Abstract
Hematopoietic stem cell (HSC) fate decisions between self-renewal and commitment toward differentiation are tightly regulated in vivo. Recent developments in HSC culture and improvements of human HSC assays have facilitated studies of these processes in vitro. Through such studies stimulatory cytokines critically involved in HSC maintenance in vivo have been demonstrated to also promote HSC self-renewing divisions in vitro. Evidence for negative regulators of HSC self-renewal is, however, lacking. Tumor necrosis factor (TNF), if overexpressed, has been implicated to mediate bone marrow suppression. However, whether and how TNF might affect the function of HSC with a combined myeloid and lymphoid reconstitution potential has not been investigated. In the present studies in vitro conditions recently demonstrated to promote HSC self-renewing divisions in vitro were used to study the effect of TNF on human HSCs capable of reconstituting myelopoiesis and lymphopoiesis in nonobese diabetic–severe combined immunodeficient (NOD-SCID) mice. Although all cord blood and adult bone marrow CD34+CD38− cells were capable of undergoing cell divisions in the presence of TNF, cycling HSCs exposed to TNF in vitro and in vivo were severely compromised in their ability to reconstitute NOD-SCID mice and long-term cultures. The negative effect of TNF was not dependent on the Fas pathway, and a similar effect could be observed using a mutant TNF exclusively targeting the p55 TNF receptor. TNF did not appear to enhance apoptosis or affect cell-cycle distribution of cultured progenitors, but rather promoted myeloid differentiation. Thus, TNF might regulate HSC fate by promoting their differentiation rather than self-renewal.
Introduction
The output of millions of blood cells per second in adult humans is strictly dependent on the presence of multipotent short- and long-term reconstituting hematopoietic stem cells (HSCs). HSC fate decisions between self-renewal and commitment toward differentiation or potentially apoptosis are tightly regulated in vivo, although the molecular mechanisms regulating these decisions remain unknown.1 However, through gene targeting studies, several growth stimulatory cytokines have been demonstrated to be critically involved in HSC maintenance in mice.2-4 The potential role of negative regulators of HSC maintenance in steady-state hematopoiesis or conditions characterized by hematopoietic failure is less clear.
Tumor necrosis factor-α (TNF) has been suggested to be involved in a number of bone marrow failure syndromes.5-7In agreement with this, a large number of previous studies have demonstrated that TNF can negatively regulate the growth of murine and human progenitors including candidate HSC populations.8-13However, the resulting effect on HSC function was not addressed in these studies.
There are 2 main reasons why studies of human HSC self-renewal in vitro have been difficult to pursue until recently. First, previously used in vitro culture conditions appeared inefficient at promoting growth of candidate human HSCs, and studies in mice suggested that such conditions were usually associated with loss of HSC potential.14-16 Second, the lack of functional assays for human HSCs addressing their ability to reconstitute myelopoiesis and lymphopoiesis made it difficult to access HSC potential. The recent cloning and characterization of the early acting cytokines c-kit ligand (KL),2 flt3 ligand (FL),2 and thrombopoietin (Tpo),17 demonstrated to be critically involved in HSC maintenance in mice,2-4,18,19 have dramatically improved the ability to promote in vitro growth of candidate human HSCs.20-23 More importantly, the use of these cytokines combined with other developments in HSC culture conditions and the ability to specifically track HSC divisions in vitro, have demonstrated that HSCs undergoing cell divisions under such conditions can preserve their HSC function.22-26 The evidence for this is particular strong in mice, in which pluripotent long-term repopulating HSCs can be directly evaluated.1 Through such studies, multiple cytokines (including KL, FL, Tpo, and interleukin-11 [IL-11]) have been demonstrated to efficiently promote in vitro murine HSC self-renewing divisions.23,26,27 The recent development and improvement of assays for candidate human HSCs have now allowed corresponding studies in humans28-31 and development of conditions, similar to those used in mice, which appear to promote self-renewing divisions of candidate human HSCs.23,25,32-34 Although it remains to be established how predictable these assays are for long-term repopulating HSC activity, the in vivo xenograft models appear particularly useful because they selectively detect very primitive progenitor/stem cells capable of in vivo reconstituting both myelopoiesis and lymphopoiesis.30,31 35 Thus, the stage has been set for meaningful studies of regulation of self-renewal of candidate human HSCs.
In the present studies we used in vitro conditions recently demonstrated to efficiently promote proliferation of murine and candidate human HSCs with sustained HSC function,23 26 to address the potential effect of TNF on self-renewal of cells capable of repopulating myelopiesis and lymphopoiesis in nonobese diabetic-severe combined immunodeficient (NOD-SCID) mice. Whereas all candidate HSCs were capable of undergoing cell divisions in the presence of TNF, they were dramatically compromised in their ability to reconstitute NOD-SCID mice in vivo and long-term cultures in vitro. Rather, the presence of TNF appeared to promote HSC differentiation. Thus, TNF appears to negatively regulate maintenance of cycling human HSCs.
Materials and methods
Isolation of CD34+ and CD34+CD38− cord blood and bone marrow cells
Cord blood (CB) cells from normal full-term deliveries were collected from mothers after informed consent was obtained, and bone marrow (BM) cells were obtained by iliac crest aspiration from normal adult volunteers after informed consent was obtained, both with approval of the ethic committee of the University Hospital of Lund. BM and CB mononuclear cells (MNCs) were isolated by Ficoll-Hypaque gradient centrifugation (Lymphoprep, Nycomed Pharma, Oslo, Norway). Positive selection of CD34+ cells was performed using a magnetically activated cell sorting (MACS) CD34 isolation kit (Miltenyi Biotec; Bergish Gladbach, Germany), as described previously.23 In most experiments CB CD34+cells were run through a second column to obtain higher purity of CD34+ cells. The purity of CB CD34+ cells in the experiments was 62% to 98% (average, 89%), and for BM cells 57% to 94% (average, 80%).
CD34+CD38−/low CB and BM cells were obtained by incubating enriched CD34+ cells with anti-CD34–fluorescein isothiocyanate (FITC) and anti-CD38–phycoerythrin (PE) monoclonal antibodies (mAbs; both from Becton Dickinson, San Jose, CA), which were subsequently sorted on a FACSVantage cell sorter (Becton Dickinson). As previously described,23 a conservative approach was taken so that only the “lowest” CD34+CD38−/low cells were sorted, for BM the 3% lowest and for CB the 6% lowest CD38-expressing cells in the CD34+ population.
Hematopoietic growth factors, TNF molecules, and antibodies
Recombinant human (rh) KL, rh interleukin-3 (IL-3), rh granulocyte colony-stimulating factor (G-CSF), and rh granulocyte-macrophage colony-stimulating factor (GM-CSF) were generously provided by Amgen (Thousand Oaks, CA). RhTpo was kindly provided by Genentech (San Francisco, CA), rh erythropoietin (Epo) by Boehringer Mannheim (Mannheim, Germany), and rhFL by Immunex (Seattle, WA). RhTNF as well as the 2 TNF mutants were kind gifts from Hoffmann-LaRoche (Basel, Switzerland). Human TNF mutants specific for TNFR55 and TNFR75 were prepared by site-directed mutagenesis.36 Solid phase binding studies have shown that the Trp32 Thr86 TNF mutant protein binds with wild-type affinity to TNFR55 and does not bind at all to TNFR75, whereas the Asn134Arg145 TNF mutant protein exclusively binds to TNFR75, although with a 5- to 10-fold lower affinity than wild-type TNF.36 A polyclonal neutralizing antibody (Ab) against human TNF was purchased from R & D Systems (Minneapolis, MN) and used at 2 μg/mL, a concentration that completely blocks the effect of rhTNF 20 ng/mL (I.D. and S.E.W.J., unpublished observations). A blocking Ab against human Fas (monoclonal mouse IgG1), clone ZB4, was purchased from Upstate Biotechnology (Lake Placid, NY) and used at a concentration (1 μg/mL) demonstrated to completely block the inhibitory effect of 1 μg/mL of an agonistic Ab against human Fas on human Jurkat cells (Figure 1).
Effects of agonistic and antagonistic anti-Fas Abs on Jurkat cells.
Jurkat cells (20 000) were cultured in 100 μL RPMI 1640 with 10% FCS in the absence or presence of 1 μg/mL CH11 (agonistic anti-Fas Ab) and/or ZB4 (blocking anti-Fas Ab) at different concentrations as indicated. Following 24 hours incubation, each well was pulsed for 24 hours with 1 μCi [3H]TdR before being harvested and evaluated for [3H]TdR uptake (cpm) as described in “Materials and methods.” Each group was performed in triplicate and error bars represent SD.
Effects of agonistic and antagonistic anti-Fas Abs on Jurkat cells.
Jurkat cells (20 000) were cultured in 100 μL RPMI 1640 with 10% FCS in the absence or presence of 1 μg/mL CH11 (agonistic anti-Fas Ab) and/or ZB4 (blocking anti-Fas Ab) at different concentrations as indicated. Following 24 hours incubation, each well was pulsed for 24 hours with 1 μCi [3H]TdR before being harvested and evaluated for [3H]TdR uptake (cpm) as described in “Materials and methods.” Each group was performed in triplicate and error bars represent SD.
[3H]TdR incorporation assay for Jurkat cells
Jurkat cells were cultured in RPMI 1640 (Life Technologies, Paisley, United Kingdom) with 10% fetal calf serum (FCS; Gibco, Paisley, United Kingdom) at 20 000 cells/100 μL in flat-bottom 96-well microtiter plates. Following 24 hours of incubation in the absence or presence of CH11, 1 μCi [3H]TdR (Amersham, Arlington Heights, IL) was added to each well. Cells were cultured for another 24 hours before being harvested onto glass fiber filters and counted in a β-scintillation counter.
Ex vivo expansion cultures
The CD34+ CB cells, CD34+CD38− CB cells, or CD34+CD38− BM cells were cultured for 2 to 12 days in serum-free (SF) medium, either X-vivo 15 (Bio-Whittaker; Walkersville, MD) supplemented with 1% detoxified bovine serum albumin (BSA; Stemcell Technologies; Vancouver, BC, Canada) or Iscoves modified Dulbecco medium (IMDM; Bio-Whittaker) supplemented with 20 mg/mL BSA, 10 μg/mL human insulin, and 200 μg/mL of human transferrin (BIT; Stemcell Technologies) as well as 40 mg/mL low-density lipoprotein (LDL; Sigma; St Louis, MO). CD34+ CB cells were seeded at 10 000 to 40 000 cells/mL, and CD34+CD38− CB and BM cells at 800 to 3500 cells/mL. Cells were cultured (in the absence or presence of TNF) in a cocktail of cytokines (rhKL 100 ng/mL, rhFL 100 ng/mL, rhTpo 100 ng/mL, and rhIL-3 20 ng/mL; KFT3) based on previous studies suggesting that these cytokines efficiently induce proliferation of candidate HSCs with sustained hematopoietic potential.23 26 Following culture, cells were enumerated and evaluated functionally in long-term cultures or transplanted into NOD-SCID mice. In some experiments cultured cells were also examined with regard to apoptosis and cell-cycle and differentiation status.
Limiting dilution assay
To investigate the direct effects of TNF on candidate stem cells, CD34+CD38− cells were seeded in Terasaki plates (Nunc; Kamstrup, Denmark) at a density of 1 cell/well in SF medium (X-vivo 15 and 1% BSA), supplemented with a cocktail of cytokines (rhKL 50 ng/mL, rhFL 50 ng/mL, rhTpo 50 ng/mL, and rhIL-3 20 ng/mL) in the absence or presence of TNF 20 ng/mL. Wells (120/group) were scored for cell growth following 10 to 12 days of incubation at 37°C in a humidified atmosphere with 5% CO2 in air. Because the statistical chance (based on Poisson probability distribution) of a well not receiving any cell is 37% by this method, the maximum expected clones were 76.23
To compare recruitment of KFT3-cultured CB CD34+CD38− cells in the absence or presence of TNF, single cells were directly sorted by an Automated Cell Deposition Unit (ACDU) coupled to a FACSVantage (Becton Dickinson), into Terasaki wells containing 20 μL/well of SF medium supplemented with rhKL 100 ng/mL, rhFL 100 ng/mL, rhTpo 100 ng/mL, and rhIL-3 20 ng/mL in the absence or presence of TNF 20 ng/mL. Each well was inspected for the presence of a single cell, and assessed on days 1, 2, 3, 5, 7, and 10 for the time of first cell division and number of cells per clone.
NOD-SCID repopulating assay
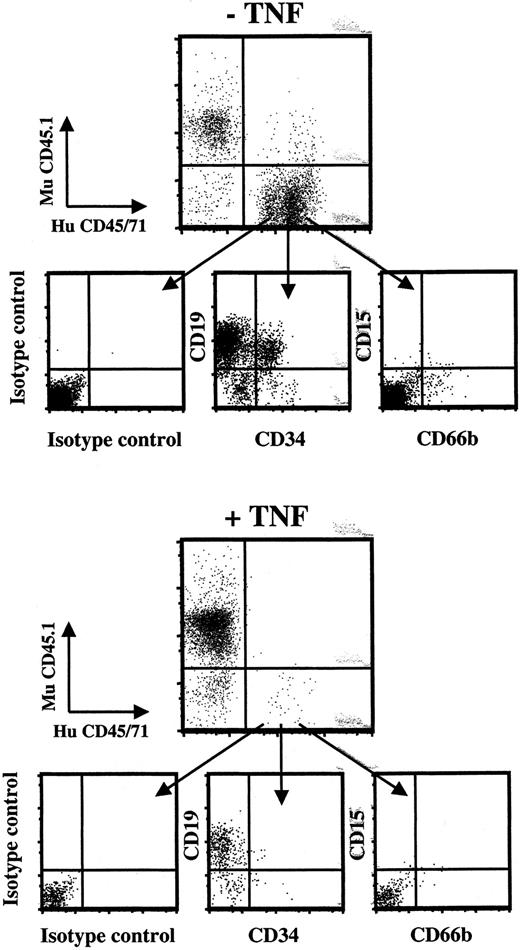
The NOD/LtSz-SCID mice (originally from The Jackson Laboratory, Bar Harbor, ME) were bred and housed under sterile conditions in microisolator cages and given irradiated food and acidified, autoclaved water. Mice were irradiated with 350 cGy from a 137Cs source at 8 to 12 weeks of age and given prophylactic ciprofloxacin 100 mg/L in the drinking water until analysis (6 weeks after transplantation). Tail vein transplantation/injection of hematopoietic cells suspended in 0.5 to 1.0 mL medium was performed within 12 hours of irradiation. In some experiments 1 × 106 irradiated (1500 cGy) accessory cells (MNC BM or CB cells or CD34+-depleted CB cells) were coinjected. After 6 weeks mice were killed by asphyxiation with CO2; femora and tibiae were collected, and engraftment was investigated by flow cytometric analysis (FACSCalibur) as described previously.35,37 38 Briefly, BM cells were counted and blocked with anti–mouse Fc-block (Pharmingen, San Diego, CA) and whole-mouse IgG (Jackson Immunoresearch Laboratories, West Grove, PA). Subsequently, cells were stained with FITC-conjugated anti–human CD45 and CD71 Abs (Becton Dickinson) as well as anti–mouse CD45.1 (Ly 5.1)–PE Ab (Pharmingen). BM cells from untransplanted mice (negative controls) and mixtures of 0.1% to 0.5% human cells in murine BM (positive controls) were always included. If engraftment was detected by CD45/CD71 analysis (detection level 0.05%), lineage analysis with anti-HuCD34–FITC (progenitors), anti-HuCD19–PE (B cells; Becton Dickinson), or anti-HuCD15–PE and anti-HuCD66b–FITC (myeloid; both Pharmingen) combined with anti-HuCD45–allophycocyanin (APC) (Becton Dickinson) was performed. For all samples 7-amino actinomycin D (7-AAD; Sigma) was included to gate out dead cells. A minimum of 50 000 BM cells were examined for each sample. Only mice with both positive myeloid and lymphoid engraftment (defined as > 10 positive events each per 50 000 viable BM cells) were evaluated as positive. Gates were set so that samples incubated with irrelevant isotype-matched control Abs, had a maximum of 1 positive event per 50 000 BM cells analyzed. If no engraftment was detected by flow cytometry or if the myeloid engraftment was questionable, BM cells were plated in methylcellulose supplemented with human-specific cytokines (rhGM-CSF 50 ng/mL, rhIL-3 25 ng/mL, rhSCF 25 ng/mL) and rhEpo 5 U/mL at a density of 105 cells/35-mm plate, 4 replicates per group. Granulocyte-macrophage colony-forming unit (CFU-GM) and erythroid burst-forming unit (BFU-E) colonies were scored after 10 to 12 days. No colonies were observed in the absence of cytokines or from BM of untransplanted mouse cultured with the same cytokines (I.D. and S.E.W.J., unpublished observation, 2000).
Long-term culture–initiating cell assay
Long-term cultures were established and maintained according to previously described procedures.39 Briefly, stroma cell feeders were established by seeding a mixture (1:1) of 2 irradiated (8000 cGy) murine fibroblast cell lines (M2-10B4 and sl/sl, kindly provided by D. E. Hogge; Vancouver, BC, Canada), engineered to produce high levels of human G-CSF, IL-3, and KL,39 into 96-wells–collagen-coated microtiter plates containing long-term culture (LTC) medium (Myelocult; Stemcell Technologies) supplemented with freshly dissolved 10−6 M hydrocortisone 21-hemisuccinate (Sigma). The cytokine production from M2-10B4 and sl/sl fibroblasts was tested (by commercially available enzyme-linked immunosorbent assay [ELISA] kits; R & D Systems) and found to be at recommended levels39 (I.D. and S.E.W.J., unpublished observation).
Freshly isolated CD34+CB cells (150 cells/well) or sorted CD34+CD38− BM/CB cells (25-75 cells/well) or the expansion equivalents (EEs) of cultured cells were seeded at 2 to 4 replicates and incubated at 37°C in a humidified atmosphere with 5% CO2 in air. Cocultures were maintained by weekly 50% medium changes. Following 6 weeks, adherent and nonadherent cells were transferred to methylcellulose cultures containing rhKL, rhGM-CSF, rhG-CSF, rhFL, rhIL-3 (all at 10 ng/mL) and rhEpo (5 U/mL). To ensure formation of a reliable number of colonies from the long-term culture, the content of each stroma coculture well was transferred to methylcellulose cultures at both a low (10%-20% of cells) and a high concentration (80%-90% of cells). LTC–colony-forming cells (LTC-CFCs) were scored after an additional 10 to 12 days in culture. Stroma cells without hematopoietic cells and cytokines were used as a negative control for the CFC assay.
Flow cytometric evaluation of apoptosis, cell-cycle status, and differentiation of cultured CB progenitors
Apoptosis was assessed by measuring redistribution of phosphatidylserine using annexin V-PE (Pharmingen) and uptake of 7-AAD.40 Briefly, CD34+ CB cells cultured for 6 days in KFT3 in the absence or presence of TNF were washed and resuspended in 100 μL annexin V binding buffer (Pharmingen) and incubated with anti-CD34–APC, annexin V–PE (5 μL), and 7-AAD (5 μg/mL) for 15 minutes, resuspended in annexin V–binding buffer and analyzed on a FACSCalibur.
The CD34+ CB cells cultured for 6 to 7 days in the absence or presence of TNF were also subjected to high-resolution cell-cycle analysis as recently described41 42 with minor modifications. Fixation and permeabilization of cells were performed with Cytfix/Cytoperm Kit (Pharmingen). After washing, cells were stained with FITC-conjugated anti–Ki-67 (Immunotech, Westbrook, ME) or an isotype-matched irrelevant control Ab. After 3 hours of incubation in phosphate-buffered saline (PBS) containing 5% FCS supplemented with 7-AAD (5 μg/mL) on ice, samples were analyzed on a FACSCalibur.
To evaluate differentiation of in vitro cultured (KFT3 in the absence or presence of TNF 20 ng/mL; 2-12 days) CD34+ CB cells were stained with anti-CD34–APC (Becton Dickinson) and a lineage cocktail containing PE- or FITC-conjugated Abs (CD3, CD14, CD15, CD19, CD20, CD56, CD66b, glycophorin A), and 7-AAD to exclude nonviable cells. Control samples of cultured cells were stained with irrelevant isotype-matched control Abs. Samples were analyzed on a FACSCalibur.
Results
CD34+CD38− CB and BM cells undergo a limited number of cell divisions before becoming sensitive to TNF-induced growth inhibition
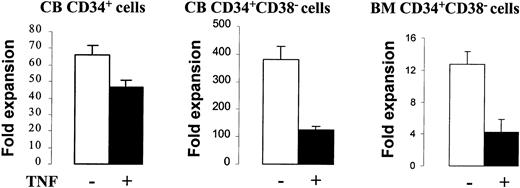
We have recently demonstrated that a combination of KL + FL + Tpo (KFT) in the absence or presence of IL-3 under SF conditions efficiently promotes cell divisions of murine and candidate human stem cells with sustained stem cell function,23,26thus representing a model for in vitro HSC self-renewal. We here used this system to study the potential effects of TNF on candidate human HSCs in CB and adult BM. When CD34+ or CD34+CD38− CB cells were cultured in KFT3 for 2 days, TNF had no effect on cell numbers (I.D. and S.E.W.J., unpublished observation, 2000), whereas following 5 to 8 days of incubation in KFT3, by which time virtually all cells have proliferated,23 KFT3 + TNF cultures contained 29% (CD34+ cells), 67% (CD34+CD38−cells), and for CD34+CD38− BM cells 65% fewer cells than KFT3 cultures (Figure 2). However, cultures containing TNF expanded many-fold (Figure2).
Effect of TNF on in vitro cellular expansion of CD34+ and CD34+CD38− cells.
Cells were cultured in SF medium in KFT3 in the absence or presence of TNF (20 ng/mL). CD34+ CB cells (5 experiments) were cultured for 5 to 6 days, CD34+CD38− CB cells (3 experiments) for 8 days, and CD34+CD38− BM cells (4 experiments) for 5 days. Data represent the mean values of all experiments for different cell types. Error bars show the SEM.
Effect of TNF on in vitro cellular expansion of CD34+ and CD34+CD38− cells.
Cells were cultured in SF medium in KFT3 in the absence or presence of TNF (20 ng/mL). CD34+ CB cells (5 experiments) were cultured for 5 to 6 days, CD34+CD38− CB cells (3 experiments) for 8 days, and CD34+CD38− BM cells (4 experiments) for 5 days. Data represent the mean values of all experiments for different cell types. Error bars show the SEM.
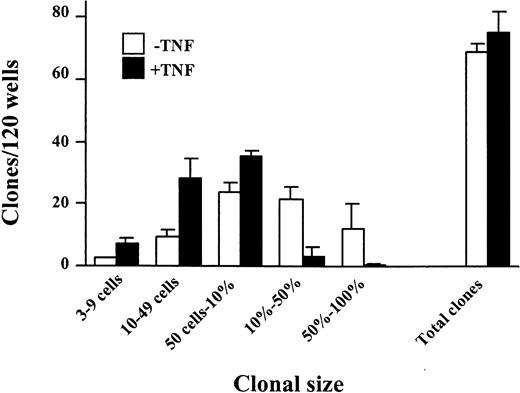
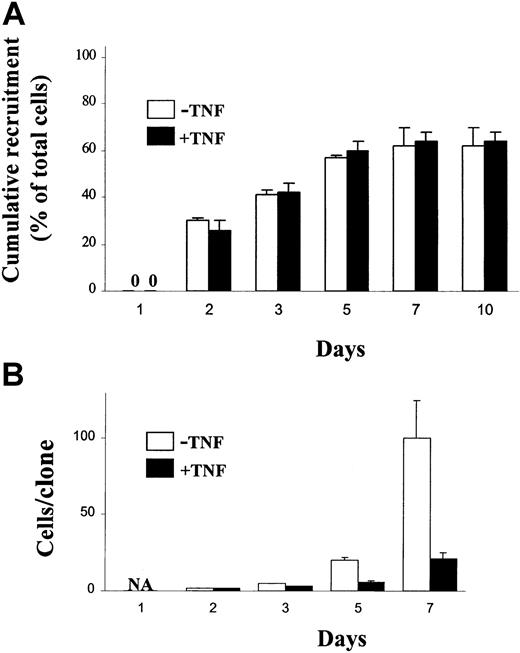
Next, single cell experiments were performed to establish whether stimulation with TNF would block the first cell divisions of CD34+CD38− CB cells. Whereas TNF reduced the size of CD34+CD38− clones, the total number of clones was not reduced (Figure 3), suggesting that all CD34+CD38− cells responsive to TNF can undergo a limited number of cell divisions before becoming sensitive to TNF-induced growth inhibition. Importantly, TNF did not affect the time to first cell division (Figure4A), but inhibited subsequent cell divisions (Figure 4B).
The effect of TNF on growth of CD34+CD38− CB cells at the single cell level.
CD34+CD38− CB cells were plated at one cell/well in SF medium supplemented with KFT3 (as described in “Materials and methods”) in the absence (open bars) or presence (black bars) of TNF (20 ng/mL). Cultures were scored for clonal size (from 3 cells to 100% of well covered by cells) and total number of positive wells as indicated following 10 to 12 days of culture. Each group consisted of 120 wells, and results presented are mean values (SEM) from 4 independent experiments.
The effect of TNF on growth of CD34+CD38− CB cells at the single cell level.
CD34+CD38− CB cells were plated at one cell/well in SF medium supplemented with KFT3 (as described in “Materials and methods”) in the absence (open bars) or presence (black bars) of TNF (20 ng/mL). Cultures were scored for clonal size (from 3 cells to 100% of well covered by cells) and total number of positive wells as indicated following 10 to 12 days of culture. Each group consisted of 120 wells, and results presented are mean values (SEM) from 4 independent experiments.
Effect of TNF on recruitment of single CB CD34+CD38− cells into proliferation.
Single CD34+CD38− CB cells were deposited with a single cell depositor, coupled to a FACSVantage, into wells in Terasaki plates containing 20 μL SF medium supplemented with KFT3 in the absence or presence of TNF (20 ng/mL). Wells were inspected on the days indicated for recruitment into proliferation (first cell division; A) and clonal size (B). Results are presented as mean values (SD) of 2 independent experiments. 0 indicates no cell division; NA, not applicable.
Effect of TNF on recruitment of single CB CD34+CD38− cells into proliferation.
Single CD34+CD38− CB cells were deposited with a single cell depositor, coupled to a FACSVantage, into wells in Terasaki plates containing 20 μL SF medium supplemented with KFT3 in the absence or presence of TNF (20 ng/mL). Wells were inspected on the days indicated for recruitment into proliferation (first cell division; A) and clonal size (B). Results are presented as mean values (SD) of 2 independent experiments. 0 indicates no cell division; NA, not applicable.
TNF negatively affects maintenance of multipotent NOD-SCID repopulating HSCs
The recent development and improvement of an assay that allows evaluation of human HSCs capable of in vivo reconstituting both myelopoiesis and lymphopoiesis in NOD-SCID mice has facilitated studies of candidate human HSCs and their regulation.31,35 When CD34+ or CD34+CD38− cells were expanded in KFT or KFT3 under SF conditions, virtually all cells were recruited into proliferation23 (Figure 3). This combined with the fact that these conditions sustained NOD-SCID repopulating cells (SRCs) capable of reconstituting human B and myeloid cells (Table1 and Figure 3), suggested that SRCs had undergone cell divisions with sustained HSC function. In a total of 3 experiments, 9 of 9 mice transplanted with the EEs of 50 000 to 85 000 CB CD34+ cells or 5000 CD34+CD38− cells cultured for 5 to 8 days in KFT3 showed human myeloid and B-cell reconstitution, with an average of as much as 25% human engraftment. In contrast, only 1 of 9 mice transplanted with the same EEs of cells cultured for 5 days in KFT3 in the presence of TNF showed human myeloid and lymphoid engraftment (1.1%; Table 1 and Figure 5). In contrast, after 2 days of culture, at which time most candidate human HSCs have not yet proliferated43 44 TNF did not affect SRCs (Table 1). In a fifth experiment TNF was neutralized following 5 days of ex vivo expansion, and cells were cultured for an additional 3 days (Table 2). Although enhancing proliferation, the neutralization of TNF did not reverse the negative effect of TNF on SRCs, suggesting that the effect of TNF is irreversible.
TNF activation compromise in vivo multilineage reconstituting activity of NOD-SCID repopulating cells
| . | Cells . | EE . | Expansion (d) . | Reconstitution . | |||
|---|---|---|---|---|---|---|---|
| −TNF . | +TNF . | ||||||
| Positive mice . | Percent human engraftment . | Positive mice . | Percent human engraftment . | ||||
| Exp. 1 | CD34+ | 85 000 | 5-8 | 4/4 | 25 (6-25-34-36) | 0/4 | 0.2 (0-0-0.3-0.5) |
| Exp. 2 | CD34+C D38− | 5 000 | 7 | 2/2 | 51 (23-79) | ||
| 2 700-5 300 | 7 | 1/2 | 0-1.1 | ||||
| Exp. 3 | CD34+ | 50 000 | 5 | 3/3 | 8 (0.3-5-18) | 0/3 | 0.3 (0-0-0.9) |
| Total | 9/9 | 25 | 1/9 | 0.3 | |||
| Exp. 3 | CD34+ | 50 000 | 2 | 3/3 | 2 (0.1-1-5) | 2/3 | 2 (0-2.5-3) |
| Exp. 4 | CD34+ | 50 000 | 2 | 3/5 | 5 (0-0.1-0.2-0.3-23) | 3/3 | 8 (0.2-3-21) |
| Total | 6/8 | 4 | 5/6 | 5 | |||
| . | Cells . | EE . | Expansion (d) . | Reconstitution . | |||
|---|---|---|---|---|---|---|---|
| −TNF . | +TNF . | ||||||
| Positive mice . | Percent human engraftment . | Positive mice . | Percent human engraftment . | ||||
| Exp. 1 | CD34+ | 85 000 | 5-8 | 4/4 | 25 (6-25-34-36) | 0/4 | 0.2 (0-0-0.3-0.5) |
| Exp. 2 | CD34+C D38− | 5 000 | 7 | 2/2 | 51 (23-79) | ||
| 2 700-5 300 | 7 | 1/2 | 0-1.1 | ||||
| Exp. 3 | CD34+ | 50 000 | 5 | 3/3 | 8 (0.3-5-18) | 0/3 | 0.3 (0-0-0.9) |
| Total | 9/9 | 25 | 1/9 | 0.3 | |||
| Exp. 3 | CD34+ | 50 000 | 2 | 3/3 | 2 (0.1-1-5) | 2/3 | 2 (0-2.5-3) |
| Exp. 4 | CD34+ | 50 000 | 2 | 3/5 | 5 (0-0.1-0.2-0.3-23) | 3/3 | 8 (0.2-3-21) |
| Total | 6/8 | 4 | 5/6 | 5 | |||
CD34+ or CD34+CD38− CB cells were cultured for 5 to 8 days (experiments 1-3) or 2 days (experiments 3 and 4) in SF medium in KFT3 in the absence or presence of TNF. NOD-SCID mice were transplanted with the EEs of 50 000 to 85 000 CD34+ cells or 2700 to 5300 CD34+CD38− cells as indicated. Data are presented as number of mice positive for human reconstitution with both B and myeloid cells. Also shown is the percentage human engraftment (representing total human reconstitution independent of whether both myeloid and B cells were observed) of individual animals within each group.
Ex vivo expanded CD34+CD38−cells exposed to TNF have severely compromised in vivo multilineage reconstituting activity.
Multilineage human engraftment of a mouse transplanted with EEs of 5000 CD34+CD38− cells cultured for 7 days in KFT3 as well as a mouse transplanted with EEs of 5300 CD34+CD38− cells cultured under identical conditions except in the presence of TNF (20 ng/mL) is shown. Of all mice transplanted with TNF-cultured cells, this was the one that showed the highest level of human reconstitution, and the only one with both myeloid and lymphoid engraftment.
Ex vivo expanded CD34+CD38−cells exposed to TNF have severely compromised in vivo multilineage reconstituting activity.
Multilineage human engraftment of a mouse transplanted with EEs of 5000 CD34+CD38− cells cultured for 7 days in KFT3 as well as a mouse transplanted with EEs of 5300 CD34+CD38− cells cultured under identical conditions except in the presence of TNF (20 ng/mL) is shown. Of all mice transplanted with TNF-cultured cells, this was the one that showed the highest level of human reconstitution, and the only one with both myeloid and lymphoid engraftment.
The suppressive effect of TNF on SRCs is irreversible
| Cytokines . | Fold expansion . | Percent human engraftment . | No. positive mice . | |
|---|---|---|---|---|
| d 1-5 . | d 6-8 . | |||
| KFT3 | KFT3+anti-TNF | 81 | 23 (0.3-5-23-36-52) | 5/5 |
| KFT3+TNF | KFT3+TNF | 29 | 0 (0-0-0-0-0-0) | 0/5 |
| KFT3+TNF | KFT3+TNF+anti-TNF | 55 | 0.1 (0-0-0-0-0.7) | 1/5 |
| Cytokines . | Fold expansion . | Percent human engraftment . | No. positive mice . | |
|---|---|---|---|---|
| d 1-5 . | d 6-8 . | |||
| KFT3 | KFT3+anti-TNF | 81 | 23 (0.3-5-23-36-52) | 5/5 |
| KFT3+TNF | KFT3+TNF | 29 | 0 (0-0-0-0-0-0) | 0/5 |
| KFT3+TNF | KFT3+TNF+anti-TNF | 55 | 0.1 (0-0-0-0-0.7) | 1/5 |
CD34+ CB cells were cultured in SF medium supplemented with KFT3 in the absence or presence of TNF (20 ng/mL). After 5 days a neutralizing Ab against TNF was added at 2 μg/mL to the groups indicated and cultured for another 3 days, at which time the EEs of 100 000 CD34+CB cells were transplanted into irradiated NOD-SCID mice. Human engraftment was investigated after 6 weeks. Data are presented as number of mice positive for human reconstitution with both B and myeloid cells. Also shown is the percentage human engraftment (representing total human reconstitution independent of whether both myeloid and B cells were observed) of individual animals within each group.
To further investigate whether TNF primarily targets actively cycling HSCs, CD34+ cells were cultured in the presence of Tpo alone, which promotes survival rather than proliferation of candidate HSCs.45 Under such conditions the addition of TNF did not adversely affect SRCs following as much as 5 days of culture (Table 3).
Effect of TNF on noncycling SRCs
| . | −TNF . | +TNF . | ||
|---|---|---|---|---|
| Percent human engraftment . | Positive mice . | Percent human engraftment . | Positive mice . | |
| Exp 1 | 0.8 (0.1-1.6) | 1/2 | 3 (4.5-1.4) | 2/2 |
| Exp 2 | 0.8 (0.5-0.9-1) | 3/3 | 0.8 (0-0.6-1.8) | 2/3 |
| . | −TNF . | +TNF . | ||
|---|---|---|---|---|
| Percent human engraftment . | Positive mice . | Percent human engraftment . | Positive mice . | |
| Exp 1 | 0.8 (0.1-1.6) | 1/2 | 3 (4.5-1.4) | 2/2 |
| Exp 2 | 0.8 (0.5-0.9-1) | 3/3 | 0.8 (0-0.6-1.8) | 2/3 |
CD34+ CB cells were cultured for 5 days in X-vivo 15 supplemented with 1% BSA and Tpo 200 ng/mL in the absence or presence of TNF (20 ng/mL). Each NOD-SCID mouse was transplanted with the equivalent of 50 000 CD34+ cells. Data are presented as number of mice positive for human reconstitution with both B and myeloid cells. Also shown is the percentage human engraftment (representing total human reconstitution independent of whether both myeloid and B cells were observed) of individual animals within each group.
Exp indicates experiment.
Maintenance of cycling CB and adult BM CD34+CD38− LTC–initiating cells is negatively regulated by TNF
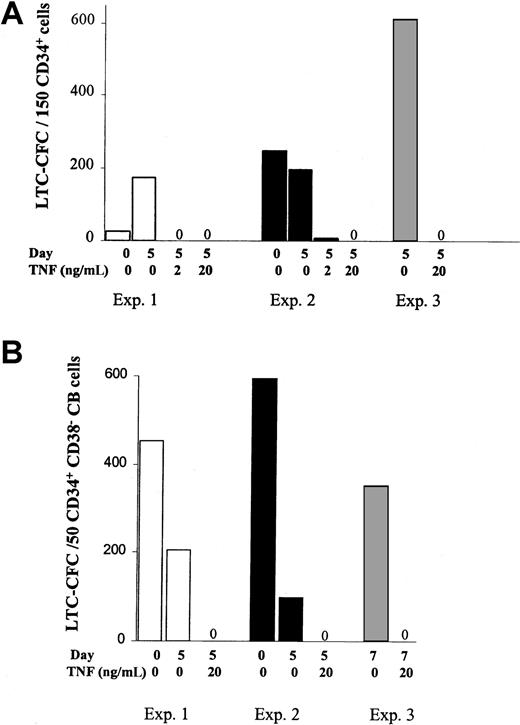
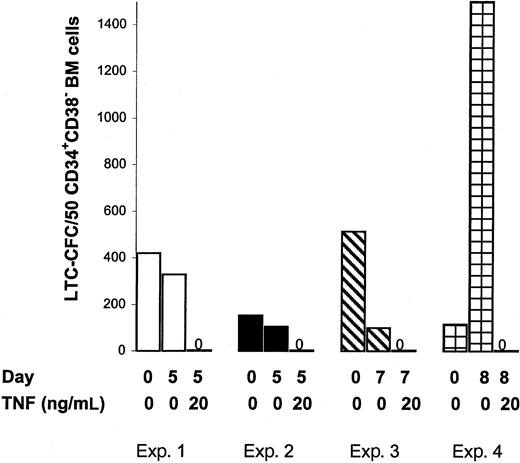
The negative effect of TNF on SRCs could potentially be due to TNF negatively affecting the crucial homing process of human HSCs to the BM in vivo. Thus next, the in vitro LTC–initiating cell (LTC-IC) assay was adapted to evaluate the effects of TNF on candidate human HSCs in which the in vitro reconstitution is unlikely to be dependent on the same homing mechanisms.28,39 In agreement with previous studies,23 high levels of LTC-CFC activity were sustained after 5 days of SF expansion of CD34+ or CD34+CD38− CB cells (Figure6A,B) or CD34+CD38− BM cells (Figure7) in KFT3. Furthermore, as observed in the in vivo reconstitution experiments, TNF at both 2 and 20 ng/mL, reduced the number of LTC-CFCs by more than 98%.
TNF activation negatively regulates maintenance of cycling CB CD34+CD38− LTC-ICs.
The 150 freshly isolated CD34+ (A) or 25 to 75 CD34+CB38− CB cells (B), or their EEs following 5 to 7 days of culture in KFT3 in the absence or presence of TNF were evaluated for 6-week LTC-CFC activity, as described in “Materials and methods.” Data are presented as mean values of 2 to 3 replicates of each group for CD34+ CB cells (panel A; 3 experiments) and CD34+CD38− CB cells (panel B; 3 experiments).
TNF activation negatively regulates maintenance of cycling CB CD34+CD38− LTC-ICs.
The 150 freshly isolated CD34+ (A) or 25 to 75 CD34+CB38− CB cells (B), or their EEs following 5 to 7 days of culture in KFT3 in the absence or presence of TNF were evaluated for 6-week LTC-CFC activity, as described in “Materials and methods.” Data are presented as mean values of 2 to 3 replicates of each group for CD34+ CB cells (panel A; 3 experiments) and CD34+CD38− CB cells (panel B; 3 experiments).
TNF negatively regulates maintenance of cycling BM CD34+CD38− LTC-ICs.
The 25 to 75 sorted CD34+CB38− BM cells and their EEs following 5 to 8 days of culture in KFT3 in the absence or presence of TNF were evaluated for 6-week LTC-CFC activity, as described in “Materials and methods.” Data represent the mean values of 2 to 3 replicates for each group in each of 4 independent experiments.
TNF negatively regulates maintenance of cycling BM CD34+CD38− LTC-ICs.
The 25 to 75 sorted CD34+CB38− BM cells and their EEs following 5 to 8 days of culture in KFT3 in the absence or presence of TNF were evaluated for 6-week LTC-CFC activity, as described in “Materials and methods.” Data represent the mean values of 2 to 3 replicates for each group in each of 4 independent experiments.
To exclude that the effect of TNF on LTC-ICs was indirect, an additional experiment was performed in which CD34+CD38− cells were cultured as single cells in KFT3 in the absence or presence of TNF (20 ng/mL) for 1 week in SF cultures. Subsequently, cells that had proliferated were transferred individually to long-term cultures. Whereas as much as 13 of the original clones cultured in KFT3 sustained LTC-IC activity, no clones from KFT3 + TNF cultures showed any LTC-CFC activity on replating (I.D. and S.E.W.J., unpublished observation, 2001).
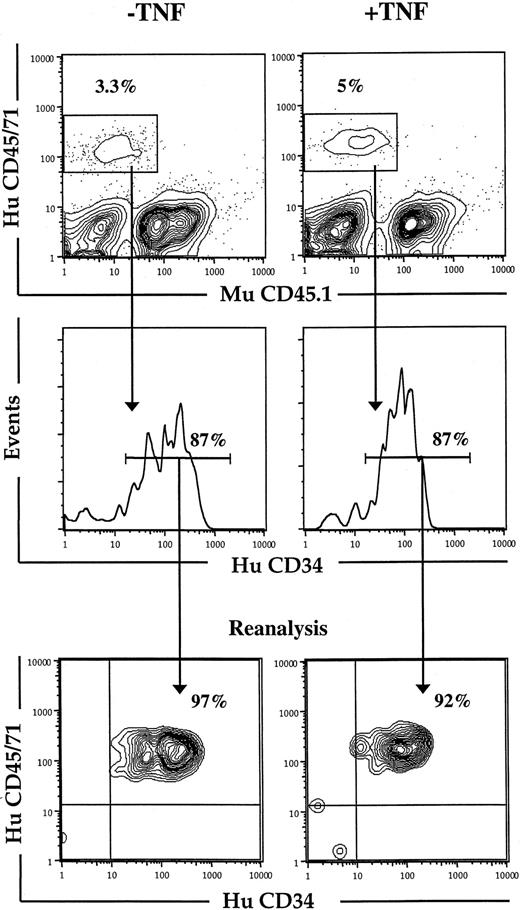
Because it was possible that the negative effect of TNF on cycling HSCs was dependent on the specific cytokines and in vitro conditions used, we also examined the effect of TNF on human HSCs in vivo. Because HSCs cycle rapidly on transplantation, NOD-SCID mice were transplanted with CD34+ CB cells and treated with TNF or PBS (control) 24, 64, and 96 hours after transplantation. Although the short-term reconstitution with human CD34+ cells was not affected by treatment with TNF (Figure 8), the number of LTC-CFCs recovered from the BM was dramatically reduced (Table4). Thus, TNF negatively regulates in vitro as well as in vivo maintenance of cycling HSCs.
Effect of in vivo TNF treatment on short-term engraftment of human CD34+ CB cells in NOD-SCID mice.
Freshly isolated CD34+ CB cells were transplanted into irradiated NOD-SCID mice that subsequently received 3 injections of PBS (−TNF) or 5 μg TNF (+TNF) 24, 64, and 96 hours after transplantation. At 24 hours after the last injection (day 5) mice were killed, and BM analyzed for human (HuCD45/71) and murine (MuCD45.1) engraftment (as described in “Materials and methods”). Cells positive for HuCD45/71 were analyzed for percentage HuCD34+cells, which were sorted on a FACSVantage for subsequent studies in LTC-IC assay (Table 4). Lower panels show purity analysis of sorted cells. Results represent data from 1 of 2 experiments with similar results.
Effect of in vivo TNF treatment on short-term engraftment of human CD34+ CB cells in NOD-SCID mice.
Freshly isolated CD34+ CB cells were transplanted into irradiated NOD-SCID mice that subsequently received 3 injections of PBS (−TNF) or 5 μg TNF (+TNF) 24, 64, and 96 hours after transplantation. At 24 hours after the last injection (day 5) mice were killed, and BM analyzed for human (HuCD45/71) and murine (MuCD45.1) engraftment (as described in “Materials and methods”). Cells positive for HuCD45/71 were analyzed for percentage HuCD34+cells, which were sorted on a FACSVantage for subsequent studies in LTC-IC assay (Table 4). Lower panels show purity analysis of sorted cells. Results represent data from 1 of 2 experiments with similar results.
Maintenance of in vivo cycling LTC-ICs is negatively regulated by TNF
| . | LTC-CFC/200 CD34+ cells . | |
|---|---|---|
| −TNF . | +TNF . | |
| Exp 1 | 183 | 9 |
| Exp 2 | 18 | 0 |
| . | LTC-CFC/200 CD34+ cells . | |
|---|---|---|
| −TNF . | +TNF . | |
| Exp 1 | 183 | 9 |
| Exp 2 | 18 | 0 |
Human CD34+ cells were sorted (as described in Figure 8) from the BM of NOD-SCID mice that had been transplanted with freshly isolated CD34+ BM cells 5 days earlier, and subsequently treated intravenously with PBS or 5 μg rhTNF 24, 64, and 96 hours after transplantation. The 200 sorted CD34+ cells were evaluated for 6-week LTC-CFC activity. Results are from 2 independent experiments and are presented as mean values of triplicates (Exp 1) and duplicates (Exp 2) determinations.
Exp indicates experiment.
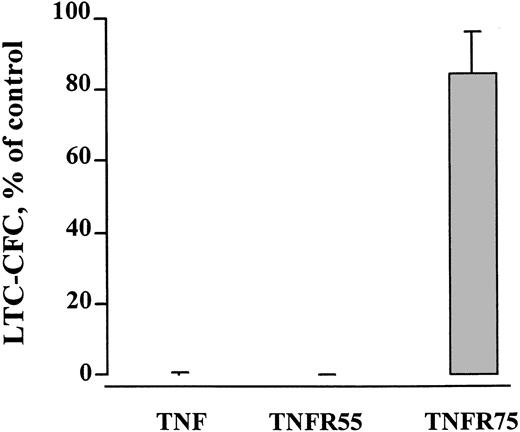
The ability of TNF to negatively regulate human HSC maintenance is signaled through TNFR55 and is not dependent on the Fas pathway
The biologic effects of TNF are mediated through 2 members of the TNF receptor superfamily, TNFR55 and TNFR75.46 To be able to dissect the relative role of these 2 receptors in regulating maintenance of candidate human HSC, mutant TNF molecules selectively binding to TNFR55 and TNFR75 were used.36 Whereas the mutant-binding TNFR55 mimicked the severe negative effect of TNF on LTC-CFC activity of ex vivo expanded CB and BM CD34+CD38− cells, the mutant targeting TNFR75 had little or no effect (Figure 9).
TNFR55 mediates the negative effect of TNF on CD34+CD38− candidate stem cells.
CD34+CD38− BM (3 experiments) or CB (1 experiment) cells were expanded in SF medium supplemented with KFT3 in the absence or presence of TNF (20 ng/mL), or mutants binding specifically to TNFR55 (200 ng/mL) or TNFR75 (500 ng/mL). After 5 to 7 days the EEs of 50 CD34+CD38− cells of each group were seeded into LTC-IC cultures, as described in “Materials and methods.” Results show the mean number of LTC-CFC (SEM) of cells expanded in the presence of TNF, or the mutants relative to the KFT3-expanded group (control). For the control group the mean number of CFCs generated in the 4 experiments varied from 106 to 350.
TNFR55 mediates the negative effect of TNF on CD34+CD38− candidate stem cells.
CD34+CD38− BM (3 experiments) or CB (1 experiment) cells were expanded in SF medium supplemented with KFT3 in the absence or presence of TNF (20 ng/mL), or mutants binding specifically to TNFR55 (200 ng/mL) or TNFR75 (500 ng/mL). After 5 to 7 days the EEs of 50 CD34+CD38− cells of each group were seeded into LTC-IC cultures, as described in “Materials and methods.” Results show the mean number of LTC-CFC (SEM) of cells expanded in the presence of TNF, or the mutants relative to the KFT3-expanded group (control). For the control group the mean number of CFCs generated in the 4 experiments varied from 106 to 350.
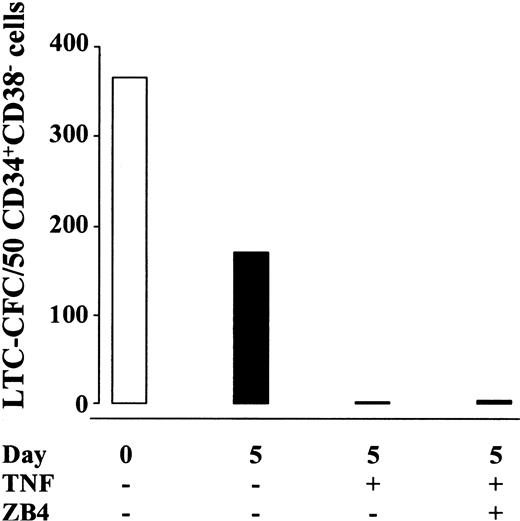
Because TNF has been demonstrated to up-regulate Fas expression on hematopoietic progenitor cells,47 48 it has thus been speculated that the negative effect of TNF on hematopoiesis might be mediated through the Fas pathway. Thus, TNF-containing cultures of CD34+CD38− CB and BM cells were supplemented with blocking anti-Fas antibodies (ZB4) capable of completely blocking the Fas receptors (as described in “Materials and methods”; Figure1). Noteworthy, the dramatic reduction in LTC-CFC activity of CD34+CD38− cells treated with TNF was not reduced by the presence of ZB4 (present both during ex vivo expansion and the long-term culture; Figure 10), supporting that the negative effect of TNF on HSC maintenance occurs independently of the Fas pathway.
The negative effect of TNF on self-renewal of candidate human HSCs is not dependent on the Fas pathway.
CD34+CB38− CB (one experiment) or BM (2 experiments) cells were cultured for 5 days in SF medium supplemented with KFT3 in the absence or presence of TNF (20 ng/mL) with or without ZB4 (1 μg/mL). Data show the mean number of CFCs generated (total of 8-10 replicates in 3 experiments) from 50 freshly isolated (open bar) or the EEs of 50 cultured (black bars) cells. ZB4 was also added to the long-term culture by the weekly medium changes to obtain the indicated concentration throughout the 6 weeks of coculture. The overall CFC values of cells cultured in the absence of TNF varied from 8 to 345 (5 of 8 replicates > 100). For cells cultured in the presence of TNF with or without ZB4, LTC-CFC varied from 0 (14 replicates) to 30 (1 replicate). ZB4 added to long-term cultures of fresh cells seeded on day 0 did not affect CFC formation (I.D. and S.E.W.J., unpublished observation, 2000).
The negative effect of TNF on self-renewal of candidate human HSCs is not dependent on the Fas pathway.
CD34+CB38− CB (one experiment) or BM (2 experiments) cells were cultured for 5 days in SF medium supplemented with KFT3 in the absence or presence of TNF (20 ng/mL) with or without ZB4 (1 μg/mL). Data show the mean number of CFCs generated (total of 8-10 replicates in 3 experiments) from 50 freshly isolated (open bar) or the EEs of 50 cultured (black bars) cells. ZB4 was also added to the long-term culture by the weekly medium changes to obtain the indicated concentration throughout the 6 weeks of coculture. The overall CFC values of cells cultured in the absence of TNF varied from 8 to 345 (5 of 8 replicates > 100). For cells cultured in the presence of TNF with or without ZB4, LTC-CFC varied from 0 (14 replicates) to 30 (1 replicate). ZB4 added to long-term cultures of fresh cells seeded on day 0 did not affect CFC formation (I.D. and S.E.W.J., unpublished observation, 2000).
Effects of TNF on apoptosis, cell-cycle distribution, and differentiation of hematopoietic progenitor cells
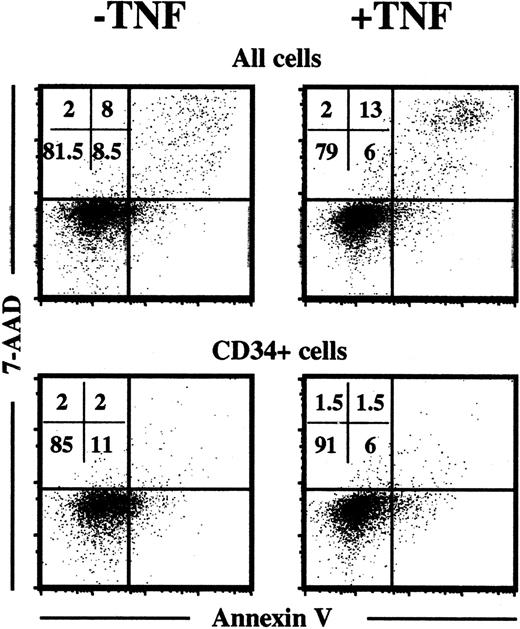
To address the potential mechanism, by which TNF might negatively affect HSC maintenance, we first examined whether TNF enhanced apoptosis of cultured progenitor cells, because the TNFR55 contains the death domain involved in apoptosis.49 Toward this aim CD34+ CB cells were cultured in KFT3 in the absence or presence of TNF (20 ng/mL) for 6 days and subsequently stained with annexin V-PE and 7-AAD, as previously described.40 Using this method “early apoptotic” cells appear as annexin V positive and 7-AAD negative, whereas “late apoptotic” cells appear as positive for both annexin V and 7-AAD. As expected,1 25 a significant fraction of cultured cells were apoptotic, but no difference was observed between cells cultured in the absence or presence of TNF (Figure11). Also, when only remaining CD34+ cells were investigated, no enhanced apoptosis was observed in response to TNF.
TNF does not enhance apoptosis of ex vivo expanded progenitors.
CD34+CB cells cultured for 6 days in KFT3 in the absence or presence of TNF (20 ng/mL) were stained with anti-CD34–APC, annexin V–PE, and 7-AAD, as described in “Materials and methods.” Annexin V and 7-AAD staining was investigated on the total cell population and on CD34+-gated cells. KFT3-treated cells expanded 55-fold, whereas KFT3 + TNF-cultured cells expanded 35-fold. One representative experiment of 2 is presented.
TNF does not enhance apoptosis of ex vivo expanded progenitors.
CD34+CB cells cultured for 6 days in KFT3 in the absence or presence of TNF (20 ng/mL) were stained with anti-CD34–APC, annexin V–PE, and 7-AAD, as described in “Materials and methods.” Annexin V and 7-AAD staining was investigated on the total cell population and on CD34+-gated cells. KFT3-treated cells expanded 55-fold, whereas KFT3 + TNF-cultured cells expanded 35-fold. One representative experiment of 2 is presented.
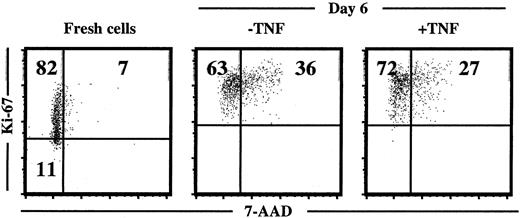
Next, the cell-cycle status of cultured progenitors was investigated with a combined 7-AAD and Ki67 staining, as previously described.41 42 In agreement with the efficient induction of proliferation, CD34+ cells cultured in KFT3 for 6 to 7 days were all in G1 or in cycle as illustrated through Ki67 expression in all cells. However, TNF did not affect the cell-cycle distribution of cultured cells (Figure12).
TNF does not affect cell-cycle distribution of ex vivo–expanded CD34+ CB cells.
Cell-cycle status of freshly isolated and cultured (in KFT3 in the absence or presence of TNF for 6 days) CD34+ CB cells stained with anti–Ki-67 and 7-AAD (as described in “Materials and methods”). KFT3 cells expanded 58-fold in the absence of TNF and 44-fold in the presence of TNF. One representative experiment of 2 is shown.
TNF does not affect cell-cycle distribution of ex vivo–expanded CD34+ CB cells.
Cell-cycle status of freshly isolated and cultured (in KFT3 in the absence or presence of TNF for 6 days) CD34+ CB cells stained with anti–Ki-67 and 7-AAD (as described in “Materials and methods”). KFT3 cells expanded 58-fold in the absence of TNF and 44-fold in the presence of TNF. One representative experiment of 2 is shown.
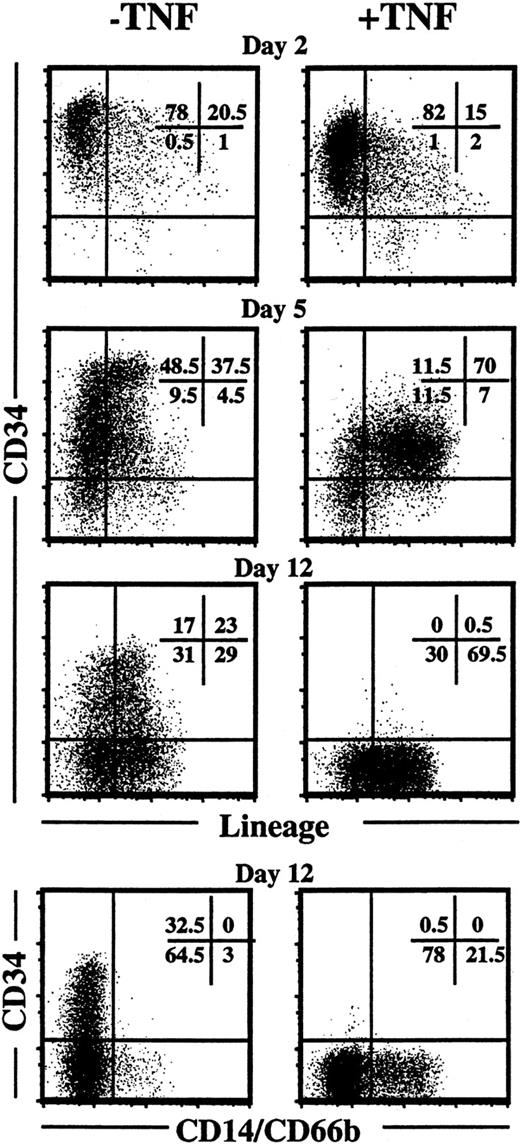
Finally, the potential effect of TNF on progenitor cell differentiation was investigated. Following 2 days of incubation, TNF had little or no effect on CD34 or lineage expression of KFT3-cultured cells, whereas following 5 days of incubation in KFT3, TNF down-regulated CD34 expression, which was accompanied by enhanced lineage differentiation (Figure 13). Strikingly, following 12 days of culture in the presence of KFT3 and TNF no CD34+cells remained, and enhanced myeloid differentiation was observed (Figure 13). In contrast, a high percentage of cells cultured in KFT3 in the absence of TNF remained CD34+Lin−.
TNF down-regulates expression of CD34 and promotes differentiation of ex vivo expanded CD34+ CB cells.
The upper 3 sets of panels show CD34 and lineage (CD3, CD14, CD15, CD19, CD20, CD56, CD66b, glycophorin A) expression on CD34+CB cells (purity 99%) cultured in KFT3 in the absence or presence of TNF (20 ng/mL) for 2, 5, and 12 days. The lower panels show staining with anti-CD34 and 2 myeloid-specific markers (CD14 and 66b) following 12 days of incubation. For each time point one representative experiment of a minimum of 2 experiments is shown.
TNF down-regulates expression of CD34 and promotes differentiation of ex vivo expanded CD34+ CB cells.
The upper 3 sets of panels show CD34 and lineage (CD3, CD14, CD15, CD19, CD20, CD56, CD66b, glycophorin A) expression on CD34+CB cells (purity 99%) cultured in KFT3 in the absence or presence of TNF (20 ng/mL) for 2, 5, and 12 days. The lower panels show staining with anti-CD34 and 2 myeloid-specific markers (CD14 and 66b) following 12 days of incubation. For each time point one representative experiment of a minimum of 2 experiments is shown.
Discussion
Tumor necrosis factor has been implicated to be involved in BM failure in aplastic anemia and graft-versus-host disease (GVHD).5-7 TNF has also been demonstrated to inhibit the in vitro growth of human hematopoietic progenitors, including primitive progenitors.10,13 However, whether targets for TNF-induced growth inhibition include HSCs, functionally defined by their ability to reconstitute myelopoiesis and lymphopoiesis, have not been investigated, but is of considerable interest, because HSCs are sufficient and required for long-term hematopoietic reconstitution.1
In the present studies we demonstrate that TNF activation of the TNFR55 negatively affects maintenance of in vitro and in vivo cycling human HSCs. Through recent studies we demonstrated that all murine and candidate human HSCs are induced to undergo proliferation in vitro in response to KL + FL + Tpo in the absence or presence of IL-3 under SF conditions.23,26 More importantly, such HSC divisions were associated with sustained HSC function. Thus, this represents a useful model for studying the in vitro regulation of HSC self-renewal promoted by cytokines demonstrated to also be crucial for in vivo HSC maintenance.2,3,18,19 The relevance of such a model is also supported by studies showing similar kinetics of HSC proliferation in vitro as in vivo following HSC transplantation.50 Using this system we here demonstrate through single cell experiments that all candidate CD34+CD38− HSCs undergo cell divisions also in the presence of TNF, and although the average number of cell divisions is reduced, the time to first cell division is not affected. This, combined with the dramatic loss in HSC function following culture in the presence of TNF (both 2 and 20 ng/mL), suggest that TNF negatively regulates HSC self-renewal. Noteworthy, a similar effect of TNF was observed in vivo on HSCs transplanted into NOD-SCID mice. That TNF can negatively affect maintenance of human LTC-IC is also supported by other recent studies.20 51 Using the NOD-SCID xenograft transplantation model, we extend this observation to show that TNF negatively affects in vivo reconstituting human HSCs with a combined myeloid and lymphoid potential. We also demonstrate that the negative effect of TNF is observed on both CB and adult BM HSCs.
The negative effect of TNF on HSCs appears to be primarily on cycling HSCs, because no effect of TNF was observed on SRCs when cells were cultured in Tpo alone, a condition demonstrated to promote survival rather than cycling of HSCs.45 Although we cannot exclude that the negative effect of TNF could be a consequence of compromised HSC engraftment or homing rather than an effect on self-renewal, it appears unlikely because in vitro LTC-ICs were compromised as much as in vivo SRCs. In addition, the negative effect of TNF on SRCs could not be reversed by neutralization of TNF and subsequent expansion, at least ruling out a reversible (cell-cycle–related) engraftment defect.
In studies such as the present one, HSCs will always represent a minority of the cells investigated. Thus, an HSC-specific effect on cell cycle or apoptosis of TNF in the present studies would have been difficult to detect. In addition, when comparing data from single cell and bulk experiments, the role of indirect effects could differ and affect the results. However, in the present studies, the negative effects of TNF observed in these 2 systems appeared comparable. Thus, although the mechanism(s) responsible for the negative effect of TNF on HSC maintenance remains to be established in more detail, the present studies provide some important clues. First, we found no evidence for enhanced apoptosis in the presence of TNF following 6 days of culture, at which time TNF had abrogated virtually all HSC reconstituting activity. This is noteworthy because suppressive effects of TNF are frequently associated with enhanced apoptosis, mediated through the death domain of TNFR55.49 However, unlike the Fas receptor, TNFR55 also signals through alternative nonapoptotic pathways.49 TNF also did not affect cell-cycle distribution of cultured progenitors, but rather facilitated differentiation, as illustrated through down-regulation of CD34 and acquisition of lineage expression. Thus, the present data provide support for the intriguing hypothesis that TNF might regulate HSC fate through promoting their differentiation rather than self-renewal.
Whether or not TNF plays a physiologic role in regulating the HSC pool remains unclear. Studies in TNFR55-deficient mice have revealed an increased number of phenotypically defined HSC,12 whereas other studies have suggested that such an increase does not necessarily translate into enhanced HSC function.52 In contrast there is ample evidence implicating involvement of TNF in the hematopoietic suppression observed in GVHD and bone marrow failure syndromes.5-7 The present findings make it possible that the targets for TNF-induced BM suppression might include HSCs, although further studies will be needed to address this.
We thank Dr Donna E. Hogge for kindly providing the murine fibroblast stroma cell lines and helpful advice regarding the use of these. We are in particular grateful to Dr Connie J. Eaves and Dr John E. Dick for helpful advice regarding the establishment and use of the LTC-IC and NOD-SCID assays. The expert assistance of Dr Lars Nilsson in the NOD-SCID assay, Gunilla Gärdebring, Lilian Wittman, and Ingbritt Åstrand-Grundström, as well as cell-sorting assistance by Carl-Magnus Högerkorp, Sverker Segrén, and Zhi Ma are highly appreciated. We also thank the staff and donors at the Department of Gynecology, Lund University Hospital and Helsingborg Hospital for help with providing CB, all volunteers for their BM contributions, and the physicians and other staff at the Department of Hematology, Lund University Hospital for performing the BM aspirations. The contributions by Dr Yutaka Sasaki, Dr Helga Björgvinsdóttir, and Dr Ewa Sitnicka through fruitful discussions and critical review of the manuscript are highly appreciated.
I.D. is supported through a fellowship from the Norwegian Cancer Society and the Faculty of Medicine, Norwegian University of Science and Technology. These studies were generously supported by grants from the Berta Kamrad Foundation; the Crafoord Foundation; the Georg Danielsson Foundation; the Gunnar, Arvid and Elisabeth Nilsson Foundation; the John and Augusta Persson Foundation; the O. and E. and Edla Johansson Foundation; the Thelma Zoega's Foundation; the Tobias Foundation; the Greta and Johan Kock's Foundations; the Royal Physiographic Society in Lund; ALF (Government Public Health Grant); Skånes Landsting; the Swedish Foundation for Strategic Research; the Swedish Cancer Society; Swedish Society of Pediatric Cancer and the Medical Faculty, University of Lund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sten Eirik W. Jacobsen, Department of Stem Cell Biology, Institute of Laboratory Medicine, University Hospital of Lund, Lund, Sweden; e-mail: sten.jacobsen@stemcell.lu.se.

![Fig. 1. Effects of agonistic and antagonistic anti-Fas Abs on Jurkat cells. / Jurkat cells (20 000) were cultured in 100 μL RPMI 1640 with 10% FCS in the absence or presence of 1 μg/mL CH11 (agonistic anti-Fas Ab) and/or ZB4 (blocking anti-Fas Ab) at different concentrations as indicated. Following 24 hours incubation, each well was pulsed for 24 hours with 1 μCi [3H]TdR before being harvested and evaluated for [3H]TdR uptake (cpm) as described in “Materials and methods.” Each group was performed in triplicate and error bars represent SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1782/7/m_h81811540001.jpeg?Expires=1767709507&Signature=xc3Hb0dBjQrL3zMPM9pgJ~iJG1QvvDdltx2HeDFGrxsrMnAKyuR9-qedaO-mof615M2BAKlqcUcXPOWE1T7-0-8FDxXs7pEmS~eQTNuaKBj2-z3qtn8r-5czBBkcmN2Th94oSgBnexIplpFHH6ZL5OWlpm7ssNbCI1y6sGNO-idpueurBGZBX1ZDRelFGBYVT1XRXjDwRAUf1xFb2pahFewDmG~VAtOVXIncqIxicFOwfxpvpsmDWa9vLwW7zE-NhlDdO8J3uuBlaGGk0vKjTTt0CHsfPeIVijHhYaKYXeXscqPGvfNRp8uShjMmXHXs3VPQt5cA-2Fe5LbkhFNV1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal