The choice of primary treatment for patients with chronic myeloid leukemia (CML) diagnosed in chronic phase has become exceedingly difficult. There is little doubt that allogeneic stem cell transplantation can eradicate the leukemia and that a graft-versus-leukemia effect makes a major contribution to this result; conversely, only a minority of patients are eligible for transplantation, which still carries an appreciable risk for death or protracted illness. For most patients, interferon-α (IFN-α) prolongs life to some degree in comparison with hydroxyurea, but it is associated with considerable toxicity. The newly introduced tyrosine kinase inhibitor STI571 induces complete hematologic remission in almost all patients and is associated with a very high rate of cytogenetic response; its capacity to prolong life in comparison with IFN-α is not yet established. Here are reviewed some factors that predict survival after nontransplantation therapy and after allografting for CML in chronic phase. Two contrasting options are considered for managing the patient with newly diagnosed disease, and it can be concluded that, for now, allogeneic stem cell transplantation soon after diagnosis should continue to be offered as an option for selected patients. Further experience with the use of STI571 as a single agent or in combination with other antileukemic agents may alter the picture in the near future.
Introduction
It is generally accepted that allogeneic stem cell transplantation (allo-SCT) has the potential to cure selected patients with chronic myeloid leukemia (CML) and that cure depends on the contribution of a poorly defined graft-versus-leukemia effect.1 However, there is a significant risk for illness or death as a direct consequence of the procedure. The introduction of interferon-α (IFN-α) in the 1980s complicated the decision about whether to recommend allo-SCT, and early clinical experience with the Abl signal transduction inhibitor STI571 has made the decision-making process yet more complex. Here we define the current problem in general terms and suggest some tentative recommendations. We recognize that these recommendations may be valid for only a limited period.
Predicting survival with nontransplantation therapy
The duration of survival after diagnosis is highly variable in a given cohort of patients with CML in chronic phase treated by nontransplantation methods; the reason for this heterogeneity is largely unknown. Studies at the molecular and cytogenetic levels may have some prognostic value. For example, it was thought at one time that the precise position of the genomic breakpoint in theBCR gene might correlate with duration of survival, but this notion has not been substantiated. Conversely, recent data suggest that the presence of genomic deletions in the vicinity of theABL-BCR gene on 9q+ may have prognostic significance.2 Moreover, the speed of telomere shortening in the leukemic clone may relate inversely to the duration of survival.3 These last 2 findings must be validated in larger clinical trials.
In the 1980s, Sokal et al4 devised a staging system based on clinical and hematologic criteria at diagnosis that correlated with duration of survival for subgroups of patients treated predominantly with busulfan. (For individual patients, the Sokal score can be calculated by accessing the Web site:http://www.nrhg.ncl.ac.uk/cgi-bin/cml/sokal.pl). More recently, Hasford et al5 introduced an analogous system for predicting survival of patients treated with IFN-α (go tohttp://www.pharmacoepi.de/cmlscore.html for an on-line calculator). Patients at low risk and treated with IFN-α had a median survival of 100 months, whereas patients at high risk had a median survival of 45 months. This low-risk group of patients, who may expect relatively long survival with IFN-α treatment, are particularly difficult to advise regarding treatment options.
In practice, one of the most effective ways of predicting survival is to assess the hematologic response to IFN-α at 6 months and the cytogenetic response at 1 year.6 The greatest survival advantage is seen in IFN-α–treated patients who achieve a major cytogenetic response (less than 35% Ph-positive metaphases), though the median time to optimal cytogenetic response may be 1 to 2 years. However, patients who do not achieve complete hematologic response at 6 months or who fail to achieve even a minor cytogenetic response (less than 65% Ph-positive) at 1 year are unlikely to obtain a major cytogenetic response. Thus, patients not achieving these landmarks could be considered candidates for alternative therapies.
Trial of therapy with interferon-α
It has been argued that patients at low risk could safely be treated with IFN-α for the first year, particularly given that the risk for blast transformation in these patients is 1% to 2%, even though they were otherwise eligible for allo-SCT. However, because patients who undergo transplantation within the first year of diagnosis have lower transplantation-related mortality rates than those who undergo transplantation at longer intervals,7 this strategy has been questioned in a decision-based analysis.8 In addition, there has been much speculation that prior treatment with IFN-α might adversely affect the result of a subsequent allograft procedure; in practice, a carefully performed multicenter study in Germany implied that patients whose IFN-α treatment was discontinued at least 90 days before allografting fare no worse than those who had never received IFN-α.9
Predicting survival after allogeneic stem cell transplantation
The range of possible outcomes for a patient undergoing allo-SCT varies widely. At one extreme, a patient may have an uneventful posttransplantation course and eventually be cured of CML. At the other extreme, a patient may die within weeks of transplantation because of acute graft-versus-host disease, opportunistic infection, or another complication. Although the probabilities of complications and death can be estimated for given patient cohorts, there is no reliable way of predicting outcome for an individual patient.
Gratwohl et al10 made use of the database maintained by the European Group for Blood and Marrow Transplantation (EBMT) to calculate a risk score that gives a probability of survival after allo-SCT. The risk score uses 5 specific pretransplantation features—phase of CML, duration of disease, patient age, degree of donor-recipient histocompatibility, and donor-recipient gender match. To this list may be added cytomegalovirus serostatus, which correlates with survival, at least if the donor is not a family member.11 12 Using the EBMT system, transplantation-related deaths for patients with total scores of 0 or 1 were approximately 20%, whereas mortality rates were as high as 70% for patients with scores of 5 or 6. The International Bone Marrow Transplant Registry is carrying out a similar analysis. This approach does not, of course, take into account the possible independent effects on transplantation-related mortality of procedure-related factors, such as details of conditioning, stem cell dose, or approach used to prevent graft-versus-host disease.
The notion that a graft-versus-leukemia effect plays a major role in leukemia eradication has led to the introduction of reduced-intensity conditioning or nonmyeloablative stem cell transplantation (NMSCT). In this procedure, the conditioning regimen is substantially reduced, and reliance is placed on the donor-derived lymphocytes for eliminating the patient's leukemia.13 14 Theoretically, this could greatly reduce the toxicity and mortality associated with the conventional procedure, allowing allografting to be offered to a wider population of CML patients. Data from different centers using a variety of reduced-intensity conditioning regimens show that some patients have achieved Ph-negativity. The rate of molecular negativity and the durability of these remissions must be determined from ongoing clinical trials before one can recommend that NMSCT, also referred to as minitransplants, should replace conventional allografting procedures for patients deemed eligible for transplantation.
STI571
STI571 is an Abl-specific tyrosine kinase inhibitor capable of inhibiting the proliferation of CML cell lines and clonogenic CML progenitor cells.15 It was first administered to patients with CML in the summer of 1998, and additional clinical trials accrued patients rapidly. The drug is given orally, is well tolerated, and has a manageable side-effect profile. Fifty-four patients with chronic-phase CML either resistant or refractory to IFN-α were treated with STI571 at a dose of 300 mg/day or greater. Almost all rapidly achieved complete hematologic responses—17 (31%) achieved major cytogenetic responses, and 7 (13%) achieved complete cytogenetic remission.16 Although the durability of these responses and the incidence of molecular remission cannot yet be assessed, an ongoing phase 2 study of 532 patients in chronic phase, for whom IFN-α therapy failed, was designed to address this issue. Thus the short-term results suggest that STI571 is a major advance in comparison with the use of IFN-α or IFN-α plus cytarabine. This notion is being tested in an international prospective study in which more than 1000 patients with newly diagnosed disease were randomized to receive either STI571 or IFN-α plus cytarabine. The definitive end-point will be survival.
Although hematologic and cytogenetic responses to IFN-α have prognostic value, one cannot automatically assume that the same relations will apply for STI571. In other words, will the patient who obtains good or complete cytogenetic response with STI571 survive as well (or better) than a patient who obtains a comparable response with IFN-α? Will such responses be equally well maintained? Patients treated with IFN-α rarely achieve complete molecular remission when a sensitive reverse transcription–polymerase chain reaction is used.17 Will STI571-treated patients do better in this regard? The ongoing randomized study comparing STI571 to IFN-α plus Ara-C is designed to address these questions, and these data are essential before we can say with certainty that a particular patient treated with STI571 is likely to gain substantial prolongation of life.
The picture is complicated further by the fact that patients treated with STI571 in combination with other agents may survive longer than those treated with STI571 alone. There are plans to launch a 3-arm multicenter study comparing STI571 with STI571 plus IFN-α and STI571 plus cytarabine in patients with newly diagnosed disease. The number of possible permutations using other drugs is enormous. Moreover, the results of allo-SCT could conceivably be improved by prior or subsequent use of STI571. Conversely, as was required with IFN-α, it remains to be determined whether prior treatment with STI571 negatively impacts survival after allo-SCT.
Patient preference
In most cases the hematologist will be asked for his or her recommendation on primary management, though the preference expressed by the informed patient must logically be the final deciding factor. For example, if the patient is determined to be cured of his or her disease, allo-SCT is the only approach clearly able to achieve this aim. Conversely, a patient for whom an allo-SCT seems advisable may deem the risks of the procedure unacceptable and may thus prefer treatment with IFN-α or STI571. These points notwithstanding, we suggest below a basis for treatment recommendations for individual patients.
Choice of primary therapy
There are 2 contrasting approaches to the treatment of patients with newly diagnosed CML.
Option 1
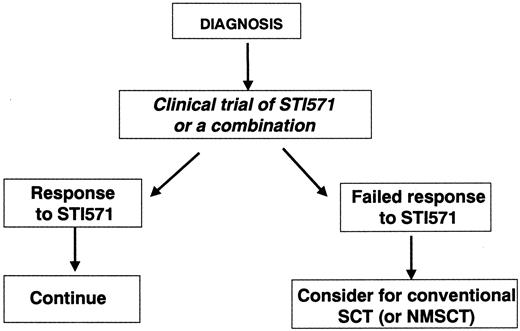
One approach is to recommend that every patient with newly diagnosed CML receive initial treatment with STI571, IFN-α, or a suitable combination, ideally in the context of a clinical trial (Figure 1). Patients in the appropriate age range for whom this trial of therapy fails and who have human leukocyte antigen (HLA)–identical siblings or HLA-matched alternative donors would then be offered allo-SCT. The problems with this approach relate in part to the difficulty in defining a meaningful response or failure to STI571 (or the combination) and in part to the risk that the inherent delay might permit time for the disease to progress. Although it now seems unlikely that prior treatment with STI571 would adversely influence the results of subsequent allograft, it is at least possible that a delay in transplantation might adversely affect the result independently of any particular prior therapy.
Schematic representation of option 1 by which all patients with newly diagnosed disease receive a “trial” of STI571.
Those who do not respond (as defined in the text) may proceed to undergo allo-SCT if they are young enough and have a suitable stem cell donor. NMSCT, nonmyeloablative stem cell transplantation.
Schematic representation of option 1 by which all patients with newly diagnosed disease receive a “trial” of STI571.
Those who do not respond (as defined in the text) may proceed to undergo allo-SCT if they are young enough and have a suitable stem cell donor. NMSCT, nonmyeloablative stem cell transplantation.
Option 2
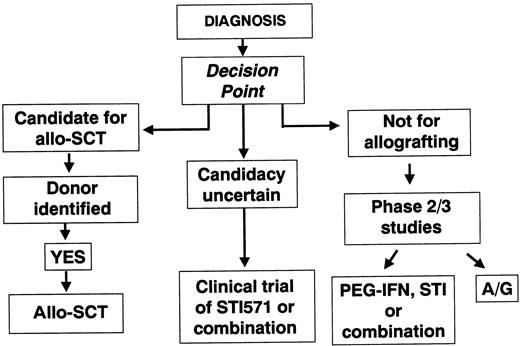
The second approach would be to try to decide within a few weeks of diagnosis whether a given patient is or is not a good candidate for allo-SCT (Figure 2). Patients newly diagnosed would likely fit into 1 of 3 categories: (1) Patients deemed eligible for transplantation (for this purpose, one must set an arbitrary level of risk for transplantation-related mortality above which early transplantation should not be recommended; one might possibly accept a risk of up to 15% or 20%); (2) Patients for whom transplantation is thought to carry a higher risk; and (3) Patients for whom transplantation could not reasonably be considered in any circumstance.
Schematic representation of option 2, by which some patients are offered treatment by allo-SCT soon after diagnosis if they are young and have a suitable donor.
Others are deemed ineligible for allo-SCT. The intermediate group is offered initial treatment with STI571 and may proceed to allo-SCT if the trial of STI571 is deemed to have failed. A/G indicates autografting.
Schematic representation of option 2, by which some patients are offered treatment by allo-SCT soon after diagnosis if they are young and have a suitable donor.
Others are deemed ineligible for allo-SCT. The intermediate group is offered initial treatment with STI571 and may proceed to allo-SCT if the trial of STI571 is deemed to have failed. A/G indicates autografting.
In this scheme, patients in category 1 would proceed to transplantation soon after diagnosis. Taking into account the various factors that impact on transplantation-related mortality, one might assume that a patient under the age of 40 with an HLA-identical sibling donor or a patient under the age of 30 years with a molecularly matched volunteer donor might be a good candidate for an early allograft. One could speculate that these upper age limits for transplantation might be reduced by 10 years for patients in the Hasford good-risk category. For patients in category 2, it would be reasonable to offer a trial of therapy with STI571 or a combination incorporating STI571 and then to assess the response after 6 or 12 months. Patients who did not achieve and maintain a reasonable degree of cytogenetic improvement would then be offered an allograft. Patients in category 3 might be offered primary treatment with STI571 or a combination of STI571 with IFN-α or cytarabine with STI571 or a combination of STI571 with IFN-α or cytarabine or an autograft procedure.
At the time of writing, we believe the best advice for the patient with newly diagnosed CML is option 2. Relatively young patients with newly diagnosed CML who have HLA-identical sibling donors or molecularly HLA-matched unrelated donors should undergo conventional transplantation within the first year of diagnosis. The role of NMSCT cannot yet be reliably assessed.
Although at present the curative potential of STI571 is unknown, it is entirely possible that STI571 alone, in combination with other agents, or in conjunction with a novel approach to immunotherapy could eradicate CML. Because clinical trials that test the various combinations will soon be initiated, patients not wanting to undergo allo-SCT should be encouraged to enroll in one or other of these studies. If 1 or 2 years from now it becomes clear that few, if any, of the patients responding to STI571 progressed to advanced disease and that the cytogenetic responses achieved are durable, then option 1 involving initial treatment with STI571 (or an STI571-containing combination) will become the treatment of choice. This view will gain additional support if some of the patients who achieve complete cytogenetic responses also achieve durable molecular remissions.
@ 2001 by The American Society of Hematology.
References
Author notes
John M. Goldman, Department of Haematology, Hammersmith Hospital, Imperial College School of Medicine, Ducane Rd, London W12 0NN, United Kingdom; e-mail: jgoldman@ic.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal