Because interleukin-18 (IL-18) is similar to IL-1 and is known to be involved in the hematopoietic progenitor cell growth, the effect of IL-18 on circulating cell populations was examined. Repeated administration of IL-18 induced significant amounts of neutrophilia in mice. In parallel, high levels of interferon-γ (IFN-γ), IL-6, and granulocyte-macrophage colony-stimulating factor (GM-CSF) were detected in the serum of these mice. Interestingly, the cytokine profiles as well as the cell populations in circulation altered around 2 weeks after the beginning of IL-18 administration. A weak but definite eosinophilia was observed concurrently with the appearance of serum IL-5. Consistent with these observations, IL-18 induced secretion of IFN-γ, GM-CSF, and IL-6 from splenocytes in culture. IL-18 also induced low levels of IL-5 in the splenocyte culture, which was inhibited by IL-12. However, markedly high levels of IL-5 were secreted into the culture medium when splenocytes from IFN-γ–deficient mice were stimulated by IL-18. CD4+ T cells strongly responded to IL-18 to secrete IL-5 and GM-CSF. IL-18 stimulated secretion of IL-6 and expression of G-CSF mRNA in splenic adherent cells. Expression of IL-18 receptors was detected in CD4+ T cells and splenic adherent cells (macrophages). These results show that IL-18 stimulates CD4+ T cells and macrophages to secrete IL-5, GM-CSF, IL-6, and granulocyte–colony stimulating factor in the absence of IL-12, which in turn induces hematopoietic cell proliferation causing neutrophilia and eosinophilia in mice.
Introduction
Dramatic changes in hematologic profiles can occur during inflammatory responses induced by various kinds of insults. Leukocyte profiles in the blood are often altered in conjunction with physiologic changes. Various mediators, cytokines, and growth factors, are involved in these changes. Interleukin-1 (IL-1) has been shown to be responsible for neutrophilia as well as fever. IL-18, originally discovered as a gamma interferon–inducing factor,1 shares many features with IL-1, including the molecular structure, processing, and signaling pathway through NF-κB. Both IL-1 and IL-18 are synthesized as inactive precursor forms which are processed by IL-1β converting enzyme to active forms.2,3 IL-18 exerts its biologic activities through a specific receptor consisting of 2 molecules, IL-1Rrp and AcP-like protein, which are members of the IL-1R family.4,5 IL-18, like IL-1, has been demonstrated to have a wide range of pathophysiologic roles in infectious diseases,6,7asthma,8,9 graft-versus-host disease (GVHD),10 and autoimmune diseases.11 This cytokine also has been shown to be detected locally or in circulation in such diseases as Crohn disease, tuberculosis, Sjogren syndrome, and lepromatous leprosy.12-15 However, biologic activities of IL-18 and IL-1 are different, probably due to different distributions of their receptors. IL-18 receptors (IL-18R) are expressed in type 1 helper T cells, whereas IL-1R are not. IL-18 plays a crucial role in host defense against infection through interferon-γ (IFN-γ) induction, whereas it is suggested to be responsible for tissue injury when operated with IL-12. IL-18 has also been reported to induce proinflammatory mediators, such as tumor necrosis factor-α (TNF-α),16 IFN-γ, and nitric oxide,17 and to augment Fas/Fas ligand (FasL)–mediated cytotoxicity by inducing FasL expression on T cells.18,19 IL-18 receptors are expressed constitutively in natural killer cells,20macrophages, and/or its lineage,21 but partly in T cells.22 In addition, expression of IL-18 receptors has been reported to be enhanced by costimulation with IL-12 in cloned T cells.23 IL-1 is known to induce secretion of several hematopoietic growth factors such as granulocyte–colony stimulating factor (G-CSF), macrophage–colony stimulating factor (M-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-6, which contribute to proliferation of hematopoietic progenitor cells.24 Recently, IL-18 has been reported to induce GM-CSF production in osteoblastic cells and IL-6 production in peripheral blood mononuclear cells,25 which implies that IL-18, like IL-1, may be involved in leukocyte recruitment, differentiation, and proliferation. Thus we are prompted to examine the effect of IL-18 on proliferation of peripheral blood cells.
Materials and methods
Animals
Female BALB/c mice were purchased from Japan SLC (Shizuoka, Japan). IFN-γ–deficient mice (BALB/c background) were kindly provided by Dr Iwakura (Institute for Medical Science, Tokyo University, Tokyo, Japan).
Preparation of CD4+ T, CD8+ T, and B cells by magnetic cell separation
Spleen cells were enriched for CD4+ and CD8+ T cells and B cells, using magnetic cell separations (MACs) according to the manufacturer's protocol (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Splenocytes were incubated on ice for 30 minutes with microbeads conjugated with anti-CD4 (L3T4) or anti-CD8 (53-6.7) antibodies. Cells were washed 3 times by MACs buffer composed of phosphate buffered saline (PBS) containing 1% fetal calf serum (FCS) and 5 mM ethylenediaminetetraacetic acid (EDTA), and applied onto the column (type VS+) in magnetic fields (VarioMACs, Miltenyi Biotec GmbH), washed with excess volumes of the buffer under magnetic fields, and eluted with the buffer without magnetic fields. For preparation of B cells, the splenocytes were treated with biotinylated antimouse CD43 antibody (S7, BD-Pharmingen, San Diego, CA) for 30 minutes on ice, and incubated with streptoavidin microbeads for 30 minutes on ice. The suspension of cells and beads was applied to the depletion column (type CS) under magnetic fields, and the noncaptured cells were gathered as B-cell fraction.
Cell cultures
Cells were suspended in RPMI 1640 medium supplemented with 10% FCS, 10 mM glutamine and 2 × 10-5M 2-mercaptoethanol, and plated on 6-well or 24-well culture dishes at a cell density of 2 × 107 or 5 × 106/mL, and cultured for appropriate lengths of time.
Analysis of cells by FACs
Cells were pretreated with the antibody-blocking Fc receptor, CD16/32 (BD-Pharmingen) to inhibit nonspecific antibody binding, and were incubated with a combination of fluorescein isothiocyanate (FITC)–conjugated antimouse CD4 (L3T4; BD-Pharmingen), phycoerythrin (PE)–conjugated antimouse CD45R/B220 (RA3-6B2; BD-Pharmingen) and CyChrome-conjugated antimouse CD8 (Ly-2; BD-Pharmingen) antibodies for 30 minutes at 4°C. Cells were washed 3 times with staining buffer composed of PBS supplemented with 2% FCS and 0.05% NaN3 and analyzed by FACs Caliber (Becton Dickinson).
Analysis of mRNA by reverse transcriptase-polymerase chain reaction
RNA was extracted from cell pellets using Isogen extraction buffer according to the manufacturer's manuals (Nippon Gene, Tokyo, Japan). Precipitated RNA was dissolved in RNase-free water and the concentrations were spectrophotometrically determined. DNA was synthesized using random hexamer primers and amplified by GeneAmp PCR system 9700 (PerkinElmer, Foster City, CA). Sequences of primers used were as follows: 5′-CGTGACAAGCAGAGATGTTG-3′ for murine IL-18Rα sense, 5′-ATGTTGTCGTCTCCTTCCTG-3′ for murine IL-18Rα antisense, 5′-ATGCTCTGTTTGGGCTGGGT-3′ for murine IL-18Rβ sense, 5′-CTGTCTTGATACAACAGGCCA-3′ for murine IL-18Rβ antisense, 5′-ATGGCTCAACTTTCTGCCCA-3′ for murine G-CSF sense and 5′-AATACCCGATAGAGCCTGCA-3′ for murine G-CSF antisense, 5′-CTGCAGGAGTGTCCATCACGGTGAAAGA-3′ for murine Fc-γ receptor I sense, 5′-GGATGTGAAACCAGACAGGAGCTGATGA-3′ for murine Fc-γ receptor I antisense and 5′-CCATCACCATCTTCCAGGAGCGAG-3′ for murine glyceraldehyde phosphate dehydrogenase (GAPDH) sense, 5′-CACAGTCTTCTGGGTGGCAGTGAT-3′ for murine GAPDH antisense. Polymerase chain reaction (PCR) amplification was performed using 35 cycles of primer extension at 72°C for 1 minute, annealing at 58°C for 30 seconds and denaturating at 94°C for 30 seconds. Products were visualized by 1.5% agarose-gel electrophoresis followed by ethidium bromide staining.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) systems for IL-3, IL-5, IL-6, GM-CSF, and IFN-γ were purchased from BD-Pharmingen. Other reagents necessary for ELISA were purchased from Sigma Chemical (St Louis, MO). The assays were carried out according to the manufacturer's manuals, using substrate solution of 3,3′,5,5′-tetramethylbenzidine (TMB) (Sigma T-8540; Sigma Chemical). OD values at 450 nm were measured by a microplate reader system (Bio-rad, Hercules, CA), and the concentrations were calculated using the computer program MPM III (Bio-rad).
Treatment of mice with IL-18 and measurement of blood cell counts
Recombinant mouse IL-18 (2 μg, 0.6 μg, and 0.2 μg in 0.2 mL PBS) or PBS was injected into 16 mice subcutaneously daily for 5 weeks. On every seventh day, blood was collected by tail cut. Blood samples (5 μL) were treated with Turk solution at 4°C to eliminate red blood cells, and the cell suspensions were plated on a slide glass by CytoSpin and stained with Diff-Quick (Midori, Osaka, Japan). White blood cells were counted under microscope.
Measurement of serum cytokine levels before and after treatment with IL-18
Forty-eight mice were treated with IL-18 or PBS as described above, killed on every seventh day, and the blood samples were collected for the determination of serum cytokines using ELISA systems.
Results
Induction of neutrophilia and eosinophilia by repetitive administration of IL-18 in mice
Daily subcutaneous administration of IL-18 at a dose of 2 μg/mouse significantly increased neutrophil counts in circulation from 2 weeks after the beginning of administration (Figure1A). Elevated neutrophil counts were maintained while IL-18 was administered, but counts reversed to the basal level within 1 week after withdrawal of administration of IL-18. The eosinophil number in circulation also increased during 3 to 5 weeks of IL-18 treatment (Figure 1B). This elevation was also reversed to the basal level within 1 week of withdrawal of IL-18. Total white blood cell counts did not change significantly during cytokine treatment (Figure 1C). Levels of lymphocytes were significantly reduced during IL-18 treatment, but were returned to the basal level within 1 week of withdrawal of IL-18 (Figure 1D).
Time course of the changes in blood cell counts induced by IL-18.
IL-18 (2 μg/mouse) was injected into 5 BALB/c mice subcutaneously once a day for 5 weeks. Five control mice were injected with PBS. Blood cells were counted after Diff-Quick (Midori) staining. Open circles show the control group and closed circles show the IL-18–treated group. Black bars inserted in each figure show the period of IL-18 treatment. Dotted lines show the changes of cell counts after the withdrawal of IL-18 treatment. Bars represent SE of 5 mice. *P < .05 and ** P < .01, compared with the control of the corresponding time point (Dunnett test for multiple comparison).
Time course of the changes in blood cell counts induced by IL-18.
IL-18 (2 μg/mouse) was injected into 5 BALB/c mice subcutaneously once a day for 5 weeks. Five control mice were injected with PBS. Blood cells were counted after Diff-Quick (Midori) staining. Open circles show the control group and closed circles show the IL-18–treated group. Black bars inserted in each figure show the period of IL-18 treatment. Dotted lines show the changes of cell counts after the withdrawal of IL-18 treatment. Bars represent SE of 5 mice. *P < .05 and ** P < .01, compared with the control of the corresponding time point (Dunnett test for multiple comparison).
Administration of lower concentrations of IL-18 (0.2 μg/mouse and 0.6 μg/mouse) caused only small increases in the number of neutrophils and eosinophils (Table 1). At 2 μg/mouse, significant increases in the number of these cells were observed. The average serum concentrations of IL-18 during administration were 201.61 ± 14.84, 79.72 ± 3.95, and 22.47 ± 3.10 ng/mL for mice that received 2 μg, 0.6 μg, and 0.2 μg of IL-18, respectively (Table 1).
Dose response of IL-18 on hematologic effect
| Treatment . | Serum IL-18 (ng/mL) . | WBC (counts/μL) . | Lymphocyte (%) . | Neutrophil (%) . | Eosinophil (%) . |
|---|---|---|---|---|---|
| Phosphate buffered saline | Not detected | 2856 ± 179.04 | 85.10 ± 2.21 | 10.90 ± 1.66 | 0.70 ± 0.25 |
| (n = 5) | (n = 5) | (n = 5) | (n = 5) | ||
| IL-18 (2.0 μg/mouse/day) | 201.61 ± 14.84* | 2260 ± 156.46† | 66.90 ± 2.59‡ | 26.70 ± 2.381-153 | 3.60 ± 0.681-153 |
| (n = 3) | (n = 5) | (n = 5) | (n = 5) | (n = 5) | |
| IL-18 (0.6 μg/mouse/day) | 79.72 ± 3.95* | 2229 ± 86.70NS | 78.67 ± 1.74NS | 16.50 ± 3.00NS | 1.33 ± 0.17NS |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | (n = 3) | |
| IL-18 (0.2 μg/mouse/day) | 22.47 ± 3.10 | 2240 ± 105.03NS | 83.00 ± 1.73NS | 12.92 ± 2.08NS | 1.00 ± 0.29NS |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | (n = 3) |
| Treatment . | Serum IL-18 (ng/mL) . | WBC (counts/μL) . | Lymphocyte (%) . | Neutrophil (%) . | Eosinophil (%) . |
|---|---|---|---|---|---|
| Phosphate buffered saline | Not detected | 2856 ± 179.04 | 85.10 ± 2.21 | 10.90 ± 1.66 | 0.70 ± 0.25 |
| (n = 5) | (n = 5) | (n = 5) | (n = 5) | ||
| IL-18 (2.0 μg/mouse/day) | 201.61 ± 14.84* | 2260 ± 156.46† | 66.90 ± 2.59‡ | 26.70 ± 2.381-153 | 3.60 ± 0.681-153 |
| (n = 3) | (n = 5) | (n = 5) | (n = 5) | (n = 5) | |
| IL-18 (0.6 μg/mouse/day) | 79.72 ± 3.95* | 2229 ± 86.70NS | 78.67 ± 1.74NS | 16.50 ± 3.00NS | 1.33 ± 0.17NS |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | (n = 3) | |
| IL-18 (0.2 μg/mouse/day) | 22.47 ± 3.10 | 2240 ± 105.03NS | 83.00 ± 1.73NS | 12.92 ± 2.08NS | 1.00 ± 0.29NS |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | (n = 3) |
The numbers of white blood cells (WBC), lymphocytes, neutrophils, and eosinophils are shown as a mean ± SE of 5 or 3 mice immediately after 5 weeks of treatment with respective doses of IL-18. Serum IL-18 concentrations are shown as a mean ± SE of 3 mice immediately after 5 weeks of IL-18 treatment.
NS indicates not significant.
P < .01, compared with IL-18 (0.2 μg/mouse/day) (Dunnett test for multiple comparison).
P < .05.
P < .001, compared with PBS.
P < .01, compared with PBS.
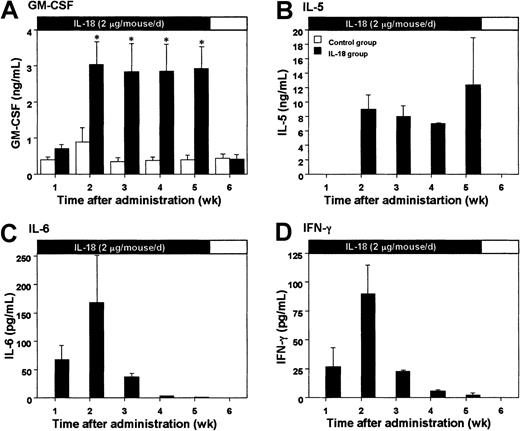
Elevation of serum cytokine levels in mice treated with IL-18
Based on the results shown in Table 1, we tested the effect of 2 μg/mouse IL-18 on serum cytokine levels in mice. Daily subcutaneous injection with IL-18 at this dose induced significant amounts of GM-CSF and IL-5 in sera 2 to 5 weeks after the first administration (Figure2A,B). Serum IL-6 levels were elevated 1 week after the IL-18 treatment, reached a peak at 2 weeks, and then rapidly decreased (Figure 2C). Similar transient elevation was observed with IFN-γ (Figure 2D). On the other hand, daily administration of IL-18 failed to induce IL-3 and IL-1β in the sera of mice (data not shown).
Serum cytokine levels in mice treated with IL-18.
IL-18 (2 μg/mouse) was injected into 18 BALB/c mice subcutaneously once a day for 5 weeks. Cytokines in the serum were analyzed by ELISA systems. Open and closed columns show control mice injected with PBS and IL-18–treated mice (3 mice each), respectively. Bars represent SE (n = 3). Closed and open bars on the top of each panel indicate the period of IL-18 treatment and withdrawal, respectively. *P < .05, compared with control of the corresponding time point (unpaired Student t test).
Serum cytokine levels in mice treated with IL-18.
IL-18 (2 μg/mouse) was injected into 18 BALB/c mice subcutaneously once a day for 5 weeks. Cytokines in the serum were analyzed by ELISA systems. Open and closed columns show control mice injected with PBS and IL-18–treated mice (3 mice each), respectively. Bars represent SE (n = 3). Closed and open bars on the top of each panel indicate the period of IL-18 treatment and withdrawal, respectively. *P < .05, compared with control of the corresponding time point (unpaired Student t test).
Effect of IL-18 on circulating blood cells in IFN-γ–deficient mice
Daily subcutaneous injection of IL-18 (2 μg/mouse) into IFN-γ–deficient mice caused significant increases in the number of neutrophils, although the major elevation occurred 3 weeks after the beginning of the IL-18 administration (Figure3A), as compared with 2 weeks with the healthy mice. The number of eosinophils started to increase at 1 week (Figure 3B), much earlier than observed with the healthy mice (3 weeks); remained high for an additional 4 weeks; and returned to the basal level within 1 week of withdrawal of IL-18 (Figure 3B). Total white blood cell count did not change significantly during cytokine treatment (Figure 3C). Levels of lymphocytes were significantly reduced 3 weeks after the beginning of administration of IL-18, but were returned to the basal level within 1 week of withdrawal of IL-18 (Figure 3D).
Time course of the changes in blood cell counts induced by IL-18 in IFN-γ–deficient mice.
IL-18 (2 μg/mouse) was injected into 4 IFN-γ–deficient mice subcutaneously once a day for 5 weeks. Four control mice were injected with PBS. Blood cells were counted after Diff-Quick (Midori) staining. Open circles show the control group and closed circles show the IL-18–treated group. Gray bars inserted in each figure show the period of IL-18 treatment. Dotted lines show the changes of cell counts after the withdrawal of IL-18 treatment. Bars represent SE of 4 mice. *P < .05 and ** P < .01, compared with the control of the corresponding time point (Dunnett test for multiple comparison).
Time course of the changes in blood cell counts induced by IL-18 in IFN-γ–deficient mice.
IL-18 (2 μg/mouse) was injected into 4 IFN-γ–deficient mice subcutaneously once a day for 5 weeks. Four control mice were injected with PBS. Blood cells were counted after Diff-Quick (Midori) staining. Open circles show the control group and closed circles show the IL-18–treated group. Gray bars inserted in each figure show the period of IL-18 treatment. Dotted lines show the changes of cell counts after the withdrawal of IL-18 treatment. Bars represent SE of 4 mice. *P < .05 and ** P < .01, compared with the control of the corresponding time point (Dunnett test for multiple comparison).
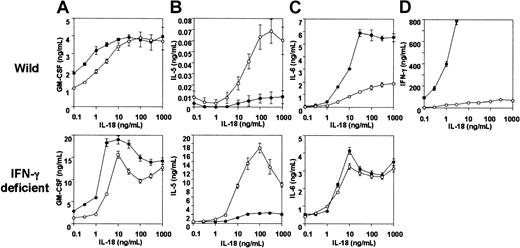
Induction of hematopoietic cytokine secretion by IL-18 in vitro
Treatment of splenocytes with IL-18 for 72 hours stimulated secretion of GM-CSF, IL-5, IL-6, and IFN-γ in a concentration-dependent manner (Figure4). IL-12 failed to stimulate secretion of these cytokines (data not shown), but it enhanced the effect of IL-18 to induce GM-CSF, IL-6 (Figure 4A,C ⊤) and, in particular, IFN-γ (Figure 4D ⊤). IL-5 secretion induced by IL-18, on the other hand, was almost completely repressed by IL-12 (Figure 4B ⊤). IL-18 induced negligible amounts of IL-3 both in the presence and absence of IL-12 (data not shown). We investigated the effect of IFN-γ on the cytokine secretion stimulated by IL-18 using the splenocytes isolated from IFN-γ–deficient mice (Figure 4). GM-CSF secretion was found to be enhanced by IL-18 (Figure 4A [bottom]). Enhanced IL-5 secretion induced by IL-18 and its inhibition by IL-12 were not affected by the IFN-γ deficiency (Figure 4B [bottom]). In contrast to wild-type splenocytes, however, IL-6 secretion induced by IL-18 was only slightly enhanced in IFN-γ–deficient splenocytes (Figure 4C [bottom]).
Effect of IL-18 on in vitro cytokine formation in wild and IFN-γ deficiency with or without IL-12.
Whole spleen cells isolated from wild and IFN-γ–deficient mice (5 × 106 cells each) were treated for 72 hours with various concentrations of IL-18 (0.1-1000 ng/mL). GM-CSF (A), IL-5 (B), IL-6 (C), and IFN-γ (D) contents in culture supernatant were measured by specific ELISA. Closed and open circles show cytokines secreted by cells incubated with IL-18 with and without IL-12, respectively. Bars represent SE of 4 experiments, using one mouse per experiment.
Effect of IL-18 on in vitro cytokine formation in wild and IFN-γ deficiency with or without IL-12.
Whole spleen cells isolated from wild and IFN-γ–deficient mice (5 × 106 cells each) were treated for 72 hours with various concentrations of IL-18 (0.1-1000 ng/mL). GM-CSF (A), IL-5 (B), IL-6 (C), and IFN-γ (D) contents in culture supernatant were measured by specific ELISA. Closed and open circles show cytokines secreted by cells incubated with IL-18 with and without IL-12, respectively. Bars represent SE of 4 experiments, using one mouse per experiment.
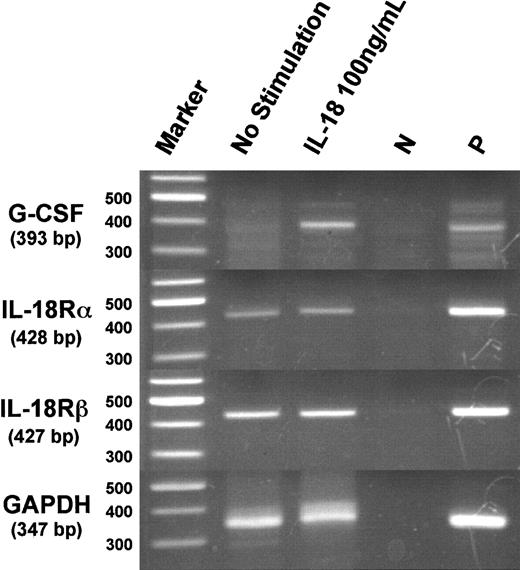
Expression of G-CSF mRNA in splenic adherent cells treated with IL-18
Because there are no ELISA kits available for detecting mouse G-CSF, we performed RT-PCR for murine G-CSF mRNA to determine the effect of IL-18 on G-CSF expression. As shown in Figure5, IL-18 was found to induce G-CSF mRNA expression in splenic adherent cells.
Expression of messenger RNAs of murine G-CSF and IL-18Rα and IL-18Rβ in splenic adherent cells following stimulation with IL-18.
Splenic adherent cells (2 × 107 cells) were treated with IL-18 (100 ng/mL) for 6 hours, and messenger RNAs of G-CSF, IL-18Rα, IL-18Rβ, and GAPDH were analyzed by RT-PCR. Messenger RNA of lipopolysaccharide (LPS)-stimulated peritoneal macrophages was used as a positive (P) control. For negative control (N), the above mRNA was subjected to PCR reaction without prior RT reaction.
Expression of messenger RNAs of murine G-CSF and IL-18Rα and IL-18Rβ in splenic adherent cells following stimulation with IL-18.
Splenic adherent cells (2 × 107 cells) were treated with IL-18 (100 ng/mL) for 6 hours, and messenger RNAs of G-CSF, IL-18Rα, IL-18Rβ, and GAPDH were analyzed by RT-PCR. Messenger RNA of lipopolysaccharide (LPS)-stimulated peritoneal macrophages was used as a positive (P) control. For negative control (N), the above mRNA was subjected to PCR reaction without prior RT reaction.
Identification of cells expressing IL-18 receptor mRNA
The expression of IL-18 receptors was analyzed in CD4+and CD8+ T cells and CD43− B cells prepared by MACs. Purity of CD4+ T cells, CD8+ T cells, and B cells, checked by the flow-cytometric analysis, were 96.8%, 96.6%, and 99.2%, respectively (Figure6A). Messenger RNAs for IL-18Rα and IL-18Rβ (AcPL) were detected in CD4+ and CD8+T cells and splenic adherent cells, but not in B cells (Figure 6B). This indicates that the expression of IL-18 receptors on these cells does not require stimulation by IL-18. Fcry receptor type I (Fc-γ RI) mRNA, the marker of NK cells and macrophages, was detected only in splenic adherent cells (Figure 6B).
RT-PCR analysis of messenger RNAs of murine IL-18Rα, IL-18Rβ, and Fc-γ RI in CD4+ and CD8+ T cells, CD43− B cells, and splenic adherent cells.
CD4+ and CD8+ T cells and CD43− B cells were isolated by magnetic cell sorter. (A) The purity of the cells was analyzed by flow cytometry after staining with cell-specific surface markers. (B) Messenger RNAs of IL-18Rα and β, Fc-γ RI, and GAPDH were analyzed by RT-PCR using specific primer sets. Fc-γ receptor I mRNA was analyzed to assess the contamination of NK cells and macrophages in T and B cells. Messenger RNA of LNK cell was used as a positive (P) control. For negative control (N), LNK mRNA was subjected to PCR reaction without prior RT reaction.
RT-PCR analysis of messenger RNAs of murine IL-18Rα, IL-18Rβ, and Fc-γ RI in CD4+ and CD8+ T cells, CD43− B cells, and splenic adherent cells.
CD4+ and CD8+ T cells and CD43− B cells were isolated by magnetic cell sorter. (A) The purity of the cells was analyzed by flow cytometry after staining with cell-specific surface markers. (B) Messenger RNAs of IL-18Rα and β, Fc-γ RI, and GAPDH were analyzed by RT-PCR using specific primer sets. Fc-γ receptor I mRNA was analyzed to assess the contamination of NK cells and macrophages in T and B cells. Messenger RNA of LNK cell was used as a positive (P) control. For negative control (N), LNK mRNA was subjected to PCR reaction without prior RT reaction.
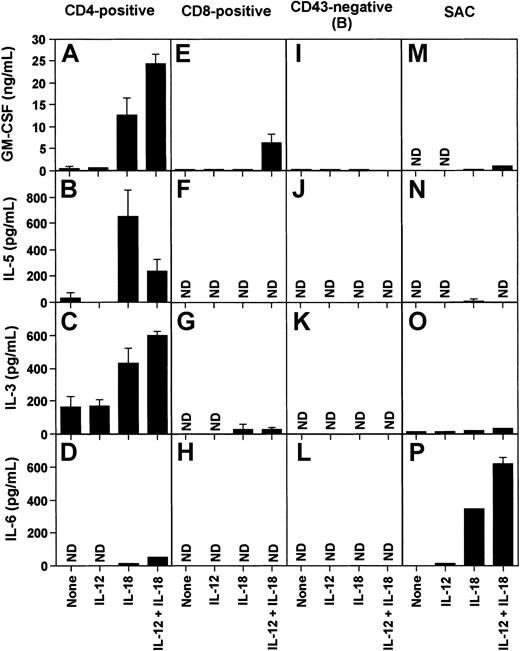
Identification of cells secreting cytokines in response to IL-18 in vitro
CD4+ and CD8+ T cells, B cells, and splenic adherent cells were cultured in the presence of IL-18, IL-12, or both for 72 hours, and several cytokines in culture supernatant were analyzed by ELISA systems (Figure 7). We found that CD4+ T cells stimulated by IL-18 secreted large amounts of GM-CSF, IL-5, and IL-3 (Figure 7A-C), whereas these cytokines were hardly detectable in the supernatant of CD8+T cells (Figure 7E-G). In CD4+ cells, IL-12 stimulated secretion of GM-CSF and IL-3 but inhibited IL-5 secretion (Figure 7A-C). IL-6 was secreted by splenic adherent cells incubated with IL-18, which was further enhanced in the presence of IL-12 (Figure 7P). Low levels of IL-6 secretion were also observed from CD4+ T cells stimulated by IL-18 and IL-12 (Figure 7D). No IL-6 was detected in the supernatant of CD8+ T cells stimulated by IL-18 and IL-12 (Figure 7H). B cells did not secrete any of the cytokines analyzed with or without treatment with IL-18 and IL-12 (Figure 7I-L).
Induction of cytokines by IL-12 and IL-18 in T and B cells and splenic adherent cells.
Whole spleen cells of BALB/c mice were separated into CD4+ and CD8+ T cells, and CD43− B cells by MACs. Cells (2 × 106cells) were treated with IL-12 (10 ng/mL), IL-18 (300 ng/mL), or both (IL-12, 10 ng/mL and IL-18, 100 ng/mL) for 72 hours, and indicated cytokines in the medium were measured by specific ELISA. Columns and bars express mean and SE of 5 experiments, respectively. None, not stimulated; ND, not detected.
Induction of cytokines by IL-12 and IL-18 in T and B cells and splenic adherent cells.
Whole spleen cells of BALB/c mice were separated into CD4+ and CD8+ T cells, and CD43− B cells by MACs. Cells (2 × 106cells) were treated with IL-12 (10 ng/mL), IL-18 (300 ng/mL), or both (IL-12, 10 ng/mL and IL-18, 100 ng/mL) for 72 hours, and indicated cytokines in the medium were measured by specific ELISA. Columns and bars express mean and SE of 5 experiments, respectively. None, not stimulated; ND, not detected.
Discussion
In the present study, we observed that continuous administration of IL-18 caused significant neutrophilia and lymphopenia with subsequent weak eosinophilia in mice (Figure 1). It also induced secretion of various cytokines into circulation, which changed parallel to the change in leukocyte populations (Figure 1 and Figure 2). In the first 2 weeks of IL-18 administration, when the number of neutrophils was elevated and that of lymphocytes was reduced, IFN-γ and IL-6 appeared in circulation (Figure 2C,D). Later, when the number of eosinophils was elevated, levels of IFN-γ and IL-6 were decreased, whereas IL-5 and GM-CSF levels remained high (Figure 2B). IL-18 was also found to induce all of these cytokines in spleen cells in vitro (Figure 3).
These observations are interesting because IL-18 has been shown to induce production of cytokines of both Th1 type (IFN-γ) and Th2 type (IL-4, IL-13),22 which have been shown to regulate the production of each other.26 IL-18, originally discovered as an IFN-γ–inducing factor, was expected to promote development of Th1 cells.1 However, it has recently been shown that IL-18 strongly induces Th1 development only in the presence of IL-1211,17 and, in the absence of IL-12, IL-18 strongly induces Th2 cytokines in various cells.8 Moreover, it has been suggested that IL-18 down-regulates the expression of IL-12.27 It is therefore possible that IL-18 may be involved in the mechanism that shifts Th1 responses to Th2 responses.
It has been observed that IL-18 is expressed in the lesions or secreted in the circulation of various diseases including autoimmune diseases, GVHD, purine nucleoside phosphorylase (PNP) deficiency, and lepromatous leprosy.10,11,15 28 However, the pathologic significance of these observations remains obscure because this cytokine seems to be involved in both destructive and compensatory pathways in inflammatory diseases. Clarification of pathophysiologic roles of IL-18 may be of value for understanding the cause and/or symptoms of various diseases.
In the present experiments, we demonstrated that IL-18 augmented the expression of hematopoietic cytokines and growth factors, such as IL-3, IL-5, IL-6, GM-CSF, and G-CSF (Figures 2, 4, and 5). All these cytokines except IL-6 have been suggested to be derived from CD4+ T cells or macrophages (Figure 7). Our in vitro experiments showed that splenic adherent cells but not CD4+and CD8+ cells secreted IL-6, suggesting that this cytokine was secreted mostly by macrophages (Figure 7). We found that IL-18 receptors were constitutively expressed on T cells (both CD4 and CD8) and splenic macrophages (Figure 5). The receptor for IL-18 is composed of inducible IL-18Rα and constitutive IL-18Rβ. IL-18 binds to its receptor and strongly activates the transcription factor NF-κB29 through a signal transduction pathway involving IRAK and TRAF6.30 The upstream regions of the genes encoding for GM-CSF,31 IL-3,32 and IL-533 contain the consensus sequence for NF-κB. It has also been demonstrated that G-CSF gene expression is regulated by NF-κB–like elements in murine macrophages.34 Moreover, expressions of IL-6 and G-CSF in synovial fibroblasts induced by thrombin requires activation of NF-κB.35 It is therefore likely that activation of NF-κB is involved in the induction of various cytokines by IL-18.
It is well known that G-CSF, GM-CSF, and IL-3 regulate the development and activation of neutrophils and promote their proliferation and survival.32,36 IL-6 also may play a role in hematopoiesis because it has been shown that Escherichia coli is unable to efficiently induce neutrophilia in IL-6–deficient mice, and antibodies to GM-CSF and IL-6 abrogate the augmentation of survival of neutrophils cultured in the supernatant of epithelial cells.37 38 In our experiments discussed here, daily administration of IL-18 resulted in induction of these cytokines in mice. It is therefore possible that the progressive neutrophilia caused by IL-18 may result from the induction of cytokines, such as IL-3, and growth factors, such as G-CSF and GM-CSF.
Lauwerys et al have shown that splenocytes stimulated by a combination of IL-12 and IL-18 produce IFN-γ, IL-3, IL-6, and TNF.39Ahn et al have reported marked synergistic effect of IL-12 and IL-18 on the induction of IFN-γ.23 In our study, the production of IL-6 and GM-CSF in splenocytes was enhanced by costimulation by IL-12 and IL-18 (Figure 4). In contrast, IL-5 production induced by IL-18 was strongly suppressed by IL-12 (Figure 4). This may be due to increased production of IFN-γ. Production of IFN-γ induced by IL-18 is strongly enhanced by IL-12, which may be responsible for the suppression of IL-5 by IL-12. This possibility was supported by the result that IL-18 stimulated IL-5 production in splenocytes from IFN-γ–deficient mice to a much greater extent than in splenocytes from the wild-type mice (Figure 4).
It is widely accepted that the hematopoietic growth factors, IL-3, IL-5, and GM-CSF, regulate the survival, maturation, and activation of eosinophils.40 It has also been demonstrated that spontaneous decreases in viability of rat peritoneal eosinophils are inhibited by recombinant IL-541 and that blockade of IL-5 results in inhibition of eosinophil accumulation in an experimental model for airway inflammation.42 Because there was a lag in the appearance of neutrophilia and eosinophilia induced by the daily administration of IL-18 which was coincident with the change in circulating cytokines from IFN-γ and IL-6 to IL-5, decreased production of IFN-γ may be responsible for IL-5 secretion and eosinophilia. However, the mechanism by which IFN-γ production was reduced after administration of IL-18 is yet to be clarified. There may be a regulatory mechanism for down-regulating IL-12 production by IL-18 so that Th1 response does not go too far.
Proliferation, differentiation, and maturation of hematopoietic cells are regulated by a variety of cytokines and growth factors, such as IL-3, IL-5, GM-CSF, G-CSF, stem cell factor, and IL-6. In this study, we demonstrated that IL-18 induced IL-5, IL-6, GM-CSF, and G-CSF. The recruitment and activation of polymorphonuclear leukocytes is the hallmark of acute inflammation. Especially, neutrophils are the most abundant cells in the blood and are essential for host defenses and for inflammatory responses. Eosinophils are present only in a trace number in the healthy blood, but increase in number in case of infection and allergy.
The primary use of colony-stimulating factors in patients receiving chemotherapy is to reduce the incidence of febrile neutropenia, leading to the reduction of use of antibiotics and hospitalization time.43-45 The fact that IL-18 increases circulating neutrophils may also suggest that it may have beneficial effects on the neutropenia during anticancer chemotherapy and agranulocytosis. On the other hand, IL-18 elevates the number of eosinophils mediating atopic dermatitis, asthma, and allergy, and thus acts as a worsening factor. In this case, IL-18 should be used as a target for neutralization in the strategy for the control of such diseases.
The authors thank Dr T. Tamaoki for important suggestions and critical reading of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Haruki Okamura, Laboratory of Host Defenses, Institute for Advanced Medical Sciences, Hyogo College of Medicine, 1-1 Mukogawa-cho, Nishinomiya, Hyogo, 663-8501, Japan; e-mail:haruoka@hyo-med.ac.jp.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal