Abstract
It was recently reported that transplantation of clonally derived murine neurosphere cells into sublethally irradiated allogeneic hosts leads to a donor-derived hematopoietic reconstitution. The confirmation of the existence of a common neurohematopoietic stem cell in the human brain will have a significant effect on stem cell research and on clinical transplantation. Here, it is demonstrated that the human fetal brain contains separate but overlapping epidermal growth factor (EGF)–responsive and basic fibroblast growth factor (FGF-2)–responsive neural stem cells. The majority (> 85%) of cells within these EGF- and/or FGF-2–generated neurospheres express characteristic neural stem/progenitor cell markers including nestin, EGF receptor, and FGF-2 receptor. These neural stem cells can be continuously passaged in vitro, and demonstrate a constant 20-fold expansion in every passage for up to the fifth passage (the longest period that has been carried out in the authors' laboratory). These neural stem cells are multipotential for neurons, astrocytes, and oligodendrocytes. After transplantation into SCID-hu mice, all neural stem cells, regardless of passages, culture conditions, and donors, are able to establish long-term hematopoietic reconstitution in the presence of an intact human bone marrow microenvironment.
Introduction
Hematopoietic stem cell transplantation (HSCT) has been shown to provide a definitive benefit for a variety of malignant and nonmalignant hematologic diseases and myelopoietic support for patients undergoing high-dose chemotherapy.1,2 Several inherent limitations associated with HSCT, however, have restricted its general use.3,4 These include (1) a lack of sufficient donors for all recipients, (2) a requirement of either operative bone marrow (BM) harvests or pheresis procedures to obtain sufficient stem cells necessary for benefit after transplantation, and (3) the potential for tumor contamination in autologous HSCT. A straightforward strategy to overcome these limitations is to develop culture systems for ex vivo expansion of transplantable hematopoietic stem cells (HSCs).5 Ex vivo–generated and –expanded HSCs could support multiple cycles of chemotherapy, provide transplantation options for patients without matched donors, facilitate transduction of vectors into HSCs for gene therapy, and provide a tumor-free product for transplantation. During the last 10 years, better ways to identify and purify HSCs and the availability of various cytokines have facilitated and improved the development of ex vivo stem cell expansion technology. Expansion of HSCs in vitro is still limited in extent and duration, and the expansion technology has not yet reached a stage where ex vivo–expanded hematopoietic progenitors and stem cells can be used routinely for replacement therapy.6-9
Another alternative strategy to overcome those limitations associated with HSCT is to identify and use stem cells from nonlymphoid tissues, which might be easier to maintain and expand in vitro and which possess hematopoietic potential such as embryonic stem cells (ESCs),10,11 neural stem cells (NSCs),12 and muscle stem cells.13,14 Mouse NSCs can easily be isolated and propagated in vitro for prolonged periods (approximately 1 year), resulting in a 107-fold increase in cell number, without losing their proliferative and multilineage potential.15-19 When the culture conditions are altered, the cells can differentiate into neurons, oligodendrocytes, and astrocytes.15-19 Surprisingly, the transplantation of clonally derived murine neurosphere culture cells into sublethally irradiated allogeneic hosts led to the late emergence and takeover of a donor-derived hematopoietic system.12 If the hematopoietic potential of cultured NSCs of human origin could be confirmed, human NSCs might truly represent an alternative source of stem cells for transplantation, thus overcoming the limitations associated with HSCT. Over the past decade, numerous studies have shown that the SCID-hu mouse model20,21 is a relevant and reliable animal model to read out long-term hematopoietic reconstitution activity derived from human hematopoietic stem cells.22-25 Because the SCID-hu mice carry transplanted human fetal bone and thymic fragments, they provide the most physiologically relevant human bone marrow and thymic microenvironments to determine if human stem cells from nonlymphoid tissues such as NSCs12 and muscle satellite cells13 14 possess in vivo hematopoietic potential. In this study, we have investigated whether human NSCs possess in vivo hematopoietic potential in SCID-hu mice. Our results demonstrate that human fetal brain tissues contain separate but overlapping epidermal growth factor (EGF)–responsive and basic fibroblast growth factor (FGF-2)–responsive NSCs. These NSCs, derived from human fetal brain tissues, express characteristic neural stem/progenitor cell markers including nestin and receptors for EGF (EGFR) and FGF-2 (FGFR1). These human NSCs can be maintained and expanded in vitro for many passages and still retain their self-proliferative and multilineage potential. Most importantly, our study demonstrates, for the first time, that cultured human NSCs do possess in vivo hematopoietic potential. Furthermore, this study is the first report to show that differentiation of cultured human NSCs into hematopoietic lineages depends on the presence of an intact human BM microenvironment. The identification of a common human neurohematopoietic stem cell and the cues in the BM microenvironment that direct the neurohematopoietic stem cell to differentiate into hematopoietic lineages will have a significant effect on stem cell research and on clinical transplantation.
Materials and methods
Primary-, secondary-, and higher-passaged neurosphere cultures
Human fetal brain and thymus tissues (from the same donors) were dissected from 17- to 24-week-old fetuses obtained by elective abortion with approved consent (Advanced Bioscience Resources, Alameda, CA). Cells from the thymus were stained with a panel of monoclonal antibodies (MoAbs) to human leukocyte antigens (HLAs) to establish the donor allotype. Although we had originally planned to dissect out the subventricular zone (SVZ) of the brain tissues for our experiments, it soon became evident that we were not able to do so because the brain tissues always arrived not as intact tissue but as many small fragments. To avoid the possibility of using different parts of the brain tissues in each of our experiments, 8 cerebral cortex fragments containing SVZ-like structures were randomly selected from each sample of incoming brain tissues. In this way, the chance of obtaining representative cells from the SVZ in each sample of human fetal brain tissues was maximized. These selected brain tissue fragments then were mechanically dissociated with a fire-polished Pasteur pipette in a 1:1 mixture of Dulbecco modified Eagle medium (DMEM) and F-12 nutrient (1:1, vol/vol; Life Technologies, Gaithersburg, MD). Viable cells were counted by trypan blue exclusion and plated as 5000 cells/200 μL per well in 96-well plates (Corning, Acton, MA) with no substrate pretreatment. The culture medium was composed of DMEM/F-12 (1:1) including Hepes buffer (5 mM), glucose (0.6%), sodium bicarbonate (3 mM), and glutamine (2 mM). A defined hormone and salt mixture composed of insulin (25 μg/mL), transferrin (100 μg/mL), progesterone (20 nM), putrescine (60 μM), and sodium selenite (30 nM) was added to the culture medium in place of serum. Human recombinant EGF and FGF-2 were purchased from R&D Systems (Minneapolis, MN). EGF and FGF-2 were added to the cultures as described in the experiments at 20 ng/mL. The number of primary spheres generated in each well in different culture conditions was assessed 7 days after plating. Some primary spheres were individually transferred to the differentiation cultures and processed for triple-antigen indirect immunocytochemistry (see below), and the remainder was used for establishing secondary cultures, mouse stromal cocultures and in vivo hematopoietic reconstitution in SCID-hu mice. Primary spheres grown in each culture condition were harvested by centrifugation at 400 rpm and resuspended in 5 mL of growth factor–free medium. The spheres were mechanically dissociated into single cells by trituration with a fire-polished Pasteur pipette, and an aliquot was counted to determine the total number of cells. For secondary cultures, 500 cells/200 μL per well were plated in each well in 96-well plates using the same culture conditions. The number of secondary spheres in each well in each culture condition was scored 7 days after plating. This procedure was repeated every 7 days 4 more times for up to the fifth passage.
Differentiation of individual primary-, secondary-, and higher-passaged neurospheres
Seven days after plating, individual spheres were removed from primary-, secondary-, and higher-passaged cultures with a pipette, placed into a 1.5- mL Eppendorf tube, and spun down at 400 rpm. Growth factor–containing medium was removed, and the spheres were individually resuspended in growth factor–free medium. Single isolated spheres were plated on poly-L-ornithine–coated (15 μg/mL) glass coverslips in individual wells of 24-well plates (1.0 mL/well) in DMEM/F-12 medium containing 1% fetal calf serum (FCS; Gemini Bio-Products, Calabasas, CA) and a hormone and salt mixture. Medium was not changed for the rest of the experiment. Coverslips were processed 7 to 8 days later for triple-antigen indirect immunocytochemistry.
Immunocytochemistry on undifferentiated and differentiated neurospheres
Indirect immunocytochemistry was carried out with individual spheres from various passages attached to glass coverslips, either immediately after plating (for nestin, EGFR, and FGFR1) or after 7 to 8 days in vitro differentiation (for triple labeling). Coverslips were fixed for 20 minutes with 4% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4, and rinsed 3 times with PBS. For immunocytochemistry with nestin, EGFR, and FGFR1, a rabbit polyclonal antiserum against nestin (1:1000; a gift from Dr R. McKay),26 27 and 2 rabbit polyclonal IgGs against EGFR (1:500) and FGFR1 (1:500) (Santa Cruz Biotechnology, Santa Cruz, CA) were diluted in PBS/3% Triton X-100/10% normal goat serum (NGS) and individually incubated with the coverslips for 2 hours at 37°C. Coverslips were washed 3 times (10 minutes each) in PBS and incubated in appropriate secondary antibodies (1:200; Sigma, St Louis, MO) for 30 minutes at 37°C. Coverslips were rinsed 3 times in PBS and one time in distilled water and mounted on glass slides with Fluoesave (Calbiochem, La Jolla, CA). For the triple-labeling immunocytochemistry of differentiated neurospheres, cells were briefly permeabilized for 5 minutes (PBS/3% Triton X-100/10% NGS) after fixation, followed by the addition of the neuron-specific mouse monoclonal antibody to microtubule-associated protein-2 (MAP-2; IgG, 1:100, Boehringer Mannheim, Indianapolis, IN) together with polyclonal rabbit antiserum to glial fibrillary acidic protein (GFAP; ready to use, Incstar, Stillwater, MN) and incubated for 90 minutes at 37°C. After thorough washing with PBS/10% NGS, cells were reacted for 45 minutes at room temperature with appropriate secondary conjugated goat anti–mouse or anti–rabbit IgG antibodies (1:100, Boehringer Mannheim). The coverslips were then incubated with monoclonal antibody to O4 (IgM, 1:200, Boehringer Mannheim). After thorough washing with PBS/10% NGS, cells were reacted for 45 minutes at room temperature with donkey anti–mouse IgM antibodies coupled to 7-amino-4-methylcoumarin-3-acetic acid (AMAC; 1:100, Jackson Immunoresearch, West Grove, PA). Coverslips were rinsed 3 times in PBS and one time in distilled water and mounted on glass slides with Fluoesave (Calbiochem). The number of GFAP-, MAP-2–, and O4-positive cells were assessed in 10 nonoverlapping fields for each sphere. The total number of cells in each field was determined by counterstaining cell nuclei with 4,6-diamidine-2-phenylindole dihydrochloride (DAPI; 1 mg/mL in methanol for 15 minutes at 37°C).
Detection of hematopoietic cells in the initial human fetal brain cell suspensions, primary-, secondary-, and higher-passaged neurospheres
Initially, to detect the potential contamination of human hematopoietic cells in the initial human fetal brain cell preparations and neurospheres of various passages, one million cells from each sample were stained with phycoerytherin (PE)–labeled MoAbs against CD45 and another million cells stained with fluorescein isothiocyanate (FITC)–labeled MoAbs against all class I human leukocyte antigens (HLA-A, -B, -C) (Pharmingen). To further detect any possible contamination of human hematopoietic cells in neurospheres derived from various passages, 1 × 105 neurosphere cells were stained with each of a panel of surface markers including CD34, CD38, thy-1, CD3, CD4, CD8, CD10, CD19, CD20, CD13, CD15, CD33, and CD71 (Pharmingen). Analysis was gated on the viable cells and the quadrants were set based on the mean fluorescence intensity of the isotype control samples. Cells were analyzed on a tri-laser fluorescence-activated cell sorter MoFlo (Cytomation, Fort Collins, CO).
In vitro human neuronal progenitor/mouse stromal cocultures
To investigate if neurosphere cells possess hematopoietic potential in vitro, neurosphere cells derived from various passages were directly cultured on a pre-established monolayer of mouse stromal cell line AC6.21.24 25 Briefly, 5 × 103 to 1 × 104 stromal cells were plated in 96-well flat-bottom plates one week before the experiment in 100 μL of long-term culture medium (LTCM) consisting of RPMI 1640, 5 × 10−5 M β-mercaptoethanol, 10 mM HEPES, 50 U/mL penicillin, 50 mg/mL streptomycin, 2 mM sodium pyruvate, 2 mM glutamine, and 10% FCS. Five thousand neurosphere cells from various passages, regardless of different culture conditions and donors, were distributed in 100 μL of LTCM into each well in 96-well flat-bottom plates with pre-established AC6.21 monolayer. Human recombinant interleukin 3 (IL-3), interleukin 6 (IL-6), granulocyte-macrophage colony-stimulating factor (GM-CSF), stem cell factor (SCF), and leukemia inhibitory factor (LIF) were added immediately after seeding the sphere cells into the microtiter plates at a concentration of 10 ng/mL of each growth factor. Human recombinant IL-3, IL-6, GM-CSF, SCF, and LIF were purchased from R&D Systems. Half of the culture medium was replaced weekly with fresh LTCM containing all 5 cytokines. To minimize disturbance to the cultures during the weekly medium change, 100 μL of old medium from each well was removed slowly from the top of the well with a multiple channel pipetter, and 100 μL of fresh medium was then slowly added to each well. Subsequently, we scaled up the experiments to 24-well plates. One million cultured neurosphere cells from various culture conditions, passages, and donors, were distributed in 500 μL of LTCM into each well with pre-established AC6.21 monolayer (final volume of LTCM in each well is 1 mL) and all 5 cytokines were added (final concentration of each cytokine is 10 ng/mL) immediately after seeding the neurosphere cells. Cultures from these scaled up experiments were carried for 2 weeks and then subjected to phenotypic analysis.
Phenotypic analysis of hematopoietic cells in the sphere/mouse stromal cocultures
The potential for the cultured human neurosphere cells to differentiate into hematopoietic lineages in this stromal coculture system was assessed by flow cytometry at the end of 5-week and 2-week culture periods for cultures initiated with 5000 and one million of various neurosphere cells, respectively.24 25 Cells from each well were harvested individually by vigorously pipetting of the wells including stromal cells. Half of the cells from each well were stained with FITC- or PE-labeled MoAbs against CD19 and CD33, and the other half were stained with antibodies against CD45 and HLA-A, -B, -C. Analysis was gated on the hematopoietic cells, excluding the stromal cells, and the quadrants were set based on the mean fluorescence intensity of the isotype control samples. FITC- or PE-labeled MoAbs against CD19, CD33, CD45, and HLA-A, -B, -C were purchased from Pharmingen. Cells were analyzed on a tri-laser fluorescence-activated cell sorter MoFlo (Cytomation).
In vivo hematopoietic reconstitution assay in SCID-hu mice
C.B-17 scid/scid mice were bled in our facility under sterile conditions. Mice used for human tissue transplantation were 6 to 8 weeks of age, and the construction of SCID-hu thymus/liver (thy/liv) and bone model mice were constructed as previously described24,25 and in accordance with the guidelines set forth by the City of Hope Research Animal Care Committee. Animals were preconditioned by total body irradiation (350 rads) 4 to 6 hours before they were subjected to NSCs or BM cell reconstitution. Initially, the ability of cultured human neurosphere cells (HLA-MA2.1–positive determined by HLA-typing of the thymus tissues from the same donors), either primary or various passages, to reconstitute thymus and BM was tested by direct inoculation into irradiated grafts (thy/liv and bone; HLA-MA2.1–negative). One million neurosphere cells in 10 μL of Hanks balanced salt solution (HBSS) were injected per human graft. For subsequent reconstitution experiments, one million human BM cells harvested from transplanted human fetal bone fragments in SCID-hu mice that were previously, 4 months earlier, injected with one million cultured human neurosphere cells, were used instead of cultured human neurosphere cells. Control animals were injected with HBSS only. Engraftment was analyzed 4 months after injection by flow cytometry as previously described.24 25
Results
Human fetal brain contains EGF- and FGF-2–responsive NSCs
It was previously reported that embryonic and adult murine forebrain contain multipotential (neuronal/glial) progenitor cells that can be induced to proliferate and form neurospheres in vitro when either EGF15-17 or FGF-218,19 is provided. These EGF- or FGF-2–responsive NSCs in embryonic and adult mouse brains possess stem cell characteristics of both self-renewal and multipotential.15-19 Recently, it has also been established that NSCs are present in the human adult brain.28-31 We are the first to demonstrate that EGF- or FGF-2–responsive NSCs are present in the human fetal brain. Our results demonstrate that NSCs that respond to EGF and FGF-2 are present in human fetal brain tissue (Figure 1A). Three different human fetal brain samples were used and compared in these experiments (Table 1). For donor no. 1, the number of spheres generated in the primary cultures in the presence of EGF (20 ng/mL), FGF-2 (20 ng/mL), and EGF plus FGF-2 was 21.8 ± 5.3 spheres per 5000 viable cells (0.45%), 17.7 ± 6.1 spheres per 5000 viable cells (0.35%), and 32.8 ± 5.2 spheres per 5000 viable cells (0.65%), respectively. Because the total number of spheres in the cultures treated with EGF plus FGF-2 (32.8 ± 5.2 per 5000 cells) is less than the sum of the number of spheres generated with EGF alone (21.8 ± 5.3 per 5000 cells) and FGF-2 alone (17.7 ± 6.1 per 5000 cells), these results suggest that a fraction of these NSCs in the fetal brain are capable of responding to both EGF and FGF-2 simultaneously. Based on results from donor no. 1, we have estimated that 33.3% of EGF-responsive NSCs are also FGF-2–responsive and 42.7% of FGF-2–responsive NSCs can also respond to EGF. The data from the other 2 donors were almost identical to donor no. 1 and showed no significant donor effect in the frequency of NSCs that respond to EGF, FGF-2, or their combination.
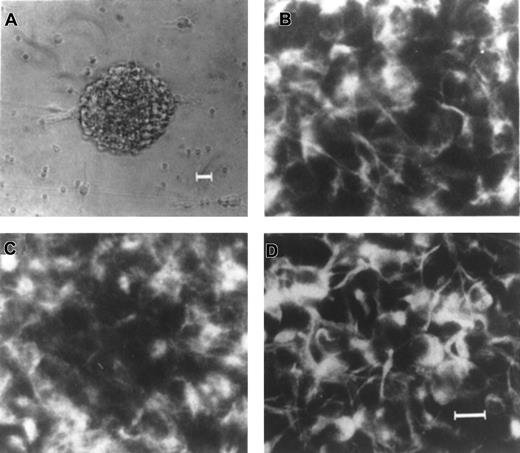
Expression of nestin, EGFR, and FGFR1 in EGF-generated primary neurospheres from human fetal brain.
(A) An example of a single EGF-generated primary human neurosphere. Immunocytochemical staining of individual EGF-generated primary human neurosphere with antibodies to nestin (B), EGFR (C), and FGFR1 (D), respectively. Scale bars: (A) 25 μm; (B-D) 10 μm.
Expression of nestin, EGFR, and FGFR1 in EGF-generated primary neurospheres from human fetal brain.
(A) An example of a single EGF-generated primary human neurosphere. Immunocytochemical staining of individual EGF-generated primary human neurosphere with antibodies to nestin (B), EGFR (C), and FGFR1 (D), respectively. Scale bars: (A) 25 μm; (B-D) 10 μm.
Frequency of NSCs from human fetal brain in response to EGF, FGF-2, and EGF plus FGF-2
| Donor no. . | EGF . | FGF-2 . | EGF + FGF-2 . | |||
|---|---|---|---|---|---|---|
| Primary cultures . | Secondary cultures . | Primary cultures . | Secondary cultures . | Primary cultures . | Secondary cultures . | |
| 1 | 21.8 ± 5.3 | 90.3 ± 7.3 | 17.7 ± 6.1 | 72.4 ± 5.4 | 32.8 ± 5.2 | 132.4 ± 9.2 |
| 2 | 22.2 ± 6.1 | 90.4 ± 6.6 | 16.8 ± 5.8 | 70.6 ± 6.3 | 33.1 ± 6.2 | 133.8 ± 9.8 |
| 3 | 23.2 ± 5.4 | 92.8 ± 6.4 | 17.6 ± 4.2 | 72.6 ± 6.4 | 31.8 ± 5.6 | 130.5 ± 8.6 |
| Donor no. . | EGF . | FGF-2 . | EGF + FGF-2 . | |||
|---|---|---|---|---|---|---|
| Primary cultures . | Secondary cultures . | Primary cultures . | Secondary cultures . | Primary cultures . | Secondary cultures . | |
| 1 | 21.8 ± 5.3 | 90.3 ± 7.3 | 17.7 ± 6.1 | 72.4 ± 5.4 | 32.8 ± 5.2 | 132.4 ± 9.2 |
| 2 | 22.2 ± 6.1 | 90.4 ± 6.6 | 16.8 ± 5.8 | 70.6 ± 6.3 | 33.1 ± 6.2 | 133.8 ± 9.8 |
| 3 | 23.2 ± 5.4 | 92.8 ± 6.4 | 17.6 ± 4.2 | 72.6 ± 6.4 | 31.8 ± 5.6 | 130.5 ± 8.6 |
Cells were plated in 96-well plates, either as 5000 cells/200 μL per well in the primary cultures or as 500 cells/200 μL per well in the secondary cultures. These wells were scored 7 days later for the presence of spheres. The total number of wells analyzed is 300 for each primary culture and 200 for each secondary culture. Data for the number of spheres per well are presented as the mean ± SD in each culture condition. These data are compiled from 3 independent experiments using different donor samples. EGF indicates epidermal growth factor; FGF-2, fibroblast growth factor.
Cultured human neurosphere cells express characteristic markers for NSCs
It has been well established that the intermediate filament protein, nestin, is expressed in all neural precursor cells in the central nerve system in rats,32 mice,15-19and humans.28-31 Because neurospheres can only be generated in the presence of EGF and /or FGF-2, these EGF- and/or FGF-2–responsive NSCs must express EGFR and/or FGFR1. The expressions of EGFR and FGFR1 have been shown to be ubiquitous throughout the cells in the mouse neurospheres, similar to nestin.33 To assure that these cultured neurospheres derived from human fetal brains do possess the characteristics of NSCs, expression of these 3 characteristic NSC markers within each human neurosphere derived from various culture conditions, passages, and donors, was determined. Our results revealed that virtually all of the cells (> 85%) within a single EGF-generated primary human neurosphere express nestin (Figure1B), EGFR (Figure 1C), and FGFR1 (Figure 1D). Similar results were obtained from all cultured human neurospheres regardless of passages, culture conditions, and donors (data not shown). Taken together, our results demonstrate that these cultured neurospheres derived from human fetal brain tissues express the 3 characteristic markers for NSCs including nestin, EGFR, and FGFR1, regardless of passages (up to the fifth passage), 3 different culture conditions, and 3 different donors.
Self-proliferation and expansion of EGF- and FGF-2–responsive NSCs
In the mouse system, there is an increase in the frequency of EGF-generated spheres from 1% in primary cultures to nearly 20% in secondary cultures derived from primary EGF-generated spheres.15-17 Similar results have also been reported for FGF-2–generated spheres in the murine system.18,19 These reports suggest that when a single NSC proliferates to form a neurosphere, it also generates more NSCs.15-19 To determine if human NSCs possess a similar self-proliferative potential, experiments were performed to determine the number of secondary spheres derived from primary spheres in response to the same culture conditions. After 7 days in vitro, the floating neurospheres from primary cultures in each culture condition were harvested, mechanically dissociated into single cells, and 500 cells were plated into each well of 96-well plates using the same culture conditions. After 7 days of secondary culture, the number of spheres in each well for each culture condition was scored. Again, 3 different fetal brain samples were used and compared in these experiments (Table 1). Our results demonstrate that primary neurospheres from human fetal brain tissues, in response to EGF and FGF-2, have become further enriched for NSCs. For donor no. 1, the number of spheres generated in each well in the secondary cultures in the presence of EGF, FGF-2, and EGF plus FGF-2 was 90.3 ± 7.3 spheres per 500 cells (18.2%), 72.4 ± 5.4 spheres per 500 cells (14.5%), and 132.4 ± 9.2 spheres per 500 cells (26.5%), respectively. Based on these results from donor no. 1, we have calculated that there is an average 40-fold expansion of NSCs from the primary neurospheres in response to EGF, FGF-2, and EGF plus FGF-2. The data from the other 2 donors were almost identical to donor no. 1 and showed no significant donor effect in self-proliferation and expansion of NSCs. Taken together, these results show that NSCs in the human fetal brain possess similar self-proliferative potential as compared with murine NSCs. We have extended the cultures to the fifth passage and our results show that the frequency of NSCs in each neurosphere remains constant (20%) from the second passage to the fifth passage (the longest culture period that has been carried out) in all culture conditions, suggesting that NSCs from human fetal brain tissues are capable of self-proliferative potential at least up to the fifth passage (data not shown).
Neurospheres have multilineage potential for neurons, astrocytes, and oligodendrocytes in vitro
In the murine system, EGF-generated progenitor cells within the spheres have multipotential for all neuronal lineages.15-17 After 7 days in vitro in the absence of EGF and in the presence of 1% FCS, triple-labeled immunocytochemistry for MAP-2, GFAP, and O4 revealed the presence of cells with the morphology and antigenic characteristics of neurons, astrocytes, and oligodendrocytes, respectively, in single spheres derived from either primary or tenth passage cultures.17 Similar data have recently been reported for FGF-2–responsive spheres in the mouse system,18,19 and for NSCs from the human adult brain.28-31 These previous results suggest that NSCs, even after the tenth passage, retain multipotential for neurons, astrocytes, and oligodendrocytes.15-19 28-31 In the present study, we demonstrate that neurospheres derived from human fetal brain tissues are also capable of multilineage differentiation for neurons, astrocytes, and oligodendrocytes. Single primary and higher passaged neurospheres from each culture condition (n = 100) were plated onto poly-L-ornithine–coated glass coverslips in 24-well culture plates in the absence of EGF and FGF-2 and in the presence of 1% serum and cultured for 8 to 10 days. The differentiated neurosphere derived from a single EGF-generated primary human neurosphere on a coverslip for a week in vitro is shown in Figure 2A. Indirect immunocytochemistry for neuronal (MAP-2) and glial cell antigens (GFAP and O4), in combination with morphologic examination, was used to identify the phenotype of cells within the differentiated neurospheres. Our results demonstrate that cells within a single EGF-generated primary human neurosphere could differentiate into GFAP-positive cells with morphology for astrocytes (Figure 2B), MAP-2–positive cells with morphology as neurons (Figure 2C), and O4-positive cells with morphology for oligodendrocytes (Figure 2D). In these differentiation cultures, 5 categories of spheres were observed, which contained: (1) astrocytes only; (2) oligodendrocytes and astrocytes; (3) neurons and astrocytes; (4) neurons and oligodendrocytes; and (5) neurons, astrocytes, and oligodendrocytes (data not shown). Three different fetal brain samples were used and results are compiled in Table 2. For donor no. 1, EGF-generated spheres have a significantly higher probability to differentiate into all 3 lineages including neurons, astrocytes, and oligodentrocytes (80% and 91% for primary and secondary spheres, respectively) than FGF-2–generated spheres (67% and 78% for primary and secondary spheres, respectively) (P < .0001). Our data also demonstrate that secondary spheres have a significantly higher probability to differentiate into all 3 lineages (91% and 78% for EGF- and FGF-2–responsive spheres, respectively) than primary spheres (80% and 67% for EGF- and FGF-2–responsive spheres, respectively) (P < .0001). Characteristics of EGF plus FGF-2–generated spheres are not significantly different from EGF-generated spheres. Data from the other 2 donors were almost identical to donor no. 1 and showed no significant donor effect (Table 2). Results obtained for neurospheres derived from higher passages, from the third to the fifth passage, are similar to the data derived from secondary neurospheres in each culture conditions for all 3 donors (data not shown). Taken together, these results suggest that human NSCs are multipotential for all neuronal lineages and they have been preferentially expanded in the spheres.
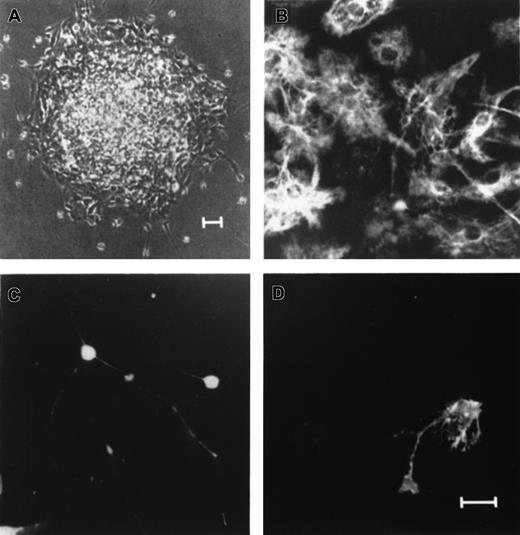
Single primary EGF-generated human neurospheres contain neurons, astrocytes, and oligodendrocytes.
Individual EGF-generated primary human neurospheres were plated on poly-L-ornithine–coated glass coverslips in differentiation cultures for 7 to 8 days in vitro. (A) An example of a single EGF-generated primary human neurosphere growing on a poly-ornitine–coated glass coverslip for one week in differentiation culture. Triple-labeled immunocytochemical staining of the differentiated neurosphere with antibodies to GFAP (B), MAP-2 (C), and O4 (D) revealed cells with the antigenic and morphologic characteristics of astrocytes (B), neurons (C), and oligodendrocytes (D). Scale bars: (A) 25 μm; (B-D) 5 μm.
Single primary EGF-generated human neurospheres contain neurons, astrocytes, and oligodendrocytes.
Individual EGF-generated primary human neurospheres were plated on poly-L-ornithine–coated glass coverslips in differentiation cultures for 7 to 8 days in vitro. (A) An example of a single EGF-generated primary human neurosphere growing on a poly-ornitine–coated glass coverslip for one week in differentiation culture. Triple-labeled immunocytochemical staining of the differentiated neurosphere with antibodies to GFAP (B), MAP-2 (C), and O4 (D) revealed cells with the antigenic and morphologic characteristics of astrocytes (B), neurons (C), and oligodendrocytes (D). Scale bars: (A) 25 μm; (B-D) 5 μm.
Frequency of clonally derived primary and secondary neurospheres from fetal brains that differentiate into multilineage brain cells in vitro
| Donor no. . | EGF-generated neurospheres . | FGF-2–generated neurospheres . | (EGF + FGF-2)–generated neurospheres . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenotype composition . | Phenotype composition . | Phenotype composition . | ||||||||||
| Primary neurospheres . | Secondary neurospheres . | Primary neurospheres . | Secondary neurospheres . | Primary neurospheres . | Secondary neurospheres . | |||||||
| N + A + O . | N + G . | N + A + O . | N + G . | N + A + O . | N + G . | N + A + O . | N + G . | N + A + O . | N + G . | N + A + O . | N + G . | |
| 1 | 80 | 13 | 91 | 4 | 67 | 22 | 78 | 14 | 88 | 9 | 91 | 6 |
| 2 | 85 | 9 | 91 | 4 | 70 | 20 | 80 | 16 | 87 | 7 | 90 | 5 |
| 3 | 82 | 11 | 93 | 5 | 68 | 20 | 76 | 15 | 88 | 9 | 91 | 5 |
| Donor no. . | EGF-generated neurospheres . | FGF-2–generated neurospheres . | (EGF + FGF-2)–generated neurospheres . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenotype composition . | Phenotype composition . | Phenotype composition . | ||||||||||
| Primary neurospheres . | Secondary neurospheres . | Primary neurospheres . | Secondary neurospheres . | Primary neurospheres . | Secondary neurospheres . | |||||||
| N + A + O . | N + G . | N + A + O . | N + G . | N + A + O . | N + G . | N + A + O . | N + G . | N + A + O . | N + G . | N + A + O . | N + G . | |
| 1 | 80 | 13 | 91 | 4 | 67 | 22 | 78 | 14 | 88 | 9 | 91 | 6 |
| 2 | 85 | 9 | 91 | 4 | 70 | 20 | 80 | 16 | 87 | 7 | 90 | 5 |
| 3 | 82 | 11 | 93 | 5 | 68 | 20 | 76 | 15 | 88 | 9 | 91 | 5 |
Individual spheres were transferred onto poly-L-ornithine–coated glass coverslips and cultured in differentiation medium for 8 to 10 days before processing for triple-labeled immunocytochemistry. The number of differentiated spheres containing all 3 lineages (N + A + O) or neurons and either astrocytes or oligodendrocytes (N + G) are presented. The data presented are the actual number in each category of a total of 100 spheres derived from each culture condition. These data are compiled from 3 independent experiments using different donor brain samples. EGF indicates epidermal growth factor; FGF-2, fibroblast growth factor; N, neurons; A, astrocytes; and O, oligodentrocytes.
The relative proportion of neurons, astrocytes, and oligodendrocytes in an EGF-generated primary sphere was determined by randomly selecting 6 clonally derived EGF-generated primary spheres (Table3). At 8 to 10 days after differentiation in vitro, 7.8 ± 0.5% of the cells in an EGF-generated sphere were neuron (MAP-2–positive), 60.3 ± 2.8% were astrocyte (GFAP-positive), and 2.9 ± 0.2% were oligodendrocyte (O4-positive). The same methodology was then employed to determine the relative proportion of neurons, astrocytes, and oligodendrocytes in the spheres derived from different culture conditions for the 3 different fetal brain samples (Table 4). The same analysis was extended to cultured neurospheres derived from the third, fourth, and fifth passages in each culture condition, and results similar to Table 4 were obtained (data not shown). Our data demonstrate a relatively constant proportion of neurons, astrocytes, and oligodendrocytes in each sphere regardless of culture conditions, passages, and donors.
Cellular composition of 6 clonally derived EGF-generated neurospheres which contain cells for all 3 lineages including neurons, astrocytes, and oligodendrocytes
| Clonally derived spheres . | Percentage of lineage-specific cells in the sphere . | ||
|---|---|---|---|
| MAP-2–positive . | GFAP-positive . | O4-positive . | |
| 1 | 8.2% (26/316) | 56.3% (178/316) | 2.5% (8/316) |
| 2 | 7.8% (31/396) | 59.3% (235/396) | 3.0% (12/396) |
| 3 | 8.4% (36/429) | 60.6% (260/429) | 3.3% (14/429) |
| 4 | 8.1% (28/347) | 58.8% (204/347) | 2.9% (10/347) |
| 5 | 7.2% (25/346) | 62.4% (216/346) | 2.9% (10/346) |
| 6 | 7.2% (30/417) | 64.3% (268/417) | 2.9% (12/417) |
| Mean ± SEM | 7.8 ± 0.5% | 60.3 ± 2.8% | 2.9 ± 0.2% |
| Clonally derived spheres . | Percentage of lineage-specific cells in the sphere . | ||
|---|---|---|---|
| MAP-2–positive . | GFAP-positive . | O4-positive . | |
| 1 | 8.2% (26/316) | 56.3% (178/316) | 2.5% (8/316) |
| 2 | 7.8% (31/396) | 59.3% (235/396) | 3.0% (12/396) |
| 3 | 8.4% (36/429) | 60.6% (260/429) | 3.3% (14/429) |
| 4 | 8.1% (28/347) | 58.8% (204/347) | 2.9% (10/347) |
| 5 | 7.2% (25/346) | 62.4% (216/346) | 2.9% (10/346) |
| 6 | 7.2% (30/417) | 64.3% (268/417) | 2.9% (12/417) |
| Mean ± SEM | 7.8 ± 0.5% | 60.3 ± 2.8% | 2.9 ± 0.2% |
The number of neurons (MAP-2–positive), astrocytes (GFAP-positive), and oligodendrocytes (O4-positive) was determined in 10 nonoverlapping fields in each sphere. Numbers in parentheses indicate the total number of lineage-specific cells divided by the total number of cells in each sphere. The mean ± SD represent the mean frequency for each of the 3 different lineages of cells among 6 clonally derived spheres examined. MAP-2 indicates microtubule-associated protein-2; GFAP, glial fibrillary acidic protein.
Cellular composition of different lineage brain cells among clonally derived neurospheres generated under different conditions
| Donor no. . | EGF-generated neurospheres . | FGF-2–generated neurospheres . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary neurospheres . | Secondary neurospheres . | Primary neurospheres . | Secondary neurospheres . | |||||||||
| MAP-2 . | GFAP . | O4 . | MAP-2 . | GFAP . | O4 . | MAP-2 . | GFAP . | O4 . | MAP-2 . | GFAP . | O4 . | |
| 1 | 7.8 ± 0.5 | 60.3 ± 2.8 | 2.9 ± 0.2 | 8.0 ± 0.6 | 60.3 ± 2.6 | 3.0 ± 0.3 | 7.6 ± 0.3 | 60.6 ± 3.1 | 2.8 ± 0.4 | 8.1 ± 0.4 | 61.8 ± 3.1 | 2.8 ± 0.4 |
| 2 | 8.1 ± 0.7 | 60.6 ± 2.2 | 3.0 ± 0.4 | 7.9 ± 0.5 | 59.8 ± 2.4 | 3.1 ± 0.5 | 7.8 ± 0.4 | 59.2 ± 2.9 | 2.8 ± 0.3 | 8.0 ± 0.2 | 58.9 ± 2.8 | 3.0 ± 0.3 |
| 3 | 7.8 ± 0.4 | 61.8 ± 2.1 | 3.0 ± 0.3 | 8.0 ± 0.6 | 59.6 ± 2.8 | 3.1 ± 0.4 | 7.7 ± 0.4 | 60.6 ± 2.8 | 2.9 ± 0.4 | 8.1 ± 0.4 | 59.9 ± 2.4 | 2.9 ± 0.4 |
| Donor no. . | EGF-generated neurospheres . | FGF-2–generated neurospheres . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary neurospheres . | Secondary neurospheres . | Primary neurospheres . | Secondary neurospheres . | |||||||||
| MAP-2 . | GFAP . | O4 . | MAP-2 . | GFAP . | O4 . | MAP-2 . | GFAP . | O4 . | MAP-2 . | GFAP . | O4 . | |
| 1 | 7.8 ± 0.5 | 60.3 ± 2.8 | 2.9 ± 0.2 | 8.0 ± 0.6 | 60.3 ± 2.6 | 3.0 ± 0.3 | 7.6 ± 0.3 | 60.6 ± 3.1 | 2.8 ± 0.4 | 8.1 ± 0.4 | 61.8 ± 3.1 | 2.8 ± 0.4 |
| 2 | 8.1 ± 0.7 | 60.6 ± 2.2 | 3.0 ± 0.4 | 7.9 ± 0.5 | 59.8 ± 2.4 | 3.1 ± 0.5 | 7.8 ± 0.4 | 59.2 ± 2.9 | 2.8 ± 0.3 | 8.0 ± 0.2 | 58.9 ± 2.8 | 3.0 ± 0.3 |
| 3 | 7.8 ± 0.4 | 61.8 ± 2.1 | 3.0 ± 0.3 | 8.0 ± 0.6 | 59.6 ± 2.8 | 3.1 ± 0.4 | 7.7 ± 0.4 | 60.6 ± 2.8 | 2.9 ± 0.4 | 8.1 ± 0.4 | 59.9 ± 2.4 | 2.9 ± 0.4 |
The data presented are the mean ± SD of the mean frequency for each of the 3 different lineages of cells among a total of 6 clonally derived spheres from each culture condition. The data are compiled from 3 independent experiments using different donor brain samples. For abbreviations, see Table 3 footnote.
No detectable hematopoietic cells in cultured human neurospheres
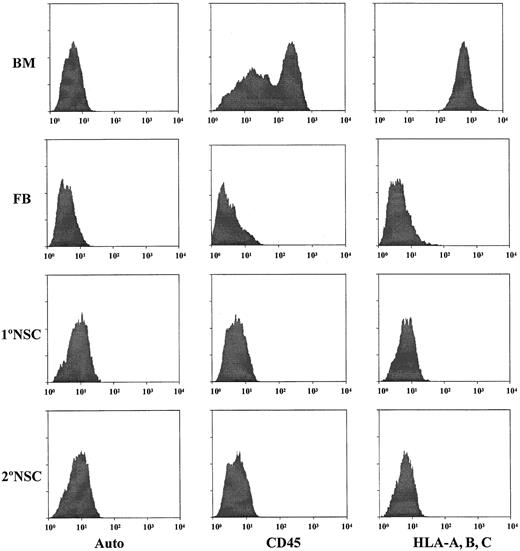
The ultimate goal of the present study is to determine if cultured neurospheres from human brain tissues truly possess hematopoietic potential as has been reported for murine NSCs.12 In order to be able to demonstrate that cultured human neurosphere cells are capable of differentiating into hematopoietic cells, it is absolutely necessary to rule out the possibility of contamination of hematopoietic cells in the cultured human neurosphere cells. Initially, we employed 2 monoclonal antibodies against 2 pan-hematopoietic cell markers, CD45 and HLA class I antigens (HLA-A, -B, -C), to determine the possible contamination of hematopoietic cells in the initial human fetal brain cell suspensions, and cultured human neurosphere cells from various passages, culture conditions, and donors. Results obtained from these analyses of samples derived from donor no. 1 are shown in Figure3. Based on the intensity of staining with human BM cells as a positive control, our results demonstrate that the initial fetal brain cell suspension contains a detectable fraction (< 0.5%) of cells which are positive for CD45 and HLA-A, -B, -C (Figure 3). However, the intensity of the staining for CD45 or HLA-A, -B, -C is about 2 logs lower than their expression on human BM cells. Our results also demonstrate that there are no detectable CD45-positive or HLA-A–, HLA-B–, HLA-C–positive cells in the EGF-generated primary and secondary neurosphere cells (Figure 3). To further confirm that there is no detectable contamination of human hematopoietic cells among EGF-generated neurosphere cells, each neurosphere sample was further subjected to the analyses with a panel of human hematopoietic markers, including stem cell markers such as CD34, CD38, and thy-1; T-cell markers such as CD3, CD4, and CD8; B-cell markers such as CD10, CD19, and CD20; and myeloid markers such as CD13, CD15, and CD33. Our results show that EGF-generated neurosphere cells from various passages do not contain any cell that has a detectable level of expression for any of the markers in this panel. These types of analyses have been extended to fetal brain cell suspensions from 3 different donors and cultured neurosphere cells derived from various passages, various culture conditions, and 3 different donors. Results from all these analyses show that there is no detectable level of contamination of human hematopoietic cells in the initial human fetal brain cell suspensions and cultured human neurosphere cells from various passages, various culture conditions, and 3 different donors.
Detection of hematopoietic cells in the initial cell preparations from fetal brain tissues and in EGF-generated primary and secondary neurospheres.
Antibodies against CD45 and HLA class I antigens (HLA-A, B, C) were used to analyze the samples for the possible contamination of human hematopoietic cells. Human fetal BM cells from the same donors of brain tissues were used as the positive controls for staining of CD45 and HLA-A, B, C hematopoietic markers. FB indicates the initial fetal brain cell suspensions. 1°NSC indicates EGF-generated primary neurospheres. 2°NSC indicates EGF-generated secondary neurospheres. Auto indicates the autofluorescence control of these samples.
Detection of hematopoietic cells in the initial cell preparations from fetal brain tissues and in EGF-generated primary and secondary neurospheres.
Antibodies against CD45 and HLA class I antigens (HLA-A, B, C) were used to analyze the samples for the possible contamination of human hematopoietic cells. Human fetal BM cells from the same donors of brain tissues were used as the positive controls for staining of CD45 and HLA-A, B, C hematopoietic markers. FB indicates the initial fetal brain cell suspensions. 1°NSC indicates EGF-generated primary neurospheres. 2°NSC indicates EGF-generated secondary neurospheres. Auto indicates the autofluorescence control of these samples.
Neurospheres cannot differentiate into hematopoietic cells in vitro
Our first approach to determine the hematopoietic potential of cultured sphere cells was to culture the neurosphere cells in a murine stromal coculture system which was previously developed in our laboratory for ex vivo stem cell expansion.24 25 Our rationale is that if this stromal coculture system is sufficient to facilitate human hematopoietic stem cell expansion and multilineage differentiation, it might also provide an appropriate environment for NSCs to differentiate into hematopoietic cells. Five thousand cultured human neurosphere cells from various culture conditions, various passages, and 3 different donors were deposited into each well of 96-well plates with the pre-established mouse stromal cell line AC6.21. Five 96-well plates were established for each neurosphere sample. The potential for the neurosphere cells to differentiate into hematopoietic cells in this stromal coculture system was assessed by flow cytometry for CD19+ B cells and CD33+ myeloid cells, and CD45+ and HLA-A–, HLA-B–, HLA-C–positive human hematopoietic cells at the end of a 5-week culture period. Our results show that there are no detectable human hematopoietic cells in any of these in vitro cultures (data not shown). Subsequently, we scaled up these experiments from 96-well plates to 24-well plates, and each well was initiated with one million cultured neurosphere cells. Three 24-well plates were established for each neurosphere sample, and cells were analyzed by flow cytometry at the end of a 2-week culture period. Again, our results demonstrate that there are no detectable human hematopoietic cells in any of these in vitro cultures. Taken together, our data suggest that cultured neurosphere cells derived from human fetal brain tissues, regardless of culture conditions, passages, and donors, are not able to differentiate into hematopoietic cells under this stromal coculture system.
Neurospheres possess a human bone marow–dependent in vivo hematopoietic potential
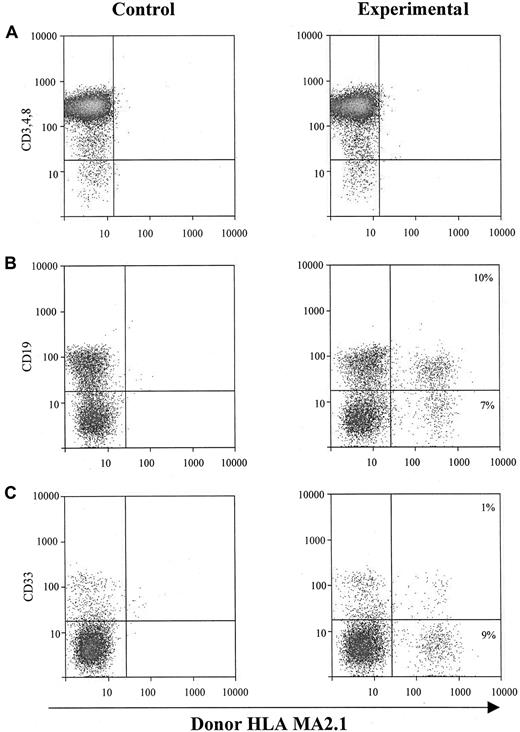
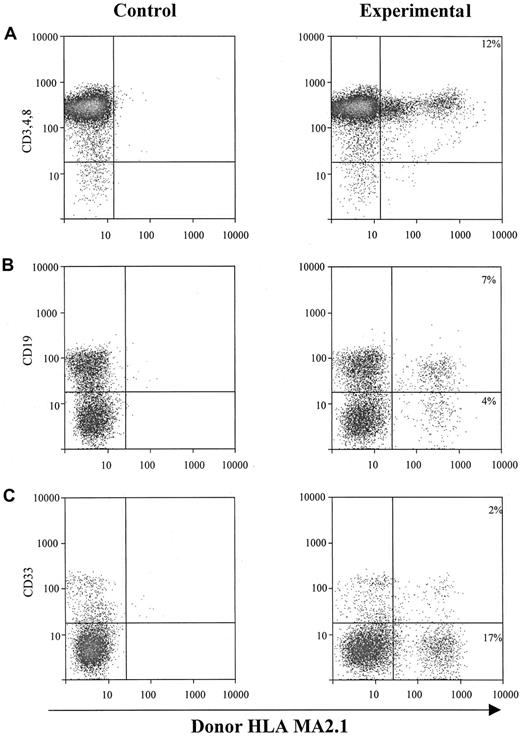
The second approach to determine the hematopoietic potential of cultured sphere cells was to test them in vivo in SCID-hu mice.20-25 One million EGF-generated primary human neurosphere cells were injected directly into each human graft, including thymus and bone fragments, in SCID-hu mice. NSC-derived hematopoietic cells (HLA-MA2.1–positive) in those injected human grafts were analyzed 4 months after neurosphere cell injection. Our results demonstrate that EGF-generated primary human neurosphere cells are able to establish a long-term hematopoiesis in the BM microenvironment with about 15% of B cells and myeloid cells derived from neurosphere cells (Figure 4B,C). EGF-generated primary human neurosphere cells, however, failed to develop into T lymphocytes in the implanted human thymus in SCID-hu mice (Figure 4A). A possible explanation for these results is that the commitment of EGF-generated primary human neurosphere cells into hematopoietic lineages might depend on the presence of an intact human BM microenvironment. To test this hypothesis, one million EGF-generated primary human neurosphere cells were first injected into bone fragments in SCID-hu mice for 4 months, then BM cells were harvested from each bone fragment. One million harvested BM cells were then injected directly into either human thymus or bone fragment grafts in SCID-hu mice. Neurosphere cell–derived hematopoietic cells (HLA-MA2.1–positive) were analyzed 4 months after injection. Our results demonstrate that long-term hematopoietic reconstitution can be detected in about 50% of the SCID-hu mice with either thymus or bone grafts (Figure 5 and Table5). In these hematopoietic-reconstituted SCID-hu thy/liv mice, about 15% of the T lymphocytes in the thymus were derived from EGF-generated primary human neurosphere cells (Figure5A). Again, in the hematopoietic-reconstituted SCID-hu bone mice, about 15% of B cells and myeloid cells in the implanted human bone fragments were derived from EGF-generated primary human neurosphere cells (Figure5B,C). The same approach was then employed to evaluate if EGF-generated secondary human neurosphere cells and FGF-2–generated primary and secondary human neurosphere cells exert a human BM–dependent hematopoietic potential similar to EGF-generated primary human neurosphere cells. Our data demonstrate that EGF-generated secondary neurosphere cells and FGF-2–generated primary and secondary human neurosphere cells display a hematopoietic potential similar to EGF-generated primary human neurosphere cells (Table 5). The hematopoietic potential is human BM–dependent, and with one million harvested BM cells per graft, about 50% of animals are reconstituted. About 15% of hematopoietic cells in the reconstituted animals were derived from cultured neurosphere cells. Similar results have been obtained from EGF plus FGF-2–generated primary and secondary neurosphere cells, and from the higher passages of neurosphere cells of various culture conditions and 3 different donors (data not shown). Taken together, our results demonstrate that cultured human neurosphere cells, regardless of culture conditions, passages, and donors, are capable of establishing a long-term hematopoiesis in SCID-hu mice, and the commitment of these cultured human neurosphere cells into hematopoietic lineages depends on the presence of a functional human BM microenvironment.
In vivo hematopoietic reconstitution with EGF-responsive NSCs from human fetal brains.
One million EGF-responsive NSCs were injected directly into each human graft in SCID-hu mice. Graft cells were harvested 4 months after injection and subjected to flow cytometry for donor-derived hematopoietic cells. (A) Intrathymic T-cell development of EGF-generated primary spheres. Graft cells were analyzed for T-cell markers CD3, CD4, and CD8, and donor marker HLA-MA2.1. (B) B-cell and myeloid-cell differentiation of EGF-generated primary spheres in implanted human fetal bone fragments. Graft cells were analyzed by flow cytometry for B-cell marker CD19 (B) and myeloid marker CD33 (C), and a donor marker for EGF-generated primary spheres (HLA-MA2.1–positive). The percentage of B and myeloid cells expressing detectable levels of donor-specific class I antigen was recorded.
In vivo hematopoietic reconstitution with EGF-responsive NSCs from human fetal brains.
One million EGF-responsive NSCs were injected directly into each human graft in SCID-hu mice. Graft cells were harvested 4 months after injection and subjected to flow cytometry for donor-derived hematopoietic cells. (A) Intrathymic T-cell development of EGF-generated primary spheres. Graft cells were analyzed for T-cell markers CD3, CD4, and CD8, and donor marker HLA-MA2.1. (B) B-cell and myeloid-cell differentiation of EGF-generated primary spheres in implanted human fetal bone fragments. Graft cells were analyzed by flow cytometry for B-cell marker CD19 (B) and myeloid marker CD33 (C), and a donor marker for EGF-generated primary spheres (HLA-MA2.1–positive). The percentage of B and myeloid cells expressing detectable levels of donor-specific class I antigen was recorded.
EGF-responsive human fetal NSC-derived BM cells are able to establish long-term hematopoietic reconstitution in SCID-hu mice.
One million EGF-responsive human fetal NSCs were injected directly into each human bone graft in SCID-hu mice, and human BM cells were harvested from those bone fragments 4 months after injection. One million of those harvested human BM cells were then directly injected into each human graft, including bone and thy/liv, in SCID-hu mice, and graft cells were harvested 4 months later and subjected to flow cytometry for donor-derived hematopoietic cells. (A) Intrathymic T-cell development of EGF-generated primary spheres. Graft cells were analyzed for T-cell markers CD3, CD4, and CD8, and donor marker HLA-MA2.1. The percentage of T cells expressing detectable levels of donor-specific class I antigen was recorded. (B) B-cell and myeloid-cell differentiation of EGF-generated primary spheres in implanted human fetal bone fragments. Graft cells were analyzed for B-cell marker CD19 and myeloid marker CD33, and a donor marker for EGF-generated primary spheres (HLA-MA2.1–positive). The percentage of B and myeloid cells expressing detectable levels of donor-specific class I antigen was recorded.
EGF-responsive human fetal NSC-derived BM cells are able to establish long-term hematopoietic reconstitution in SCID-hu mice.
One million EGF-responsive human fetal NSCs were injected directly into each human bone graft in SCID-hu mice, and human BM cells were harvested from those bone fragments 4 months after injection. One million of those harvested human BM cells were then directly injected into each human graft, including bone and thy/liv, in SCID-hu mice, and graft cells were harvested 4 months later and subjected to flow cytometry for donor-derived hematopoietic cells. (A) Intrathymic T-cell development of EGF-generated primary spheres. Graft cells were analyzed for T-cell markers CD3, CD4, and CD8, and donor marker HLA-MA2.1. The percentage of T cells expressing detectable levels of donor-specific class I antigen was recorded. (B) B-cell and myeloid-cell differentiation of EGF-generated primary spheres in implanted human fetal bone fragments. Graft cells were analyzed for B-cell marker CD19 and myeloid marker CD33, and a donor marker for EGF-generated primary spheres (HLA-MA2.1–positive). The percentage of B and myeloid cells expressing detectable levels of donor-specific class I antigen was recorded.
Neurospheres possess in vivo hematopoietic potential
| Source of neurospheres . | Primary neurospheres . | Secondary neurospheres . | ||||||
|---|---|---|---|---|---|---|---|---|
| Hematopoietic reconstitution . | Hematopoietic reconstitution . | |||||||
| EGF-generated . | FGF-2–generated . | EGF-generated . | FGF-2–generated . | |||||
| Donor no. . | Frequency . | Percentage . | Frequency . | Percentage . | Frequency . | Percentage . | Frequency . | Percentage . |
| Bone SCID-hu model | ||||||||
| 1 | 6/10 | 15.6 ± 3.3 | 5/10 | 14.4 ± 3.4 | 6/10 | 16.5 ± 3.9 | 5/10 | 15.4 ± 2.6 |
| 2 | 5/10 | 16.6 ± 3.5 | 5/10 | 12.6 ± 2.7 | 5/10 | 15.7 ± 2.4 | 6/10 | 13.6 ± 3.1 |
| 3 | 5/10 | 15.1 ± 2.8 | 5/10 | 13.1 ± 2.6 | 6/10 | 16.2 ± 3.8 | 5/10 | 14.3 ± 3.5 |
| Thy/liv SCID-hu model | ||||||||
| 1 | 5/10 | 14.8 ± 3.5 | 6/10 | 12.6 ± 3.5 | 5/10 | 16.6 ± 3.1 | 5/10 | 13.1 ± 3.3 |
| 2 | 6/10 | 13.6 ± 2.2 | 6/10 | 13.8 ± 2.6 | 6/10 | 15.3 ± 3.2 | 5/10 | 12.8 ± 2.7 |
| 3 | 5/10 | 14.1 ± 2.8 | 5/10 | 13.6 ± 3.2 | 6/10 | 15.8 ± 3.4 | 5/10 | 14.1 ± 3.2 |
| Source of neurospheres . | Primary neurospheres . | Secondary neurospheres . | ||||||
|---|---|---|---|---|---|---|---|---|
| Hematopoietic reconstitution . | Hematopoietic reconstitution . | |||||||
| EGF-generated . | FGF-2–generated . | EGF-generated . | FGF-2–generated . | |||||
| Donor no. . | Frequency . | Percentage . | Frequency . | Percentage . | Frequency . | Percentage . | Frequency . | Percentage . |
| Bone SCID-hu model | ||||||||
| 1 | 6/10 | 15.6 ± 3.3 | 5/10 | 14.4 ± 3.4 | 6/10 | 16.5 ± 3.9 | 5/10 | 15.4 ± 2.6 |
| 2 | 5/10 | 16.6 ± 3.5 | 5/10 | 12.6 ± 2.7 | 5/10 | 15.7 ± 2.4 | 6/10 | 13.6 ± 3.1 |
| 3 | 5/10 | 15.1 ± 2.8 | 5/10 | 13.1 ± 2.6 | 6/10 | 16.2 ± 3.8 | 5/10 | 14.3 ± 3.5 |
| Thy/liv SCID-hu model | ||||||||
| 1 | 5/10 | 14.8 ± 3.5 | 6/10 | 12.6 ± 3.5 | 5/10 | 16.6 ± 3.1 | 5/10 | 13.1 ± 3.3 |
| 2 | 6/10 | 13.6 ± 2.2 | 6/10 | 13.8 ± 2.6 | 6/10 | 15.3 ± 3.2 | 5/10 | 12.8 ± 2.7 |
| 3 | 5/10 | 14.1 ± 2.8 | 5/10 | 13.6 ± 3.2 | 6/10 | 15.8 ± 3.4 | 5/10 | 14.1 ± 3.2 |
One million neurosphere cells were injected directly into each human bone fragment in SCID mice, and BM cells were harvested from each bone fragment 4 months later. One million harvested BM cells were then injected into each human graft in the SCID-hu mice, including bone and thy/liv mice. Hematopoietic reconstitution derived from human fetal neurosphere cells was determined by flow cytometry in these SCID-hu mice 4 months later. The frequency of hematopoietic reconstitution is presented as the total number of hematopoietic-reconstituted animals divided by the total number of animals used in the experiments. The percentage of neurosphere-derived hematopoietic cells represents the mean ± SD of all hematopoietic-reconstituted animals from the same experiments. These data are compiled from 3 independent experiments using different donor brain samples.
Discussion
Hematopoietic stem and progenitor cells from BM, growth factor mobilized peripheral blood (MPB), and umbilical cord blood (UCB) have been successfully transplanted into patients with a variety of malignant and nonmalignant diseases. These procedures, however, are not without limitations. In some cases, patients do not mobilize sufficient stem cells to meet minimum cell requirements for transplantation. In the case of UCB, the number of primitive hematopoietic cells in an average harvest is probably insufficient for transplantation into most adults.34 There is, therefore, a need for strategies to provide ex vivo–expanded stem and progenitor cell populations for transplantation. Lessons learned over the past 10 years from investigations focused on developing optimal ex vivo stem cell expansion systems have contributed to a much better understanding of stem cell biology. However, ex vivo stem cell expansion technology is not yet ready for prime time in the clinic for replacement therapy.35,36 NSCs can be easily isolated from both embryonic and adult mouse brains15-19 and human adult brains,28-31 and propagated in vitro for prolonged periods under simple conditions (they require only serum-free medium and either EGF or FGF-2).15-19 28-31 If NSCs from human brains possess in vivo hematopoietic potential as shown in the mouse system, human NSCs might truly represent a feasible source of stem cells for transplantation to overcome the limitations associated with HSCT.
In the present study, we have demonstrated that NSCs in response to EGF and FGF-2 are present in human fetal brain tissues (Table 1). However, the frequency of those NSCs in the human fetal brain tissues (0.35%-0.45%) is about 2- to 3-fold less than in the mouse system.15-19 Human fetal brain tissues contain separate but overlapping EGF- and FGF-2–responsive NSCs, which is consistent with what has been reported in the mouse system (Table1).33,37 The likely interpretation of this lower frequency of NSCs in human fetal brain tissues is that we have used brain tissue fragments containing cerebral cortex, SVZ, and hippocampus instead of only SVZ in our experiments. To assure that these EGF- and/or FGF-2–generated NSCs derived from human fetal brain tissues are truly NSCs, we have further shown that these NSCs express characteristic neural stem/progenitor cell markers such as nestin, EGFR, and FGFR1 (Figure 1), and can be maintained and expanded in vitro for many passages and still sustain their proliferative (Table 2) and multilineage potential (Figure 3; Tables 3 and 4). Because our goal in this study is to determine if cultured neurosphere cells from human brain tissues possess hematopoietic potential as it has been reported for murine neurospheres, it is clear that we need to rule out the possibility that there is a contamination of human hematopoietic cells in the neurospheres and that these contaminating hematopoietic cells, instead of cultured human neurospheres, might contribute to the presence of human hematopoietic cells in the experimental systems. Two pan-hematopoietic cell markers, CD45 and HLA-A, -B, -C, were used to detect the content of human hematopoietic cells in the initial human fetal brain cell preparations, and human neurospheres derived from various culture conditions, passages, and donors. Our data show that there is a minor fraction (< 0.5%) of cells expressing CD45 or HLA-A, -B, -C markers only in the initial human fetal brain cell preparations, but not in any of neurospheres, regardless of culture conditions, passages, and donors (Figure 3). However, the expression level of CD45 or HLA-A, -B, -C on these cells is about 2 logs lower than their expression levels on human BM cells (Figure 3). At the present time, we do not yet know (1) if these cells are truly hematopoietic cells; (2) if these cells are hematopoietic cells, why the expression of CD45 or HLA-A, -B, -C on these cells is 2 logs lower than the normal BM cells; and (3) if these cells are not hematopoietic cells, what is the origin of these cells. Our results from flow cytometric analyses demonstrate that there is no detectable fraction of cells in any cultured neurosphere sample expressing any of the following hematopoietic markers: CD45; HLA-A, -B, -C; CD34; CD38; thy-1; CD3; CD4; CD8; CD10; CD19; CD20; CD13; CD15; and CD33. Our results from in vitro coculturing of neurosphere cells with stromal cells (either 5000 or 1 000 000 cells per well) show that there are no detectable human hematopoietic cells in any of these cultures. Based on our extensive experience with this stromal coculture system,24,25 we have shown that this coculture system is able to support the growth and expansion of all hematopoietic cells. Because there are no detectable human hematopoietic cells in any of the wells derived from neurosphere cells (either initiated with 5000 or 1 000 000 cells per well) in this coculture system, it is clear that there is no contamination of human hematopoietic cells in cultured human neurospheres, which is consistent with our data from flow cytometric analyses of human neurosphere cells. From these results, we can conclude that there is no contamination of any human hematopoietic cells in any of these neurosphere samples, and that the stromal coculture system is not sufficient to provide all necessary signals to drive the human neurosphere cells to differentiate into hematopoietic cells in vitro. Finally, we have established, for the first time, that NSCs from the human fetal brain possess a human BM–dependent in vivo hematopoietic potential (Figure 4, Figure 5, and Table 5). With the injection of one million BM cells derived from NSC-reconstituted bone fragments in SCID mice per human graft, about 50% of animals were reconstituted (Table 5). Because cultured neurosphere cell–derived BM cells are capable of establishing long-term hematopoietic reconstitution in the secondary recipients of SCID-hu mice, these results suggest that cultured neurosphere cells can commit and differentiate into hematopoietic stem cells in intact human BM in SCID-hu mice. Extrapolated from our previous limiting dilution experiments in SCID-hu mice with purified human hematopoietic stem cells,24 25 the in vivo hematopoietic reconstitution activity with one million harvested BM cells derived from NSC-reconstituted human bone fragments in SCID mice (50% reconstitution rate) is equivalent to 5000 transplantable human hematopoietic stem cells.
It was recently shown that adult mouse NSCs adopted a hematopoietic fate after transplantation into irradiated hosts.12 To control for successful repopulation of the BM in sublethally irrradiated recipient mice, the transplantation of adult clonal NSCs was compared with that of bulk NSCs from the embryonic and adult brain, freshly-isolated BM cells from ROSA 26 mice, which are transgenic forlacZ and therefore express β-galactosidase in all tissues.12 In all cases, systemic injection revealed a repopulation of the BM with a variety of myeloid, lymphoid, and early hematopoietic cells.12 One difference between the transplantation of BM cells and that of NSCs was the longer time period (3 weeks) needed for repopulation by NSCs.12 This result suggested that NSCs might undergo additional steps of fate determination, differentiation, and maturation with respect to BM cells to produce hematopoietic progeny. However, the signals that direct NSCs to differentiate into hematopoietic cells are not known.12Our study extends the observation of this previous report in the mouse system12 and demonstrates that NSCs from human fetal brains do possess in vivo hematopoietic potential. Furthermore, our study demonstrates, for the first time, that an intact human BM microenvironment is a prerequisite for directing NSCs to commit and differentiate into hematopoietic lineages in vivo. There are 2 possible explanations for how an NSC might adopt a hematopoietic fate. The cells of origin could be more primitive than the pluripotent NSCs, closer to totipotent embryonic stem cells, somatic stem cells, or primordial germ cells.38 Such primitive stem cells from both mice and humans can be propagated in vitro and differentiate into a variety of cell types, including neural and hematopoietic lineages. Alternatively, lineage-defined progenitor cells in adult tissues may be more plastic than hitherto thought. They might have the capacity to de-differentiate, or be reprogrammed, becoming totipotent stem cells.39 Hematopoietic reconstitution in the SCID-hu mice with clonally derived and retroviral insertion marked human NSCs will permit the elucidation of the nature of those neurohematopoietic stem cells and the signals in the BM microenvironment that direct the neurohematopoietic stem cells to commit to the hematopoietic system.
Given the fact that human NSCs can be continuously expanded for extended periods of time while maintaining their proliferative and multilineage potential,40-42 human NSCs have obvious potential for neural regeneration and the treatment of neural disease.43-45 Because human NSCs also possess in vivo hematopoietic potential (this study), human NSCs may also provide a practical source of cells that could be used in treatments aimed at hematopoietic reconstitution in various blood diseases and disorders.46 Recently, another report demonstrated that NSCs from the adult mouse brain can contribute to the formation of chimeric chick and mouse embryos and give rise to cells of all germ layers.47 Taken together, results from those 2 previous studies12 47 and this study demonstrate that NSCs have a very broad developmental capacity and may potentially be used to generate a variety of cell types for transplantation for diseases other than various neural and blood disorders.
We thank Drs Michael Barish, Paul Salvaterra, and David DiGiusto for review of the manuscript; Drs Jeffrey Longmate and Joycelynne Palmer for their assistance in statistical analysis; Lucy Brown and Jim Bolen for assistance in FACS analysis; supportive team members in the Department of Molecular Biology at Beckman Research Institute at City of Hope for their administrative assistance; and members in the Animal Research Center at City of Hope for their assistance in animal care.
Supported by grants NCI PPG CA 30206, NCI CA 33572, and NCI CA 71866 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Chu-Chih Shih, Division of Hematology/Bone Marrow Transplantation, City of Hope National Medical Center, and Department of Molecular Biology, Beckman Research Institute at the City of Hope, 1500 East Duarte Rd, Duarte, CA 91010-3000; e-mail:cshih@coh.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal