF is a 23-kd glycoprotein that stimulates the growth and differentiation of granulocyte and macrophage progenitor cells, both in vitro and in vivo.12 Moreover, GM-CSF acts on mature granulocytes and macrophages, increasing their survival, migration, and antimicrobial activity.13 A number of different cell types including T and B lymphocytes, macrophages, fibroblasts, and endothelial cells can produce GM-CSF when stimulated. This production can be the result of increased transcription, as in T lymphocytes, or posttranscriptional stabilization of the mRNA, as in macrophages, eosinophils, and fibroblasts. In the latter cases, in vitro studies have shown that this stabilization resulted from inhibition of ARE-dependent degradation.8 14 It is therefore conceivable that the ARE plays an important role in the control of GM-CSF production in vivo. The fact that multiple regulator checkpoints govern the expression of GM-CSF suggests that impaired regulation of this cytokine might have untoward consequences.
In the present study, we have investigated the role of GM-CSF ARE in vivo by generating transgenic mice bearing mouse GM-CSF gene constructs, either containing or lacking the ARE. Because other regulatory sequences located within the mRNA could influence ARE activity, we kept in the constructs the ARE in its natural context.15,16 Moreover, to analyze when and where the ARE controls the level of GM-CSF mRNA expression, the constructs were placed under the transcriptional control of the cytomegalovirus (CMV) early immediate promoter, which has been shown to be active early in development and in most tissues.17 18 Because both constructs led to major lethality before birth, we analyzed transgene expression during late embryonic development. Our results reveal the fundamental role of the ARE in the control of GM-CSF mRNA accumulation in vivo and point to the consequences of inappropriate GM-CSF expression on the differentiation of myeloid cells.
Materials and methods
Cell cultures and transfection
HeLa and L929 cells were transfected using lipofectamine (Life Technologies, Merelbeke, Belgium) and stable clones were selected with 1 mg/mL G418 (Life Technologies). Cycloheximide treatment (100 μg/mL) was performed for a period of 2.5 hours. Semiconfluent nontransfected or transfected HeLa cells were washed 3 times with Dulbecco modified Eagle medium (DMEM), covered with 15 mL medium without fetal bovine serum (FBS), and further incubated for 3 to 5 days. The cell culture supernatants were collected and the GM-CSF concentration was determined using the mouse GM-CSF enzyme-linked immunosorbent assay (ELISA) kit (Endogen, Woburn, MA) and c-Myc Tag ELISA. Monoclonal antimouse GM-CSF antibody (BD Pharmingen, San Diego, CA) was coated (10 μg/mL) overnight at 4°C. GM-CSFTag detection was performed using biotinylated 9E10 antibody (10 μg/mL), extravidine peroxidase conjugate (Sigma Chemical, St Louis, MO) 1:1000, and o-phenylenediamine dihydrochloride (Sigma) as peroxidase substrate. The supernatants were concentrated 30 × using Amicon centriprep-10 concentrator (Millipore, Brussels, Belgium) for subsequent Western blot analysis.
The biologic activity of the GM-CSFTag protein was assessed in a proliferation assay using the GM-CSF–dependent cell line NFS-60.19 Briefly, these cells were cultured during 3 days in RPMI medium containing 10% WEHI 3 conditioned culture medium (containing interleukin-3 [IL-3]) to induce IL-3 receptor decrease. Supernatant to be tested (100 μL) was added in triplicate in the first lane of wells of a 96-well plate containing 100 μL RPMI medium in each well. Then, serial 2 × dilutions were performed and 100 μL RPMI medium containing 20 000 NFS-60 cells was added to each well. After 24 hours of incubation, [3H]-thymidine (0.37 MBq) was added and cells were further incubated for 12 hours. Cells were harvested and [3H]-thymidine incorporation was measured. In parallel, the GM-CSFTag-mediated biologic activity was verified by performing the proliferation assay in the presence of decreasing concentrations of anti–GM-CSF antibody.
Western blot analysis
Fifteen microliters of 30 × concentrated supernatants in sodium dodecyl sulfate (SDS) sample buffer were fractionated on 15% SDS–polyacrylamide gel (SDS-PAGE). After electroblotting, the nitrocellulose membranes (Schleicher & Schuell, Gent, Belgium) were incubated in phosphate-buffered saline (PBS) containing 2% dried milk overnight and then 3 hours at room temperature in a PBS, 10% FBS, 0.5% Tween-20 solution containing 10 μg/mL polyclonal goat anti–GM-CSF antibody (R&D Systems, Abingdon, Oxon, United Kingdom). The membranes were then washed in PBS containing 0.5% Tween, and incubated with horseradish peroxidase (HPR) antigoat antibody (Sigma) 1:4000 in PBS, 10% FBS, 0.5% Tween-20 for 1 hour 30 minutes. Detection was performed using enhanced chemiluminescence (ECL) plus Western blotting detection system (Amersham, Pharmacia Biotech, Roosendaal, The Netherlands).
RNA extraction and reverse transcription–polymerase chain reaction
Total RNA was extracted as reported in Chomczynski and colleagues.20 Reverse transcription–polymerase chain reaction (RT-PCR) analysis was performed using 7 μg total RNA, GM3 primer (5′AATTTATTATTCTGGTAAGACATTC3′; Figure1A) and 200 U Superscript RTII (Life Technologies) for 1 hour 30 minutes at 37°C. PCR was performed with a quarter of the RT using either GM2 (5′ AAGCTAACATGTGTGCAGACCCGC3′) and GM3 primers or GM1 (5′CTCAGAGAGAAAGGCTAAGG3′) and GM4 (5′AAAGTTTTAATAATTTATTATTCTGG3′) primers. The PCR products were cloned into pSP64 plasmids, which were then appropriately linearized for in vitro transcription.
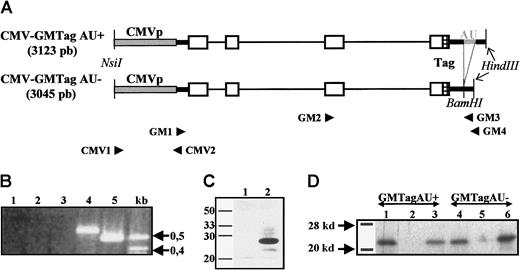
GM-CSF gene constructions with or without ARE.
(A) Schematic representation of the CMV-GMTag AU+and AU− DNA constructs. Gray box indicates CMV promoter; thick black lines, 5′ and 3′ UTRs; thin lines, introns; open boxes, coding region; gray thick line, ARE; and hatched box, c-Myc Tag. Primers used for PCR or RT-PCR and relevant restriction sites are indicated. (B) RT-PCR products derived from RNA of L-929 cells untransfected or transfected with both constructs. Lane 1, untransfected L-929 cells; lane 2, L929 cells treated with cycloheximide; lane 3, L929 cells stably transfected with pBK-CMV–GMTag AU+; lane 4, pBK-CMV–GMTag AU+transfected L-929 cells treated with cycloheximide; lane 5, L929 cells stably transfected with pBK-CMV–GMTag AU−. (C) Detection of GMTag by Western blot; 15 μL 30 × concentrated supernatant of nontransfected (lane 1) or pBK-CMV–GMTag AU− transfected HeLa cells was loaded on 15% SDS-PAGE and blotted on nylon membrane. The membrane was incubated with goat antimouse GM-CSF primary antibody and HPR-coupled rabbit antigoat secondary antibody and revealed by ECL. (D) Translation of in vitro transcribed GM-Tag AU+ and AU− mRNAs in rabbit reticulocyte lysate. After translation, 10 μL of the reaction was immunoprecipitated with anti–GM-CSF or anti-Tag antibodies. The immunoprecipitated products were analyzed by SDS-PAGE and autoradiography. Lanes 1, 2, and 3, GMTag AU+ RNA translation product immunoprecipitated with antimouse GM-CSF antibody (lane 1), anti-Tag antibody (lane 3), or no antibody (lane 2); lanes 4, 5, and 6, GMTag AU−translation product immunoprecipitated with anti–GM-CSF (lane 4), anti-Tag (lane 6) antibodies, or no antibody (lane 5).
GM-CSF gene constructions with or without ARE.
(A) Schematic representation of the CMV-GMTag AU+and AU− DNA constructs. Gray box indicates CMV promoter; thick black lines, 5′ and 3′ UTRs; thin lines, introns; open boxes, coding region; gray thick line, ARE; and hatched box, c-Myc Tag. Primers used for PCR or RT-PCR and relevant restriction sites are indicated. (B) RT-PCR products derived from RNA of L-929 cells untransfected or transfected with both constructs. Lane 1, untransfected L-929 cells; lane 2, L929 cells treated with cycloheximide; lane 3, L929 cells stably transfected with pBK-CMV–GMTag AU+; lane 4, pBK-CMV–GMTag AU+transfected L-929 cells treated with cycloheximide; lane 5, L929 cells stably transfected with pBK-CMV–GMTag AU−. (C) Detection of GMTag by Western blot; 15 μL 30 × concentrated supernatant of nontransfected (lane 1) or pBK-CMV–GMTag AU− transfected HeLa cells was loaded on 15% SDS-PAGE and blotted on nylon membrane. The membrane was incubated with goat antimouse GM-CSF primary antibody and HPR-coupled rabbit antigoat secondary antibody and revealed by ECL. (D) Translation of in vitro transcribed GM-Tag AU+ and AU− mRNAs in rabbit reticulocyte lysate. After translation, 10 μL of the reaction was immunoprecipitated with anti–GM-CSF or anti-Tag antibodies. The immunoprecipitated products were analyzed by SDS-PAGE and autoradiography. Lanes 1, 2, and 3, GMTag AU+ RNA translation product immunoprecipitated with antimouse GM-CSF antibody (lane 1), anti-Tag antibody (lane 3), or no antibody (lane 2); lanes 4, 5, and 6, GMTag AU−translation product immunoprecipitated with anti–GM-CSF (lane 4), anti-Tag (lane 6) antibodies, or no antibody (lane 5).
In vitro transcription and translation
In vitro transcription was performed with the SP6 mMESSAGE mMACHINE kit (Ambion, Austin, TX). Five hundred nanograms of each RNA were translated for 30 minutes in the rabbit reticulocyte lysate system (Promega Benelux, Leiden, The Netherlands) supplied with 0.555 MBq/20 μL reaction L-[35S]-methionine (Amersham, Pharmacia Biotech).
Transgenic DNA constructs
The CMV-GMTag AU+ and CMV-GMTag AU−constructs were generated using the mouse GM-CSF genomic clone PLGM-CSF12 (a generous gift from Dr P. Vandenabeele). Briefly, c-Myc Tag was inserted at the C-terminal end of GM-CSF coding sequence by PCR amplification of GM-CSF genomic clone using (1) a 86-bp long primer A (forward) starting at the EcoRV site at the end of the GM-CSF coding sequence and containing the c-Myc Tag encoding sequence before the TGA stop codon and (2) the reverse primer B (5′AAGCTTTGACAGCATTGACTCTCATCAATAC3′), which hybridized to the sequence located 91 nucleotides downstream from the poly(A) signal. The AU−construct was obtained after separated amplification of the 2 sequences adjacent to the ARE (defined as the sequence located between nucleotides 1-203 and 283-418 downstream from the stop codon using primers A and C (5′GGATCCGTCCCTATCAGTAGAAAATATCTC3′) and primer D (5′GGATCCAATGTCTTACCAGAATAATAAATT3′) and B, respectively. The 2 PCR products were then ligated and inserted into the PLGM-CSF12 plasmid (details are available on request). The modified GMTag AU+and AU− fragments were inserted into the pBK-CMV plasmid (Stratagene, La Jolla, CA) in which the lac promoter was deleted. The resulting pBK-CMV–GMTag AU+ and AU− constructs were verified by sequencing.
Generation of transgenic mice
The 3-kb CMV-GMTag AU+ and AU−fragments without plasmid sequences were isolated and injected into one of the pronuclei of (C57Bl/6XCBA) fertilized eggs.21Transgenic embryos or adult mice were identified by PCR with placenta or tail DNA using primers CMV1 (TCTGACGGTTCACTAAACCA) and CMV2 (ATCAATTACGGGGTCATTAG) (Figure 1A). Southern blot analysis was performed with a 600-pb probe corresponding to the CMV promoter to confirm the presence of the transgene.
Fourteen and 18-day-old embryos (E14 and E18) and their placentas were collected and directly frozen on dry ice, and stored at −80°C. Genetic screen was performed on placenta DNA to identify transgenic embryos. Transgenic embryos and nontransgenic control animals were totally cut in 20-μm sections in a cryostat and sections kept at −80°C until use for in situ hybridization and immunohistochemistry.
The transgene copy number was evaluated by semiquantitative PCR amplification of a transgene-specific fragment (AU+, 364 bp; AU− , 290 pb) containing the Tag sequence (primers: forward: 5′GAGCAAAAGCTCATTTCTGA3′ and reverse 5′ AATTTATTATTCTGGTAAGACATTCTCAATAAATAG3′) and compared to the amplified product specific of the GM-CSF endogenous gene (primers: forward: 5′ TGAATGCAAAAAACCAGGCCAAAAATGAGG3′ and the same reverse primer as for the transgene-specific amplification). The amplified products were submitted to electrophoresis in a 2% agarose gel, followed by Southern blot analysis. The Southern blot was hybridized with a labeled oligonucleotide hybridizing to the PCR fragments and the radioactive signal was quantified using an Instantimager (Packard Instrument, Meriden, CT).
In situ hybridization
[α-35S] uridine triphosphate (UTP) (Amersham Pharmacia Biotech) labeled riboprobes were synthesized by in vitro transcription. In situ hybridization and emulsion were performed as previously described22 using antisense and sense riboprobes as negative controls.
Immunohistochemistry
Slides were air-dried for 10 minutes, fixed in acetone for 10 minutes at room temperature, and hydrated by 100%, 95%, and 75% ethanol and H2O baths (1 minute/bath). For peroxidase detection, slides were treated 35 minutes in methanol, 0.3% H202 at room temperature to inhibit endogenous peroxidases, rinsed in PBS pH 7.4 (1 minute), and blocked with 100 μL PBS containing 1% blocking reagent (Roche Diagnostics, Brussels, Belgium) pH 7.4 (1 hour) at room temperature. Three rinsing steps in PBS, pH 7.4, were performed before covering the slides with 100 μL biotinylated antibody (10 μg/mL) in PBS, 1% blocking reagent, pH 7.4, and incubation overnight at room temperature in a humidified chamber. For detection, vectastain ABC-peroxidase and ABC-alkaline phosphatase kits (Vector Laboratories, Burlingame, CA) were used as described, with DAB (Sigma) and vector blue substrate kit for substrate, respectively.
Peroxidase detection
Peroxidase activity was assayed by treating the slides as for immunohistochemistry (without endogenous peroxidase-inhibiting treatment) until blocking step. After blocking, slides were directly incubated with 100 μL peroxidase DAB substrate (Sigma).
Results
Comparative analysis of GM-CSF AU+ and AU− constructs exvivo
Two mouse GM-CSF gene constructs containing or lacking the ARE were generated, GM-CSF AU+ and GM-CSF AU−, respectively. The 2 constructs were both under the control of the CMV promoter, which has been previously shown to promote the strong expression of reporter genes in tissue-cultured cells and the quasi-ubiquitous (although at variable levels) expression of trangenes in transgenic mice.17 Moreover, they both contained a sequence encoding a human c-Myc epitope before the stop codon to allow the distinction between the transgenes and the endogenous gene as well as their respective products (Figure 1A, and “Materials and methods”). Before generating transgenic animals with these constructs, their capacity to generate correct transcripts as well as biologically active GM-CSF was tested. Therefore, L-929 or HeLa cells were stably transfected with both constructs and RT-PCR was performed to detect transgenic GM-CSF AU+ and AU− mRNAs (see “Materials and methods”). Although RT-PCR products derived from the pBK-CMV–GMTag AU− transfected cells could easily be detected, those corresponding to the pBK-CMV–GMTag AU+transcripts could only be observed once the cells were treated with cycloheximide, which is known to stabilize ARE-containing mRNAs7 8 (Figure 1B). Likewise, the GMTag protein could only be detected in the culture medium of pBK-CMV–GMTag AU-transfected Hela cells by ELISA (data not shown) or by Western blot (Figure 1C), confirming thus the lack of GM-CSF AU+ transcripts in pBK-CMV–GMTag AU+ transfected cells. The ability of each construct to yield tagged GM-CSF mRNA and protein was tested using HeLa cells transfected with pBK-CMV–GMTag AU− and cycloheximide-treated HeLa cells transfected with pBK-CMV–GMTag AU+. After RT-PCR, the complementary DNAs (cDNAs) were in vitro transcribed and translated in reticulocyte lysate (see “Materials and methods”). As illustrated in Figure 1D, both cDNAs led to the synthesis of tagged GM-CSF, which was immunoprecipitated both with anti–GM-CSF and anti-Myc Tag antibodies. Finally, the biologic activity of the tagged GM-CSF was analyzed by measuring the proliferation rate of the GM-CSF–dependent NFS-60 cells in the presence of varying dilutions of culture media from pBK-CMV–GMTag AU− transfected Hela cells. Whereas culture medium from untransfected cells did not significantly promote cell proliferation, culture medium from transfected cells induced cell proliferation in a dose-dependent manner. Moreover, this proliferative effect was due to the tagged GM-CSF because it was counteracted by increasing doses of anti–GM-CSF antibody (Figure2). Altogether, these results show that both constructs encode biologically active, tagged GM-CSF but that the ARE imposes strong posttranscriptional regulation that prevents accumulation of GM-CSF AU+ transcripts in the in vitro cultured cells.
Analysis of GMTag protein biologic activity.
The GM-CSF/IL-3 proliferation-dependent NFS-60 cells were incubated with decreasing concentrations of nontransfected (○) or pBK-CMV–GMTAg AU− transfected (●) HeLa cell supernatants. The first dilution of the pBK-CMV–GMTag AU−transfected cell supernatant contained 10 ng/mL GM-CSF as determined by ELISA. (■) Cells were incubated with 100 μL supernatant of pBK-CMV–GMTAg AU− transfected HeLa cells in the presence of decreasing concentrations of antimouse GM-CSF antibody. The transfected cell supernatant contained 20 ng/mL GM-CSF as determined by ELISA. Cell proliferation was measured by [3H]-thymidine incorporation (see “Materials and methods”).
Analysis of GMTag protein biologic activity.
The GM-CSF/IL-3 proliferation-dependent NFS-60 cells were incubated with decreasing concentrations of nontransfected (○) or pBK-CMV–GMTAg AU− transfected (●) HeLa cell supernatants. The first dilution of the pBK-CMV–GMTag AU−transfected cell supernatant contained 10 ng/mL GM-CSF as determined by ELISA. (■) Cells were incubated with 100 μL supernatant of pBK-CMV–GMTAg AU− transfected HeLa cells in the presence of decreasing concentrations of antimouse GM-CSF antibody. The transfected cell supernatant contained 20 ng/mL GM-CSF as determined by ELISA. Cell proliferation was measured by [3H]-thymidine incorporation (see “Materials and methods”).
Comparative analysis of GM-CSF AU+ and AU−transgene expression in vivo
The 2 constructs were microinjected to derive transgenic mice. The results are summarized in Table 1. For GM-CSF AU+, 15 mice were recovered from 642 reimplanted eggs; 2 transgenic pups were born, but did not survive for more than 3 weeks. Death was associated with partial paralysis and failure to thrive. For GM-CSF AU−, 14 mice were recovered from 598 reimplanted eggs; none were transgenic. Statistical comparison of the transgenic yield obtained for both constructs compared to the yield that we currently obtained with other constructs revealed a strong lethality associated with the 2 transgenes. To circumvent this problem, we derived transient transgenic embryos and analyzed transgene expression at day 14 and 18 of development by in situ hybridization with GM-CSF antisense riboprobe (see “Materials and methods”).
Summary of the microinjection experiments
| . | Injected DNA construct . | |
|---|---|---|
| CMV-GMTagAU+ . | CMV-GMTagAU− . | |
| Experiment 1 | ||
| Total no. of reimplanted eggs | 642 | 598 |
| No. of births | 15 | 14 |
| Transgenic animals | 2 | 0 |
| Survivals after 3 wks | 0 | 0 |
| Experiment 2 | ||
| Total no. of reimplanted eggs | 266 | 465 |
| No. of E14 embryos | 12 | 28 |
| E14 transgenic embryos | 2 | 3 |
| Experiment 3 | ||
| Total no. of reimplanted eggs | 257 | |
| No. of E18 embryos | 10 | |
| E18 transgenic embryos | 2 | |
| . | Injected DNA construct . | |
|---|---|---|
| CMV-GMTagAU+ . | CMV-GMTagAU− . | |
| Experiment 1 | ||
| Total no. of reimplanted eggs | 642 | 598 |
| No. of births | 15 | 14 |
| Transgenic animals | 2 | 0 |
| Survivals after 3 wks | 0 | 0 |
| Experiment 2 | ||
| Total no. of reimplanted eggs | 266 | 465 |
| No. of E14 embryos | 12 | 28 |
| E14 transgenic embryos | 2 | 3 |
| Experiment 3 | ||
| Total no. of reimplanted eggs | 257 | |
| No. of E18 embryos | 10 | |
| E18 transgenic embryos | 2 | |
At E14, we obtained 2 (of 12) and 3 (of 28) transgenic embryos for GM-CSF AU+ and AU−, respectively (Table 1). Transgene expression was clearly detectable in GM-CSF AU−embryos (Figure 3A-D) but not in AU+ (Figure 3B) or control nontransgenic embryos (Figure3C); this result confirmed the data previously obtained with in vitro cultured cells. Beside widely distributed isolated cells, clusters of cells containing high levels of transgenic mRNA were found in tissues where the CMV promoter is highly active at this developmental stage, in particular the choroid plexus (Figure 3E), the olfactory epithelium (Figure 3F), and the heart (Figure 3G).18 In the latter case, cells expressing the transgene appeared to be in clusters in the lumen of heart-associated blood cavities, as shown by superimposing in situ hybridization and histologic analysis (compare Figure 3G and Figure 3H, control). The expression profile in the 3 transgenic embryos was observed to be identical and independent of the copy number of the transgene (which varied between 1 and 5 copies, see “Materials and methods”).
Transgene expression.
Detection under dark field illumination (magnification × 6) of CMV-GMTag AU− (A) and AU+ (B) transgene expression in E14 embryo by in situ hybridization using [35S]-labeled GM-CSF antisense riboprobe; (C) nontransgenic embryo. The white dots observed in the CMV-GMTag AU− sample correspond to dispersed transgenic mRNA-containing cells. White lines appearing in the CMV-GMTag AU+ and nontransgenic samples correspond to artifacts due to dust and manipulation. (D-G) Detection under bright field illumination of CMV-GMTagAU− transgene expression (black dots) (D) and expression foci at greater magnification (× 100) in the choroid plexus (E), the olfactory epithelium (F), and heart (G). (H) Staining of the heart by hemalum-eosin to show transgene expression by cells in clusters in blood vessels (arrows); br indicates brain; cp, choroı̈d plexus; he, heart; oe, olfactory epithelium; and ve, ventricule. These expression patterns are representative of the different embryos analyzed.
Transgene expression.
Detection under dark field illumination (magnification × 6) of CMV-GMTag AU− (A) and AU+ (B) transgene expression in E14 embryo by in situ hybridization using [35S]-labeled GM-CSF antisense riboprobe; (C) nontransgenic embryo. The white dots observed in the CMV-GMTag AU− sample correspond to dispersed transgenic mRNA-containing cells. White lines appearing in the CMV-GMTag AU+ and nontransgenic samples correspond to artifacts due to dust and manipulation. (D-G) Detection under bright field illumination of CMV-GMTagAU− transgene expression (black dots) (D) and expression foci at greater magnification (× 100) in the choroid plexus (E), the olfactory epithelium (F), and heart (G). (H) Staining of the heart by hemalum-eosin to show transgene expression by cells in clusters in blood vessels (arrows); br indicates brain; cp, choroı̈d plexus; he, heart; oe, olfactory epithelium; and ve, ventricule. These expression patterns are representative of the different embryos analyzed.
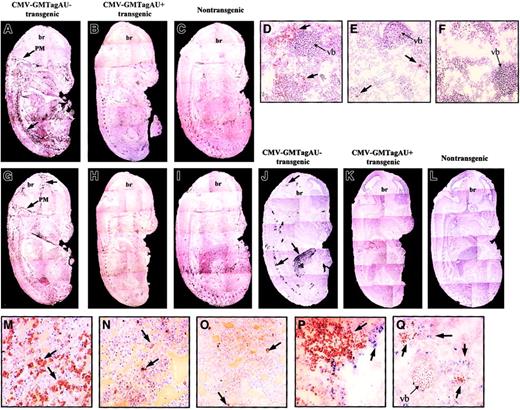
Because granulocytes and macrophages constitute the main cell populations responsive to GM-CSF, their distribution and abundance in the transgenic embryos were investigated. Immunohistochemical staining with antibodies specific for the granulocyte marker Ly-6G (Gr1)23 showed an overall increase in this cell population (Figure 4A). Granulocytes were distributed in a dispersed manner within the embryos but also concentrated within the pia mater of the central nervous system (CNS), between vertebrae along the spinal column, and around the blood vessels. In contrast, the GM-CSF AU+ embryos were similar to the nontransgenic animals in which the granulocytes were much less abundant and mainly localized in the liver (compare Figure 4A to Figure 4B-C and Figure 4D to Figure 4E-F).
Phenotype of CMV-GMTag AU+ and AU− E14 transgenic embryos.
(A-C) Immunohistochemical detection (black peroxidase staining) of mature granulocytes using Gr1 antibody in CMV-GMTagAU−(A), CMV-GMTagAU+ (B), or nontransgenic (C) embryos. Gr1+ cells appear like black points forming dark areas (magnification × 5). (D-F) Immunohistochemical analysis at greater magnification (× 200) of Gr1+ cells (brown peroxidase staining) in vertebra area of CMV-GMTagAU− (D), CMV-GMTagAU+ (E), or nontransgenic (F) embryos. (G-I) Immunohistochemical detection of macrophages using F4/80 antibody in CMV-GMTagAU− (G), CMV-GMTagAU+ (H), or nontransgenic (I) embryos (black peroxidase staining). (J-L) Detection of endogenous peroxidase activity by histochemistry. Peroxidase-positive cells appear as black spots. (J) CMV-GMTagAU− transgenic embryo; (K) CMV-GMTagAU+ transgenic embryo; (L) nontransgenic embryo. (M-O) analysis of peroxidase-positive cells (brown peroxidase staining) at greater magnification (× 200) in the liver of CMV-GMTagAU− (M), CMV-GMTagAU+ (N), and nontransgenic (O) embryos. (P,Q) Double staining showing different localization of Gr1+ cells (blue alkaline phosphatase staining) and peroxidase-positive cells (brown peroxidase staining), in the liver (P) and close to vertebral bone (Q) of E14 CMV-GMTagAU− transgenic embryo. Arrows point to stained organs (A-C, G-L) or to stained cells (D-F, M-Q). br indicates brain; PM, pia mater; vb, vertebral bone. The phenotypes illustrated in this figure are representative of the different embryos analyzed.
Phenotype of CMV-GMTag AU+ and AU− E14 transgenic embryos.
(A-C) Immunohistochemical detection (black peroxidase staining) of mature granulocytes using Gr1 antibody in CMV-GMTagAU−(A), CMV-GMTagAU+ (B), or nontransgenic (C) embryos. Gr1+ cells appear like black points forming dark areas (magnification × 5). (D-F) Immunohistochemical analysis at greater magnification (× 200) of Gr1+ cells (brown peroxidase staining) in vertebra area of CMV-GMTagAU− (D), CMV-GMTagAU+ (E), or nontransgenic (F) embryos. (G-I) Immunohistochemical detection of macrophages using F4/80 antibody in CMV-GMTagAU− (G), CMV-GMTagAU+ (H), or nontransgenic (I) embryos (black peroxidase staining). (J-L) Detection of endogenous peroxidase activity by histochemistry. Peroxidase-positive cells appear as black spots. (J) CMV-GMTagAU− transgenic embryo; (K) CMV-GMTagAU+ transgenic embryo; (L) nontransgenic embryo. (M-O) analysis of peroxidase-positive cells (brown peroxidase staining) at greater magnification (× 200) in the liver of CMV-GMTagAU− (M), CMV-GMTagAU+ (N), and nontransgenic (O) embryos. (P,Q) Double staining showing different localization of Gr1+ cells (blue alkaline phosphatase staining) and peroxidase-positive cells (brown peroxidase staining), in the liver (P) and close to vertebral bone (Q) of E14 CMV-GMTagAU− transgenic embryo. Arrows point to stained organs (A-C, G-L) or to stained cells (D-F, M-Q). br indicates brain; PM, pia mater; vb, vertebral bone. The phenotypes illustrated in this figure are representative of the different embryos analyzed.
The use of antibodies specific for macrophages (F4/80)24 indicated that these cells were also found in greater number in GM-CSF AU− embryos, particularly in the meninges (compare Figure 4G and Figure 4H) than in GM-CSF AU+ or nontransgenic embryos (compare Figure 4H and Figure4I, control). In the course of phenotypic analysis of the embryos, we noticed a strong endogenous peroxidase activity in AU−embryos (compare Figure 4J to Figure 4K-L), particularly in the liver (Figure 4M), between vertebra along the vertebral column, around the epithelium of blood vessels, and in the brain, near the choroid plexus and in the meninges. This activity was undetectable in AU+and control embryos, except in a few cells of their liver (Figure 4N-O). Although the distribution of the peroxidase-expressing cells appeared very similar to that of Gr1+ and F4/80+ cells, they did not colocalize (Figure 4P-Q and data not shown). Thus, this high peroxidase activity might correspond to myeloid progenitors, which are known to produce high levels of myeloperoxidase,25 26 the proliferation of which is increased in GM-CSF AU− embryos.
Altogether, the differences of expression and phenotype observed between E14 GM-CSF AU+ and AU− transgenic embryos clearly indicate that at this developmental stage, GM-CSF ARE in its natural context, imposes a strong inhibitory influence on the amount of GM-CSF transcripts. Its deletion causes accumulation of GM-CSF transcripts, the translation of which causes excessive and deleterious myeloid differentiation.
Because the GM-CSF AU+ construct did not lead to detectable expression and did not result in a change of phenotype in E14 embryos but appeared to impair the viability of transgenic animals, its expression was analyzed at a later stage of development, in E18 embryos. We obtained 2 (of 10) transgenic embryos, which contained 1 to 2 copies of the transgene (Table 1). As shown in Figure5A, GM-CSF AU+ transcripts were now clearly detectable in the embryos, the main expressing foci corresponding to the olfactory epithelium (Figure 5B) and the gut. Phenotypic analysis showed widespread proliferation of neutrophils. These Gr1+ cells were disseminated throughout the embryos, with more dense foci around the olfactory epithelium (Figure 5C), in the bone marrow, the lungs (compare Figure 5D to Figure 5G, control), the spleen, and around blood vessels (not shown). Cells containing strong peroxidase activity were abundantly present in E18 GM-CSF AU+ embryos, as previously observed in E14 GM-CSF AU− embryos. These cells were located in the spleen (compare Figure 5E to Figure 5H, control), the gut (compare Figure 5F to Figure 5I, control), the liver, lungs, and around some blood vessels (not shown). In all the organs analyzed, peroxidase activity (see “Materials and methods”) was higher in transgenic compared to nontransgenic embryos at degrees varying from 4-fold (liver, spleen) to 200-fold (lungs) (Table 2). Altogether, our results show that overexpression of GM-CSF observed in E14 CMV-GMTag AU− and in E18 CMV-GMTag AU+ embryos is accompanied by severe hyperproliferation of macrophages and granulocytes, which seems to compromise further development.
Detection of transgene expression in E18 CMV-GMTagAU+ transgenic embryo and associated phenotypes.
(A) In situ hybridization using [35S]-labeled GM-CSF antisense riboprobe showing transgene expression foci in olfactory epithelium (oe) and gut (gu) (magnification × 2). (B) Transgene expression in olfactory epithelium at greater magnification. (C,D,G) Immunohistochemical detection of granulocytes with anti-Gr1 antibody (brown peroxidase staining) surrounding olfactory epithelium (C), lungs and the rib bone marrow (D, arrows and BM, respectively), and lung and rib bone marrow of nontransgenic embryo (G). (E,F,H,I) Detection of peroxidase-positive cells (black staining) in spleen (E) and gut (F) of transgenic compared to nontransgenic embryo (respectively, H and I); br indicates brain; BM, bone marrow; gu, gut; oe, olfactory epithelium; and sp, spleen (B, C, E, F, H, I, magnification × 100; D, G, magnification × 200). The expression pattern and the phenotype are representative of the 2 embryos analyzed.
Detection of transgene expression in E18 CMV-GMTagAU+ transgenic embryo and associated phenotypes.
(A) In situ hybridization using [35S]-labeled GM-CSF antisense riboprobe showing transgene expression foci in olfactory epithelium (oe) and gut (gu) (magnification × 2). (B) Transgene expression in olfactory epithelium at greater magnification. (C,D,G) Immunohistochemical detection of granulocytes with anti-Gr1 antibody (brown peroxidase staining) surrounding olfactory epithelium (C), lungs and the rib bone marrow (D, arrows and BM, respectively), and lung and rib bone marrow of nontransgenic embryo (G). (E,F,H,I) Detection of peroxidase-positive cells (black staining) in spleen (E) and gut (F) of transgenic compared to nontransgenic embryo (respectively, H and I); br indicates brain; BM, bone marrow; gu, gut; oe, olfactory epithelium; and sp, spleen (B, C, E, F, H, I, magnification × 100; D, G, magnification × 200). The expression pattern and the phenotype are representative of the 2 embryos analyzed.
Relative frequencies of peroxidase-positive cells in E18 CMV-GMTag AU+ transgenic compared to nontransgenic embryos
| . | Brain . | Olf epith . | Heart . | Lungs . | Liver . | Spleen . | Bone marrow . | Gut . |
|---|---|---|---|---|---|---|---|---|
| AU+ | + | + | − | 200 | 4 | 4 | 6 | 6 |
| NT | − | − | − | 1 | 1 | 1 | 1 | 1 |
| . | Brain . | Olf epith . | Heart . | Lungs . | Liver . | Spleen . | Bone marrow . | Gut . |
|---|---|---|---|---|---|---|---|---|
| AU+ | + | + | − | 200 | 4 | 4 | 6 | 6 |
| NT | − | − | − | 1 | 1 | 1 | 1 | 1 |
Peroxidase-positive cells were counted under light microscopy on equivalent surface areas in different tissues of transgenic and nontransgenic embryos. The frequencies were expressed as relative values, 1 being attributed to the nontransgenic samples. In absence of positive cells in the nontransgenic samples, the presence of peroxidase-positive cells was expressed as a +.
AU+ indicates CMV-GMTag AU+ E18 embryos; NT, nontransgenic E18 embryos; Olf epith, olfactory epithelium.
Discussion
Several in vitro studies have demonstrated the crucial role played by AREs in the posttranscriptional regulation of gene expression.2,7-11 However, their physiologic importance has been investigated in vivo only for tumor necrosis factor (TNF) mRNA. Indeed, it was recently shown that deletion of the TNF ARE affected mechanisms responsible for TNF mRNA instability and translational repression in hematopoietic and stromal cells, resulting in the development of chronic inflammatory arthritis and Crohn-like inflammatory bowel disease.27 In the present report, we attempted to evaluate the importance of GM-CSF ARE in vivo and constructed 2 CMV/GM-CSF transgenes that differed only by the absence or the presence of the ARE. Because transgenic adult mice carrying either construct could not be generated, we analyzed the importance of GM-CSF ARE during embryonic development. The posttranscriptional control exerted by this element was revealed by comparing the level of transgene expression in E14 GM-CSF AU+ and AU−embryos; whereas high levels of transgenic mRNA could be observed in AU− embryos, no signal was detected in AU+embryos. Thus, the GM-CSF ARE imposes a strong negative control on the level of transgenic mRNAs at this developmental stage. This control is widespread because it is observable not only in the natural sites of GM-CSF expression but also in any place where the CMV promoter is strongly active.18 However, later on, this ARE-mediated control is weakened because accumulation of transgenic GM-CSF AU+ mRNA could be easily observed in E18 embryos. A number of trans-acting factors interacting with AREs have been identified and cloned28; in particular AUF1/hnRNPD (heterogeneous nuclear ribonucleoprotein D) was shown to be involved in ARE-mediated GM-CSF mRNA degradation.29Interestingly, GM-CSF mRNA half-life appears to be longer in mononuclear cells from adult blood than from neonatal umbilical cord, a difference attributable to a decreased level of AUF1,30 which is also observable in most tissues of mouse embryos between E10 and E16.5.31 Thus, the progressive weakening of GM-CSF ARE-mediated posttranscriptional control, beginning in the embryo and continuing through postnatal life, might be due, at least in part, to a concomitant decrease in AUF1 synthesis.
Accumulation of transgenic GM-CSF transcripts led to profound phenotypic alterations in E14 AU− and E18 AU+embryos. Indeed, GM-CSF AU− E14 embryos displayed an abnormally high number of granulocytes (Gr1+) infiltrating most tissues and accumulation of macrophages (F4/80+), particularly in the meninges. In GM-CSF AU+ E18 embryos, a large number of neutrophils were found all over the embryo with particularly dramatic concentrations around the olfactory epithelium, near blood vessels, and in the liver and brain. We also noticed a significant increase in peroxidase-expressing cells. These Gr1− cells were present in the liver, along the vertebral column, and in the CNS of AU− E14 embryos and, in addition, in the gut and lung of AU+ E18 embryos. This peroxidase activity most probably resulted from myeloperoxidase, which is known to be transiently expressed by myeloid progenitors during myeloid differentiation.25,26 Surprisingly, none of these activities were observed in previously described GM-CSF transgenic mice32; mice were healthy during development but at 2 to 4 months of age developed blindness and a fatal syndrome of tissue damage. Most probably, due to the tissue-specificity of the retroviral promoter used to drive GM-CSF, transgene expression was restricted to macrophages in this earlier study, whereas in our study, the CMV promoter is active early and in many cell types, including the natural sites of GM-CSF synthesis. The phenotype we have observed in the transgenic embryos thus reflects the known pleiotropic activities of GM-CSF on hematopoietic cells in an exaggerated form. Our data extend to embryogenesis the previous observations made in adult mice in which GM-CSF overexpression resulted in increased numbers of macrophages and granulocytes.33 34
Due to the ubiquitous activity of the CMV promoter, high levels of transgene expression result in abnormally high levels of GM-CSF throughout the embryos, which, in turn, leads to generalized proliferation of GM-CSF–sensitive cells. In some cases, such as CNS and olfactory epithelium, the location of these cells is superimposed on that of transgene expression, probably resulting from chemoattractant activity of the GM-CSF. In other cases, GM-CSF overproduction stimulates the proliferation of myeloid progenitors and mature cells in their resident locations and their subsequent migration. In addition, GM-CSF overexpression most probably induces myeloid cell activation, leading to a systemic inflammatory state accompanied by progressive destruction of tissues infiltrated by granulocytes and macrophages. The hematopoietic disorders observed in E14 AU− transgenic embryos and later on in AU+embryos most probably preclude further development and explain why viable transgenic mice cannot be generated.
In conclusion, this study shows for the first time that the GM-CSF ARE is a physiologic regulatory element with developmentally controlled activity. In addition, the GM-CSF transgenic embryos are a valuable model to analyze the effect of myeloid hyperplasia similar to that induced by GM-CSF overexpression during embryonic development.
The AU+ and AU− transgenic mice were obtained from the Service de transgenèse CNRS Toulouse. We thank J. Auriol for the screening of transgenic mice and Drs D. Caput and N. Walker for sharing their expertise in the analysis of the transgenic embryos.
Supported by grants from the EC Biotech Program (BIO4-CT95-0045), the Fund for Medical Scientific Research (Belgium, grant 3.4586.93), the Actions de Recherches Concertées (grant 94-99/181), the Belgian Banque Nationale, and a Télévie grant of the Fonds National Belge de la Recherche Scientifique (FNRS).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Véronique Kruys, Laboratoire de Chimie Biologique, IBMM, Université Libre de Bruxelles, Rue des Profs Jeener et Brachet 12, 6041 Gosselies, Belgium; e-mail:vkruys@dbm.ulb.ac.be.

![Fig. 2. Analysis of GMTag protein biologic activity. / The GM-CSF/IL-3 proliferation-dependent NFS-60 cells were incubated with decreasing concentrations of nontransfected (○) or pBK-CMV–GMTAg AU− transfected (●) HeLa cell supernatants. The first dilution of the pBK-CMV–GMTag AU−transfected cell supernatant contained 10 ng/mL GM-CSF as determined by ELISA. (■) Cells were incubated with 100 μL supernatant of pBK-CMV–GMTAg AU− transfected HeLa cells in the presence of decreasing concentrations of antimouse GM-CSF antibody. The transfected cell supernatant contained 20 ng/mL GM-CSF as determined by ELISA. Cell proliferation was measured by [3H]-thymidine incorporation (see “Materials and methods”).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/5/10.1182_blood.v98.5.1281/5/m_h81711446002.jpeg?Expires=1769162519&Signature=r~OOwtYTH7mAzaimSITIYbbRFMUYOVNX5HV6WlwRCxvE5soGWhdXis9XnYqdQJTwyg0SBpiILko3L11Qmil2-50U9-x~4a3pl9yKjleCVq1S-DpbLX5a3jRxgg3oDvz5eo~TJgXeCYvIrH411DL-1Es1zlYX5YZ6KNT9PAwGdxSWZlSN-LMqnfjyBNa7uG2mmzvS5c4cgz5tAlWfTbm83-E6Woha2cpVPqyxG0yDgC-6FB3h4kXK9893CHdOmMxGDRA~iJtugzZNZTu6YpVWa6WiPn4npPje6r4mqZCvbc5lDkIjG79Eas1JAxh0T5pByWF5KrFlb5M3FpP4juLVTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Transgene expression. / Detection under dark field illumination (magnification × 6) of CMV-GMTag AU− (A) and AU+ (B) transgene expression in E14 embryo by in situ hybridization using [35S]-labeled GM-CSF antisense riboprobe; (C) nontransgenic embryo. The white dots observed in the CMV-GMTag AU− sample correspond to dispersed transgenic mRNA-containing cells. White lines appearing in the CMV-GMTag AU+ and nontransgenic samples correspond to artifacts due to dust and manipulation. (D-G) Detection under bright field illumination of CMV-GMTagAU− transgene expression (black dots) (D) and expression foci at greater magnification (× 100) in the choroid plexus (E), the olfactory epithelium (F), and heart (G). (H) Staining of the heart by hemalum-eosin to show transgene expression by cells in clusters in blood vessels (arrows); br indicates brain; cp, choroı̈d plexus; he, heart; oe, olfactory epithelium; and ve, ventricule. These expression patterns are representative of the different embryos analyzed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/5/10.1182_blood.v98.5.1281/5/m_h81711446003.jpeg?Expires=1769162519&Signature=3Bubx2QMFp4u-hnWlBmUuMpHwRkTBbA6AGUFZuPxhM8uoHGGYSFA7mWpGRDcoDl907d0dDivDExupgt-mJpMKy4E6IujeuWhGEz685AjcyBJWRSzlnJVXrum5pvYtEjUw3VHGESrJ-RVv8Qix-EhQ-rBQijyS0vuDA3Lignse5co68vuwT9W7Zmrg1VDvFrM~11SuGpZ9nB6AIzKEhblfmWGH0AUcJ~dyByNgl3camWs~1kT6hhiRYTARlwrB2uBukYgrOBlX-vUfXzKDtIrUGhpDk3qzOrHR2Mf~D~gi5SgGfHRE-m~BXPlZtdA9Uh6Ldk0GkkiE1uumTSLXSm6MA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Detection of transgene expression in E18 CMV-GMTagAU+ transgenic embryo and associated phenotypes. / (A) In situ hybridization using [35S]-labeled GM-CSF antisense riboprobe showing transgene expression foci in olfactory epithelium (oe) and gut (gu) (magnification × 2). (B) Transgene expression in olfactory epithelium at greater magnification. (C,D,G) Immunohistochemical detection of granulocytes with anti-Gr1 antibody (brown peroxidase staining) surrounding olfactory epithelium (C), lungs and the rib bone marrow (D, arrows and BM, respectively), and lung and rib bone marrow of nontransgenic embryo (G). (E,F,H,I) Detection of peroxidase-positive cells (black staining) in spleen (E) and gut (F) of transgenic compared to nontransgenic embryo (respectively, H and I); br indicates brain; BM, bone marrow; gu, gut; oe, olfactory epithelium; and sp, spleen (B, C, E, F, H, I, magnification × 100; D, G, magnification × 200). The expression pattern and the phenotype are representative of the 2 embryos analyzed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/5/10.1182_blood.v98.5.1281/5/m_h81711446005.jpeg?Expires=1769162519&Signature=bCXi6gig~WdH6PzTNJxSIfAUoj~ECmA1ewkdfnJBvwaZfGFGvhrOM6eTHCp4NGPQMcWXO4R0iIc8Jc8ETbtRacmUXKWHYJKxJEviPu6p26YQrJtFfh9~Jed10TKMRSW0iyc14BgX1T~b8T3h~ZbyiY8NanTe-f9F1M1EXFcCmZ30BwBLc~sDqL1qe-YQCmksH5-R9NpGA4Zp2plpj4ROfW~TEFqVt70nmC61HhGq23guJxYE0RWEYK07e3vNoJ7nB6WNI5~jcEdNmSfsqpOTv7Wn5Q6O2cN4OBdoYp-LM-KT6WAInu3cRxx-jbIjXyq08Rf6lRAxGKt1XuuvSB33EA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal