Abstract
Retrospective studies of patients with thrombotic microangiopathies (TMAs) have shown that a deficient activity of von Willebrand factor (vWF)–cleaving protease is involved in thrombotic thrombocytopenic purpura (TTP) but not in the hemolytic-uremic syndrome (HUS). To further analyze the relevance of this enzymatic activity in TMA diagnosis, a 20-month multicenter study of vWF-cleaving protease activity was conducted in adult patients prospectively enrolled in the acute phase of TMA. Patients with sporadic (n = 85), intermittent (n = 21), or familial recurrent (n = 5) forms of TMA (66 manifesting as TTP and 45 as HUS) were included. TMA was either idiopathic (n = 42) or secondary to an identified clinical context (n = 69). vWF-cleaving protease activity was normal in 46 cases (7 TTP and 39 HUS) and decreased in 65 cases (59 TTP and 6 HUS). A protease inhibitor was detected in 31 cases and was observed only in patients manifesting TTP with a total absence of protease activity. Among the 111 patients, mean vWF antigen levels were increased and the multimeric distribution of vWF was very heterogeneous, showing either a defect of the high-molecular-weight forms (n = 40), a normal pattern (n = 21), or the presence of unusually large multimers (n = 50). Statistical analysis showed that vWF-protease deficiency was associated with the severity of thrombocytopenia (P < .01). This study emphasizes that vWF-cleaving protease deficiency specifically concerns a subgroup of TMA corresponding to the TTP entity.
Introduction
Thrombotic microangiopathies (TMAs) are uncommon disorders but the young age of patients at onset, acute presentation, difficult diagnosis, and sometimes fatal outcome make them of great interest. TMAs define syndromes of microangiopathic hemolytic anemia and thrombocytopenia associated with platelet aggregation in the microcirculation responsible for ischemic manifestations.1The pathophysiology of TMA involves endothelial damage associated with an occlusion of small arterioles and capillaries by platelet plugs containing high quantities of von Willebrand factor (vWF).2 vWF is a large glycoprotein essential for platelet adhesion and aggregation, especially at the high shear stress-associated hemodynamic conditions of the microcirculation.3 It is synthesized by megakaryocytes and endothelial cells as a multimeric protein and released in plasma. The largest multimers of vWF are the most biologically active. In plasma, the size of the multimers is regulated by a specific metalloprotease,4,5 which cleaves the peptide bond between Y842-M843 (single-letter amino acid codes) of vWF mature subunit6 and prevents the spontaneous interaction of the largest multimers with platelets. In plasma of patients with TMA, unusually large (UL) multimers of vWF have been observed.7Because of their ability to induce platelet aggregation at high shear stress of blood flow,8 9 they were thought to be involved in the formation of the microvascular thrombi.
Clinically, TMAs constitute a heterogeneous group of diseases including either sporadic forms (acute single episode), intermittent forms (relapsing episodes separated by symptom-free periods of months or years), or recurrent forms (mostly familial, with frequent relapsing episodes recurring after regular symptom-free intervals of about 3 or 4 weeks).1,10,11 Acute phases of TMA may be idiopathic or secondary to various clinical conditions such as pregnancy, infections, systemic diseases, cancer, and drugs.1,11 Classically, TMAs are described as encompassing 2 main syndromes: thrombotic thrombocytopenic purpura (TTP) and the hemolytic-uremic syndrome (HUS).1 However, the distinction between TTP and HUS remains a matter of controversy.12 Is the classical bipolar symptomatology (predominance of neurologic symptoms in TTP and of renal failure in HUS) a reality because both syndromes often overlap? Do TTP and HUS involve distinct pathophysiologic mechanisms? Finally, is it useful to discriminate TTP from HUS because reports of large series of patients showed that plasmatherapy was beneficial in both syndromes,12 13 in contrast to old dogma considering plasmatherapy effective in TTP and globally disappointing in HUS?
In 1998, however, a major breakthrough in the understanding of TMA pathophysiology occurred with the discovery of a deficient activity of vWF-cleaving protease (either constitutional or acquired via an autoantibody) specific for TMA presenting as TTP.14,15Hence, vWF-cleaving protease was found normal not only in HUS14 but also in various other diseases different from TTP.15 These results were obtained from retrospective studies involving selected patients and they represent an essential first step in identifying vWF-cleaving protease as a new potential tool for the biologic diagnosis of TMA.
The aim of the present study was to focus on the acute phase of TMA in unselected patients prospectively enrolled to answer the following questions: (1) Is vWF-cleaving protease significantly different between TMAs presenting as TTP and those presenting as HUS? (2) Do vWF-cleaving protease levels correlate with any other clinical or laboratory feature in patients with TMA?
Patients, materials, and methods
Human plasma collection
Venous blood was collected into 1:10 final volume of 3.8% sodium citrate, before any treatment. Platelet-poor plasma (PPP) was obtained by centrifugation at 2500g for 20 minutes and aliquoted samples were stored at −80°C until tested.
Patients
From May 1999 to December 2000, all adult patients (age > 15 years) with an acute event of TMA were prospectively enrolled from the 27 national participating centers after obtaining appropriate consent. For each patient, a questionnaire was completed, allowing initial clinical and laboratory evaluation. The participating centers were asked to choose between TTP and HUS presentation using the classical criteria (hemolytic anemia, thrombocytopenia, renal failure, neurologic symptoms, fever). Some patients could also be studied after several months of remission.
Controls
Healthy volunteers (40 men, 62 women; mean age, 35 years) were used as controls to determine the normal ranges of vWF-cleaving protease.
Antibodies
Monoclonal antibodies (MoAbs) anti-vWF were prepared and characterized as described16 and used as purified IgG. MoAb 453 is directed to the C-terminal part of vWF. A pool of 4 MoAbs to the N-terminal end of vWF (anti-N-ter MoAbs) and a polyclonal monospecific antibody to vWF (polyAb) were also used in this study.
Radiolabeling of antibodies
Anti N-ter MoAbs and polyAb IgG were labeled using Na125I (Amersham, Les Ulis, France) and Iodo-Gen (Pierce Chemical, Rockford, IL) as described.17 Specific radioactivities were 5 μCi/μg.
Recombinant vWF
Standard assays
Routine laboratory tests including platelet count, measurement of hemoglobin level, percentage of schistocytes, lactate dehydrogenase (LDH), total bilirubin, haptoglobin, and creatinine were performed according to conventional methods in each center.
vWF antigen
The vWF antigen (vWFAg) levels in plasma were measured using an enzyme-linked immunosorbent assay (ELISA Asserachrom vWF, Diagnostica Stago, Asnières, France).
Multimeric analysis of vWF
The multimeric distribution of vWF was studied using 0.1% sodium dodecyl sulfate, 1% agarose (IEF, Amersham Pharmacia Biotech AB, Uppsala, Sweden) gel electrophoresis under nonreducing conditions as previously described20 21 with some modifications. About 1 mIU vWFAg was loaded in each well. After migration, the gels were washed with water, dried, and incubated in 1% skim milk, 25 mM Tris-HCl, and 150 mM NaCl, pH 7.4. Staining was performed by incubating the dried gel with 125I-polyAb (106cpm/mL) in the previous buffer. After extensive washing with 150 mM NaCl followed by water, the vWF-associated radioactivity was revealed by autoradiography. The relative proportion of low- (≤ 5-mers, LMW), intermediate- (6- to 10-mers, IMW), and high-molecular-weight (> 10-mers, HMW) multimers of vWF was determined using a densitometric analysis of the autoradiographs (Hyrys 2 densitometer, Sebia, Issy-les-moulineaux, France) and expressed as a percentage of the total multimers. Normal ranges for each category of multimers were determined from the extreme values of 20 healthy individuals (LMW, 28%-45%; IMW, 34%-45%; HMW, 20%-36%). A pool of PPP from 25 healthy volunteers (NP) was used as reference in each autoradiograph. The proportion of UL multimers in patients was calculated as follows: ULpatient(%) = HMWpatient(%) − HMWNP(%).
Assay of vWF-cleaving protease activity by immunoradiometric assay
Measurement of vWF-cleaving protease activity in plasma was performed in blind, as previously described21 with minor modifications concerning the hydrolysis step. Briefly, the principle of the method relies on the hydrolysis of a constant amount of rvWF WT used as substrate by serial dilutions of tested plasma used as protease provider. Plasma samples were treated with Pefabloc (2 mM final) for 10 minutes and serially diluted from 1:10 to 1:640 in 5 mM Tris-HCl, pH 8, 1.5 M urea. Aliquots of 90 μL were preincubated with 10 μL 100 mM BaCl2 for 5 minutes at room temperature. Sixty microliters of this mixture was added to 40 μL rvWF WT (0.05 IU) previously dialyzed against 5 mM Tris-HCl, pH 8, 1.5 M urea, into wells of microtitration plates coated with 1% bovine serum albumin, and then incubated 48 hours at 37°C. The digestion was stopped by addition of 5 μL 200 mM EDTA in water. The residual rvWF WT Ag contained in the hydrolysate was then estimated by 2-site immunoradiometric assay (IRMA) as described,21 using the MoAb 453 for coating and125I-anti-N-ter MoAbs as second antibody. NP was arbitrarily defined as containing 100% of vWF-cleaving protease and used as an internal control.
Inhibitor for the vWF-cleaving protease was assayed by measuring the residual protease activity in mixtures of TMA patient plasma and NP at 3 different volume/volume ratios, 1:1, 2:1, and 3:1, after a 30-minute preincubation at room temperature. Titer was arbitrarily defined as high, medium, or low for no residual activity of vWF-cleaving protease in 1:1, 2:1, and 3:1 mixtures, respectively.
Statistical analysis
Statistics were performed using computer-assisted program Statview (Abacus Concepts, Berkeley, CA) in patients with definitive diagnosis of TMA. Means and SDs were calculated for each continuous variable. Comparisons were performed using an independent samplet test for parametric continuous variables, a Kruskal-Wallis z test for nonparametric continuous variables, and a χ2test for categorical variables. Relationships between vWF-cleaving protease activity and other variables were studied using univariate and multivariate analysis; both linear and logistic regression analysis were used as a function because vWF-cleaving protease was considered as a continuous variable or a binary variable (normal/abnormal and nil/nonnil), respectively.
Results
Clinical features of patients with TMA
Over a 20-month period, 111 consecutive unselected patients with TMA were included. The mean age of the 64 women and 47 men was about 40 years. Features of TMA are presented in Table1; 85 patients exhibited a sporadic form, 21 patients an intermittent form, and 5 patients a recurrent familial form (3 unrelated propositus with recurrent disease since childhood, associated with a familial history of hemolytic anemia with thrombocytopenia and/or multiple organ dysfunction in 2 relatives at least and 2 related cases who were brother and sister). TMA was either idiopathic (n = 42) or associated with one or several clinical conditions (n = 69) such as neoplasia, immunologic disorders, bacterial or viral infections, pulmonary hypertension, pregnancy, bone marrow transplantation, drugs (benzodiazepine, oral contraceptives, nonsteroid anti-inflammatories, cannabis), and Castleman syndrome. Sixty-six patients were initially diagnosed as having TTP and 45 as having HUS, showing a similar distribution among the 3 various forms of TMA.
Clinical data in 111 patients with TMA
| . | Sporadic TTP . | TMA (n = 85) HUS . | Intermittent TTP . | TMA (n = 21) HUS . |
|---|---|---|---|---|
| Idiopathic TMA | 18 | 11 | 4 | 4 |
| Secondary TMA | 35 | 21 | 6 | 7 |
| Neoplasia | 12 | 4 | 0 | 2 |
| Immunologic disorders | 7 | 4 | 3 | 1 |
| Infections | 7 | 5 | 0 | 1 |
| Pulmonary hypertension | 2 | 4 | 0 | 1 |
| Pregnancy | 3 | 1 | 1 | 0 |
| Bone marrow transplantation | 2 | 0 | 0 | 2 |
| Drugs | 2 | 0 | 2 | 0 |
| Castleman syndrome | 0 | 3 | 0 | 0 |
| Total | 53 | 32 | 10 | 11 |
| . | Sporadic TTP . | TMA (n = 85) HUS . | Intermittent TTP . | TMA (n = 21) HUS . |
|---|---|---|---|---|
| Idiopathic TMA | 18 | 11 | 4 | 4 |
| Secondary TMA | 35 | 21 | 6 | 7 |
| Neoplasia | 12 | 4 | 0 | 2 |
| Immunologic disorders | 7 | 4 | 3 | 1 |
| Infections | 7 | 5 | 0 | 1 |
| Pulmonary hypertension | 2 | 4 | 0 | 1 |
| Pregnancy | 3 | 1 | 1 | 0 |
| Bone marrow transplantation | 2 | 0 | 0 | 2 |
| Drugs | 2 | 0 | 2 | 0 |
| Castleman syndrome | 0 | 3 | 0 | 0 |
| Total | 53 | 32 | 10 | 11 |
vWF-cleaving protease activity
In the 102 healthy volunteers, the values of vWF-cleaving protease activity ranged from 49% to 200% (mean ± SD = 114% ± 32%) with an apparent parametric distribution. Thus, normal ranges were defined as the mean ± 2 SD (50%-178%).
In the 111 patients with TMA, vWF-cleaving protease activity was normal in 46 cases (7 TTP and 39 HUS) and decreased in 65 cases (59 TTP and 6 HUS). Figure 1 shows the values of vWF-cleaving protease activity as a function of the progressive form of TMA. Among the 85 sporadic forms, protease activity was normal in 35 cases (7 TTP and 28 HUS) and decreased in 50 cases (46 TTP and 4 HUS), including 14 partial defects (range, 10%-45%) and 36 total defects (undetectable activity). In the 21 intermittent forms of TMA, protease activity was normal in 9 cases (9 HUS) and undetectable in 12 cases (10 TTP and 2 HUS). In the 5 recurrent familial forms, protease activity was normal in the 2 HUS cases and undetectable in the 3 TTP cases. Figure 2 shows the distribution of vWF-cleaving protease activity as a function of the clinical context associated with TMA. In the 42 idiopathic forms, vWF-protease was either undetectable in all TTP (n = 25) and normal in all HUS (n = 17). In the 69 secondary forms, results are less clearcut because partial deficiencies of vWF-protease are present, involving TTP (n = 12) as well as HUS (n = 2). Undetectable values are predominant in TTP (22 cases) but also observed in 4 HUS cases. In contrast, normal values are mainly observed in HUS (22 cases versus 7 TTP).
vWF-cleaving protease activity in plasma from 111 patients with TMA as a function of the progressive form.
vWF-cleaving protease activity was measured during an acute event in 85 sporadic, 21 intermittent, and 5 familial recurrent forms of TMA. Patients were presenting as TTP (●) or HUS (○). The dashed line shows the lower limit of normal (50%).
vWF-cleaving protease activity in plasma from 111 patients with TMA as a function of the progressive form.
vWF-cleaving protease activity was measured during an acute event in 85 sporadic, 21 intermittent, and 5 familial recurrent forms of TMA. Patients were presenting as TTP (●) or HUS (○). The dashed line shows the lower limit of normal (50%).
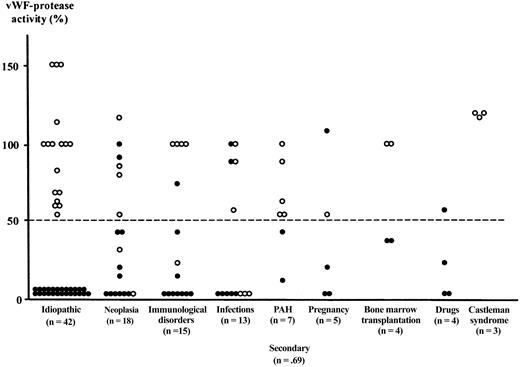
vWF-cleaving protease activity in plasma from 111 patients with TMA as a function of the clinical context.
vWF-cleaving protease activity was measured during an acute event in 42 idiopathic and 69 secondary forms of TMA. Patients were presenting as TTP (●) or HUS (○). The dashed line shows the lower limit of normal (50%). PAH indicates pulmonary arterial hypertension.
vWF-cleaving protease activity in plasma from 111 patients with TMA as a function of the clinical context.
vWF-cleaving protease activity was measured during an acute event in 42 idiopathic and 69 secondary forms of TMA. Patients were presenting as TTP (●) or HUS (○). The dashed line shows the lower limit of normal (50%). PAH indicates pulmonary arterial hypertension.
Inhibitor to vWF-cleaving protease
Among the 65 patients with a decreased activity of vWF-cleaving protease, a protease inhibitor was identified in 31 cases (48%). Inhibitor titer was either high (n = 5), medium (n = 8), or low (n = 18). These 31 patients with a protease inhibitor were all previously diagnosed as having TTP and demonstrated an undetectable protease activity. They had either recurrent familial (n = 1), intermittent (n = 9), or sporadic forms (n = 21). In 14 cases, TTP was idiopathic, whereas in 17 cases, a clinical context was identified consisting of 7 immunologic disorders (lupus, primary antiphospholipid syndrome, thyroiditis, psoriasis, Crohn disease, vaccination against yellow fever), 4 infections (human immunodeficiency virus [HIV], hepatitis C virus), 3 neoplasias (osteosarcoma, breast cancer, acute myeloid leukemia), 2 drug ingestions (benzodiazepines, cannabis), and 1 pregnancy.
Clinical and biologic analysis of the 111 patients with TMA
Using the files from the participating centers, retrospective clinical analysis of patients with TMA revealed that neurologic symptoms were present in 90% of those with TTP and in 15% of those with HUS. These signs were very heterogeneous (headaches, confusion, convulsions, sensory or motor deficiencies), but neurologic deficiencies relative to ischemic stroke were observed only in TTP patients. Fever was present in 50% of the patients with TTP and 21% of those with HUS.
Analysis of routine laboratory assays given by the participating centers and of vWF assays (vWFAg levels, vWF multimeric distribution, and vWF-cleaving protease activity) as a function of TTP or HUS is presented in Table 2. The totality of patients demonstrated a Coombs-negative hemolytic anemia (elevated LDH and bilirubin with undetectable haptoglobin; data not shown) with a more decreased hemoglobin level in TTP. Also, in the TTP group, thrombocytopenia was more frequent and more severe. In contrast, acute renal insufficiency (creatinine level > 350 μmol/L) was almost constant in HUS although very rare in TTP. Mean vWFAg levels were similarly increased in both groups. Mean proportions of each category of multimers were not significantly different between TTP and HUS (Kruskal-Wallis z test). However, vWF multimeric patterns were heterogeneous, showing 3 types of multimeric distribution (Figure3). The proportion of HMW multimers was either increased, with UL multimers (n = 27 TTP and 23 HUS), normal (11 TTP and 10 HUS), or decreased, concomitantly with an increase of the LMW multimers (25 TTP and 15 HUS). The activity of vWF-cleaving protease was decreased in 89% of TTP and in 13% of HUS cases.
Laboratory parameters in 111 TMA patients
| . | TTP (n = 66) . | HUS (n = 45) . | P* . |
|---|---|---|---|
| Hemolytic anemia | 66 (100%) | 45 (100%) | NS |
| Hemoglobin (N: 12-16 g/dL) | 7.2 ± 1.5 (4.7-10.3) | 8.8 ± 1.8 (5.3-11.8) | .01 |
| Schistocytes (percent of erythrocytes) | 4.0 ± 3.0 (0-18) | 2.0 ± 1.4 (0-4) | NS |
| Thrombocytopenia | 62 (94%) | 27 (60%) | .005 |
| Platelet count (N: 150-400 × 109/L) | 35 ± 27 (5-178) | 95 ± 60 (3-272) | .002 |
| Acute anuric renal failure | 1 (1.5%) | 44 (98%) | < .0001 |
| Creatinine (N: 40-110 μmol/L) | 162 ± 140 (54-700) | 360 ± 207 (120-800) | .001 |
| vWF antigen (N: 50-150 IU/dL) | 230 ± 94 (70-540) | 241 ± 156 (94-727) | NS |
| vWF multimers | |||
| LMW (N: 28%-45%) | 39 ± 13 (26-68) | 40 ± 12 (13-63) | NS |
| IMW (N: 34%-45%) | 32 ± 5 (23-44) | 33 ± 4 (28-42) | NS |
| HMW (N: 20%-36%) | 25 ± 6 (5-44) | 25 ± 8 (4-52) | NS |
| UL (N: 0%) | 5 ± 4 (0-18) | 5 ± 4 (0-16) | NS |
| Decreased vWF-cleaving protease | 59 (89%) | 6 (13%) | < .0001 |
| Inhibitor to vWF-protease† | 30 (51%) | 0 (0%) | < .0001 |
| . | TTP (n = 66) . | HUS (n = 45) . | P* . |
|---|---|---|---|
| Hemolytic anemia | 66 (100%) | 45 (100%) | NS |
| Hemoglobin (N: 12-16 g/dL) | 7.2 ± 1.5 (4.7-10.3) | 8.8 ± 1.8 (5.3-11.8) | .01 |
| Schistocytes (percent of erythrocytes) | 4.0 ± 3.0 (0-18) | 2.0 ± 1.4 (0-4) | NS |
| Thrombocytopenia | 62 (94%) | 27 (60%) | .005 |
| Platelet count (N: 150-400 × 109/L) | 35 ± 27 (5-178) | 95 ± 60 (3-272) | .002 |
| Acute anuric renal failure | 1 (1.5%) | 44 (98%) | < .0001 |
| Creatinine (N: 40-110 μmol/L) | 162 ± 140 (54-700) | 360 ± 207 (120-800) | .001 |
| vWF antigen (N: 50-150 IU/dL) | 230 ± 94 (70-540) | 241 ± 156 (94-727) | NS |
| vWF multimers | |||
| LMW (N: 28%-45%) | 39 ± 13 (26-68) | 40 ± 12 (13-63) | NS |
| IMW (N: 34%-45%) | 32 ± 5 (23-44) | 33 ± 4 (28-42) | NS |
| HMW (N: 20%-36%) | 25 ± 6 (5-44) | 25 ± 8 (4-52) | NS |
| UL (N: 0%) | 5 ± 4 (0-18) | 5 ± 4 (0-16) | NS |
| Decreased vWF-cleaving protease | 59 (89%) | 6 (13%) | < .0001 |
| Inhibitor to vWF-protease† | 30 (51%) | 0 (0%) | < .0001 |
Continuous variables are expressed as mean ± SD; extreme values are between brackets. Categorical variables are given as numbers and percentage of cases.
N indicates normal (value); NS, not significant.
Comparisons between the TTP group and the HUS were performed using a χ2 test for categorical variables and an independent sample t test for continuous variables.
The percentage of patients with an inhibitor is calculated among patients with a vWF-protease deficiency.
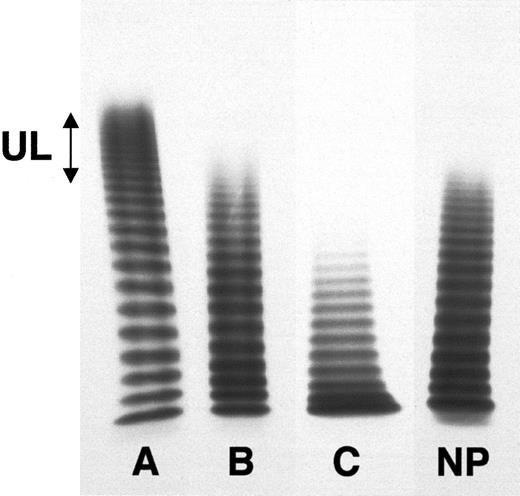
Various multimeric patterns of plasma vWF observed in patients with acute TMA.
The multimeric distribution of vWF was studied using 0.1% sodium dodecyl sulfate, 1% agarose gel electrophoresis under nonreducing conditions. About 1 mIU vWFAg was loaded in each well. After migration and washing, the gels were incubated with a 125I-goat polyclonal antibody to vWF. After extensive washing, vWF-associated radioactivity was revealed by autoradiography. Patients with acute TMA show, respectively, an increased proportion of HMW multimers, including UL forms (A), a normal multimeric distribution (B), and a partial loss of the HMW (C). NP indicates normal pooled plasma.
Various multimeric patterns of plasma vWF observed in patients with acute TMA.
The multimeric distribution of vWF was studied using 0.1% sodium dodecyl sulfate, 1% agarose gel electrophoresis under nonreducing conditions. About 1 mIU vWFAg was loaded in each well. After migration and washing, the gels were incubated with a 125I-goat polyclonal antibody to vWF. After extensive washing, vWF-associated radioactivity was revealed by autoradiography. Patients with acute TMA show, respectively, an increased proportion of HMW multimers, including UL forms (A), a normal multimeric distribution (B), and a partial loss of the HMW (C). NP indicates normal pooled plasma.
Statistical analysis of vWF-cleaving protease activity in patients with TMA
Using vWF-cleaving protease activity as a continuous variable, univariate linear-regression analysis demonstrated a relationship with both platelet count (P = .004) and creatinine (P = .01). Similar results were obtained with an univariate logistic-regression analysis, using vWF-cleaving protease activity as a binary variable, either normal/abnormal or nil/nonnil. Multivariate linear regression analysis was used to examine the association between vWF-cleaving protease activity and platelet count after adjustment for the LMW and UL multimers of vWF. These factors were chosen because they may influence platelet count because they have an increased affinity for platelet receptors. This adjustment did not affect the significance of the association between vWF-cleaving protease and platelet count (P = .001). The link with platelet count persisted with a multivariate logistic-regression analysis using vWF-cleaving protease as a binary variable.
Follow-up of vWF-cleaving protease activity in patients with TMA in remission
We had the opportunity to obtain samples in remission from 15 patients (8 sporadic, 5 intermittent, and 2 recurrent familial forms). Among those, 14 patients demonstrated a total lack of vWF-protease activity during the acute phase and 1 patient had a partial decrease. Their main features are summarized in Table3. In remission, vWF-protease activity normalized concomitantly with the disappearance of the inhibitor (when initially present) in the 8 patients with a sporadic TMA (6 TTP and 2 HUS) and in 4 of 5 patients with an intermittent TTP. Interestingly, despite a clinical and biologic remission, vWF-protease activity remained undetectable in one patient with an intermittent TTP with no inhibitor and in both patients with a recurrent familial form, including one with an inhibitor.
Follow-up of vWF-protease in 15 patients in remission of TMA
| Sex/age . | TMA . | Associated context . | Treatment . | vWF-protease percent/inhibitor . | |
|---|---|---|---|---|---|
| Acute phase . | Remission . | ||||
| F/51 | Sporadic TTP | None | Plasmapheresis and corticosteroids | 0/− | 100/− |
| F/53 | Sporadic TTP | None | Plasmapheresis and corticosteroids | 0/+ | 100/− |
| F/23 | Sporadic TTP | Neoplasia | Chemotherapy | 0/+ | 100/− |
| F/61 | Sporadic TTP | Neoplasia | Chemotherapy | 0/− | 75/− |
| F/18 | Sporadic TTP | Neoplasia | Plasma infusion and corticosteroids | 0/+ | 50/− |
| F/28 | Sporadic HUS | Infection | Plasmapheresis | 0/− | 100/− |
| M/32 | Sporadic HUS | Infection | Plasmapheresis | 0/− | 100/− |
| F/36 | Sporadic TTP | Pregnancy | Plasmapheresis | 20/− | 70/− |
| F/53 | Intermittent TTP | None | Plasmapheresis | 0/+ | 70/− |
| M/32 | Intermittent TTP | Drug | Plasmapheresis | 0/+ | 50/− |
| M/36 | Intermittent TTP | Drug | Plasma infusion | 0/+ | 100/− |
| F/35 | Intermittent TTP | Lupus | Plasmapheresis and corticosteroids | 0/+ | 100/− |
| F/35 | Intermittent TTP | Pregnancy | Plasma infusion and corticosteroids | 0/− | 0/− |
| F/19 | Recurrent TTP | None | Plasmapheresis | 0/− | 0/− |
| M/18 | Recurrent TTP | None | Plasmapheresis | 0/+ | 0/+ |
| Sex/age . | TMA . | Associated context . | Treatment . | vWF-protease percent/inhibitor . | |
|---|---|---|---|---|---|
| Acute phase . | Remission . | ||||
| F/51 | Sporadic TTP | None | Plasmapheresis and corticosteroids | 0/− | 100/− |
| F/53 | Sporadic TTP | None | Plasmapheresis and corticosteroids | 0/+ | 100/− |
| F/23 | Sporadic TTP | Neoplasia | Chemotherapy | 0/+ | 100/− |
| F/61 | Sporadic TTP | Neoplasia | Chemotherapy | 0/− | 75/− |
| F/18 | Sporadic TTP | Neoplasia | Plasma infusion and corticosteroids | 0/+ | 50/− |
| F/28 | Sporadic HUS | Infection | Plasmapheresis | 0/− | 100/− |
| M/32 | Sporadic HUS | Infection | Plasmapheresis | 0/− | 100/− |
| F/36 | Sporadic TTP | Pregnancy | Plasmapheresis | 20/− | 70/− |
| F/53 | Intermittent TTP | None | Plasmapheresis | 0/+ | 70/− |
| M/32 | Intermittent TTP | Drug | Plasmapheresis | 0/+ | 50/− |
| M/36 | Intermittent TTP | Drug | Plasma infusion | 0/+ | 100/− |
| F/35 | Intermittent TTP | Lupus | Plasmapheresis and corticosteroids | 0/+ | 100/− |
| F/35 | Intermittent TTP | Pregnancy | Plasma infusion and corticosteroids | 0/− | 0/− |
| F/19 | Recurrent TTP | None | Plasmapheresis | 0/− | 0/− |
| M/18 | Recurrent TTP | None | Plasmapheresis | 0/+ | 0/+ |
Discussion
Diagnosis of TMA is a challenge because the usual clinical and biologic criteria as well as specific analysis of vWF are often defective in distinguishing them from other diseases and discriminating their clinical variants, especially in the early stage of the acute phase. In 1996, a metalloprotease purified from human plasma was demonstrated to cleave vWF within its main physiologic proteolysis site, and thus to play a major role in the modulation of vWF multimeric distribution in plasma.4,5 Because UL multimers of vWF are commonly found in plasma from patients with TMAs, vWF-cleaving protease was investigated as a new candidate for TMA pathophysiology. Indeed, a deficient activity of the enzyme was found, surprisingly, not in all TMAs but specifically in TTP.14 15 However, because these results relied on retrospective studies, both the sensitivity and specificity of this new biologic tool may be debatable. In the present study, vWFAg, vWF multimers, and vWF-cleaving protease activity were analyzed in consecutive unselected patients with suspected TMA, prospectively enrolled in the acute phase of the disease.
Over a 20-month period, 111 adult patients with TMA were included, corresponding to an incidence of about 1/1 000 000 residents. This value is lower than that previously reported,22 because we did not include pediatric cases and the totality of national cases has probably not been enrolled. Routine laboratory abnormalities identified in our patients were similar to those previously reported.23 Analysis of both vWFAg and vWF multimers demonstrated heterogeneous results, with no clear tendency as a function of recurrent familial, intermittent, or sporadic cases. The pathogenic role of plasma UL multimers of vWF is controversial in TMA,24-26 because they are not constantly found in the acute phase, may persist in remission, and are present in other physiologic or pathologic conditions without inducing microthrombotic manifestations.27,28 In contrast, an increase of the LMW multimers of vWF, reflecting an excessive proteolysis, was reported as a consistent finding of TMA acute phase.25,26 In the present study, neither UL nor increased LMW multimers of vWF were consistently found in TMA. This discrepancy concerning the LMW multimers may be methodologic because we chose the first 5-mers to define the LMW forms,29 whereas Galbusera and colleagues chose the 2 fastest migrating bands of the multimeric profile.25,26 Furthermore, searching for statistical relationships between vWF-cleaving protease and other laboratory features may be interesting to establish a pathogenic link between vWF-cleaving protease deficiency and the variation of the multimeric profile of vWF. The lack of correlation between vWF-cleaving protease and UL forms of vWF underlines the complex association of factors involved in the regulation of vWF multimeric distribution during acute TMA: platelet activation inducing an adsorption of vWF onto their membrane receptors, endothelial injury responsible for a secretion of highly multimerized vWF, release of enzymes inducing an abnormal proteolysis of vWF.30 31 However, the relationship identified between vWF-cleaving protease deficiency and the severity of thrombocytopenia strongly suggests that UL multimers of vWF act as a crucial bridging key in TMA pathogenesis even though they cannot be considered as a sensitive and specific marker of TMA.
Thus, in the current study, the sensitivity and the specificity of a deficient activity of vWF-cleaving protease were prospectively analyzed in unselected patients with acute TMAs. Interestingly, our results are globally concordant with previous reports relying on retrospective selected cases.14,15 Concerning the distinction between TTP and HUS, we found an 89% sensitivity and a 91% specificity of vWF-cleaving protease defect in TMA manifesting as TTP, including familial recurrent, intermittent, and sporadic cases. Our results may be surprising when considering the reported failure of clinical criteria to differentiate TTP from HUS.10 12 This concordance may reflect the experience of the participating centers, but it also strongly suggests that 2 different entities are distinguishable among the TMAs. However, the presence of 6 cases manifesting as HUS despite a vWF-cleaving protease deficiency underlines that intermediate cases are unavoidable. In these 6 patients, the HUS-associated contexts were heterogeneous, consisting of 3 infections (gastroenteritis, HIV), 2 neoplasia (lymphoma and chronic myeloid leukemia), and a single lupus, thus allowing no obvious link between vWF-cleaving protease deficiency and a common group of diseases. In none of them did the outcome reveal neurologic symptoms evoking a switch to a diagnosis of TTP, and HUS remained the definitive diagnosis.
Among the progressive forms of TTP (Figure 1), a total vWF-cleaving protease deficiency was constantly found in both familial recurrent and intermittent forms but not in sporadic forms where partial defects and even rare normal levels were present. This result may be interpreted either as a limit for vWF-cleaving protease deficiency sensitivity in TTP or as a difficulty in making a differential diagnosis between sporadic TTP and other diseases, especially at the early stage of symptoms. In that regard, the definitive diagnosis of our 7 patients with presumed TTP and exhibiting normal vWF-cleaving protease levels remains obscure. Indeed, in some cases, the associated context (metastatic neoplasia, lupus, septicemia, drug-induced renal failure) by itself may be an explanation for the clinical and biologic symptoms used for TMA diagnosis. In only one patient with pregnancy (vWF-protease activity = 109%), the initial TTP diagnosis was revisited as a HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome.
On the other hand, when considering the clinical contexts associated with TMA (Figure 2), total deficiencies of vWF-cleaving protease were observed in idiopathic as well as in secondary forms, especially in neoplasia, immunologic disorders, infections, pregnancy, and drug-related TTP. In contrast, partial deficiencies of vWF-cleaving protease were found only in secondary forms of TMA. Interestingly, we demonstrated a partial deficiency of vWF-cleaving protease (35%) in 2 patients with TTP related to bone marrow transplantation, which contrasts with a previous report from van der Plas and coworkers32 who found normal vWF-protease levels in 7 of 8 patients with TTP related to bone marrow transplantation. However, the heterogeneity of secondary forms of TMA associated with partial deficiencies of vWF-cleaving protease activity raises the question of the interpretation of the latter as a function of both physiologic and pathologic contexts. To date, the cellular origin as well as the regulating mechanisms of this metalloprotease are still unknown and the influence of factors, such as hormonal status or visceral dysfunction, on the activity of vWF-cleaving protease measured in plasma remains to be evaluated. In a previous report,33 we showed that vWF-cleaving protease activity was normal in pulmonary hypertension. In contrast, Oleksowicz and associates demonstrated that decreased vWF-protease levels were observed in metastatic cancers.34 The variation of vWF-protease activity in other clinical contexts independently of the presence of a TMA has been reported only in a short series of cases.15 Thus, the significance of partial functional deficiencies of vWF-protease in TMA is open to question because they may be related only to the associated clinical context.
The mechanisms described for vWF-cleaving protease deficiency are either potentially constitutional or acquired, thus related in most cases to an inhibitory autoantibody against the protease.14,15 In the present study, among the 65 patients with a vWF-cleaving protease deficiency, we found a 48% prevalence of inhibitor, which is lower than those previously reported by Tsai and coworkers15 and Furlan and colleagues14 (67% and 80%, respectively). Interestingly, we identified inhibitors to vWF-cleaving protease in sporadic and intermittent forms of TTP, which is in agreement with previous reports,14,15,35 but also in one case of familial recurrent TTP (inhibitor purified as an IgG; data not shown). The latter patient exhibited a relapsing TTP since childhood, treated by extensive plasma infusions, associated with a familial history of hemolytic anemia and thrombocytopenia in 2 relatives (who were unfortunately not available for investigation at the time of study). In this case, the presence of an alloantibody acquired following plasma infusion may not be excluded and will perhaps be confirmed in future studies. In the current study, the prevalence of a protease inhibitor was more important in intermittent (90%) than in sporadic TTP (40%). Globally, protease inhibitors were associated with an autoimmune context in almost 50% of cases, suggesting that autoantibodies are a common mechanism that accounts for vWF-cleaving protease activity deficiency. In the other cases, inhibitors to vWF-protease were associated with various contexts in which association with TMA was previously reported, such as HIV infection,36neoplasia, or drugs.37 Among drug-associated TMA,37 ticlopidine was recently involved in TTP related to an inhibitor to vWF-protease38 and clopidogrel was demonstrated to be associated with TTP in 11 patients.39In the present study, no TMA linked to antiplatelet drugs was identified. However, the absence of detectable inhibitor in 52% of patients with a vWF-cleaving protease deficiency–related TMA, especially the sporadic forms, remains unexplained and suggests that still unknown mechanisms may account for an acquired decrease of vWF-cleaving protease activity.
Even if too limited to establish prognostic factors, the follow-up of some patients in remission (Table 3) is interesting for speculation on the potential constitutional or acquired mechanism for vWF-cleaving protease deficiency. The 8 patients with sporadic TMA and 4 of 5 with intermittent TTP recovered a normal vWF-protease activity, which suggests a transitory acquired mechanism (corresponding to an inhibitor in about half the cases). Surprisingly, one patient with an intermittent TTP (first relapse occurring at the end of a pregnancy), who had no inhibitor despite a total vWF-cleaving protease deficiency in the acute phase, kept the same phenotype in remission. Such a status may be concordant with a constitutional defect, even though no familial history was highlighted by the anamnesis. As expected, in both recurrent familial cases, vWF-cleaving protease activity remained nil in remission, which corroborates the likely constitutional defect and underlines the status of vWF-cleaving protease deficiency as a risk factor more than as an univocal etiology for TMA.
In conclusion, our study emphasizes that vWF-cleaving protease deficiency specifically concerns a subgroup of TMA and thus allows new insights in the pathophysiology of this complex and heterogeneous group of diseases. In that regard, HUS may correspond to a kidney-limited TMA involving local mechanisms, whereas TTP may be considered as a systemic TMA related to a general enzymatic system abnormality. Moreover, besides the well-defined sporadic and familial recurrent TTP, the intermittent forms of the disease probably constitute a heterogeneous group including acquired as well as constitutional forms. In the latter, the belated expression of the first event may be related to specific abnormalities of the gene encoding vWF-cleaving protease, for which cloning will probably be helpful to elucidate critical pieces of the TMA puzzle.
The authors acknowledge all the physicians from the participating centers for providing clinical and biologic data and plasma samples. These investigators and their institutions are listed in the.
Hôpital Bichat, Paris: Pr M.C. Guillin, Dr N. Ajzenberg, Laboratoire d'Hématologie; Hôpital Broussais, Paris: Pr M. Aiach, Dr M. Alhenc-Gelas, Laboratoire d'Hémostase; Hôpital Cochin, Paris: Dr F. Heshmati, ETS, Dr P. Blanche, Service de Médecine interne; Institut Curie, Paris: Pr P. Pouillard, Dr E. Blot, Service d'Oncologie médicale; Hôpital Necker, Paris: Pr B. Varet, Pr O. Hermine, Dr A. Buzyn, Service d'Hématologie clinique, Pr J.P. Grünfeld, Pr P. Lesavre, Dr L. Lidove, Dr D. Chauveau, Dr D. Jolly, Service de Néphrologie; Hôpital de la Pitié-Salpétrière, Paris: Pr JP Vernant, Service d'Hématologie Clinique, Pr J.C. Piette, Dr Z. Amoura, Dr P. Hausfater, Service de Médecine interne; Hôpital Saint Antoine, Paris: Pr A. Najman, Dr L. Garderet, Service d'Hématologie clinique, Dr J. Guglielminotti, Service de Réanimation médicale; Hôpital Saint Louis, Paris: Pr J.P. Clauvel, Dr A. Bussel, Dr P. Coppo, Service d'Immunologie clinique I, Pr L. Degos, Dr P. Rousselot, Service des Maladies du sang, Pr F. Sigaux, Dr M.L. Scrobohacci, Service d'Hématologie biologique, Dr V. Hugo, Dr F. Martinez, Service de Néphrologie; Hôpital Tenon, Paris: Pr J.D. Sraer, Dr K. Akposso, Service de Néphrologie A, Pr P. Ronco, Pr E. Rondeau, Dr J. Rossert, Service de Néphrologie B; Hôpital Antoine-Béclère, Clamart: Dr C. Boyer-Neumann, Dr M. Wolf, Service d'Hématologie biologique, Dr F. Brivet, Service de Réanimation médicale, Pr G. Simonneau, Pr M. Humbert, Service de Pneumologie; Hôpital Avicenne, Bobigny: Pr L. Guillevin, Dr L. Mouthon, Dr M.H. André, Dr P. Cohen, Service de Médecine interne, Dr S. Brechignac, Hémothérapie; Hôpital Beaujon, Clichy: Pr A. Bezeau, Dr MH. Denninger, Service d'Hématologie biologique, Dr C. Gardin, Service d'Hématologie clinique; Hôpital Bicêtre, Le Kremlin Bicêtre: Pr G. Tchernia, Dr M. Dreyfus, Service d'Hématologie biologique, Pr B. Charpentier, Service de Néphrologie, Dr A.Mercat, Service de Réanimation Médicale, Pr X. Mariette, Service de Rhumatologie, Pr J. Delfraissy, Service de Médecine interne; Hôpital Foch, Suresnes: Dr P. Loirat, Dr D. Hurel, Dr M. Djibre, Service de Réanimation médicale; Hôpital Jean Verdier, Bondy: Dr Guirafac, Service de Médecine interne; Hôpital Louis Mourier, Colombes: Dr A. Khechai, Dr Lebret-Lerolle, Service d'Hématologie biologique; Hôpital d'Angers: Dr C. Ternisien, Laboratoire d'Hémostase, Pr M. Boasson, Dr M. Hunault-Berger, Service de Médecine Interne; Hôpital Saint André, Bordeaux: Pr C. Combe, Dr C. Level, Service de Néphrologie; CHU de Dijon: Dr Caillot, Service d'Hématologie clinique, Dr Mousson, Service de Néphrologie; Hôpital de Dunkerque: Dr M.H. Weillaert, Laboratoire de Biologie; CHU La Timone, Marseille: Pr I. Juhan-Vague, Dr K. Pouymayou, Service d'Hématologie biologique, Dr Poullin, Hémaphérèse; Pr Weiller, Service de Médecine Interne; CHU de Nancy: Pr P. Bordigoni, Service de Médecine infantile, Pr T. Lecompte, Laboratoire d'Hématologie; CRTH-CHRU Hôtel Dieu, Nantes: Pr JL. Harrousseau, Dr E. Fressinaud, Laboratoire d'Hématologie, Dr F. Mechinau, Service d'Oncologie pédiatrique; CHU de Reims: Dr A. Wynckel, Service de Néphrologie; CHU de Strasbourg: Dr Haffner, Dr Rondeau, Dr Caillard, Service de Réanimation Médicale A, Dr J.C. Weber, Service de Médecine interne, Dr Chantrel, Service de Néphrologie; CHU Trousseau, Tours: Pr Y. Gruel, Dr C. Pouplard, Laboratoire d'Hématologie; Hôpital de Valenciennes: Dr Binaut, Service de Néphrologie.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dominique Meyer, INSERM Unité 143, 84, rue du Général Leclerc 94 276 Le Kremlin Bicêtre Cedex, France; e-mail: dmeyer@infobiogen.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal