Abstract
Baseline platelet production is dependent on thrombopoietin (TPO). TPO is constitutively produced and primarily regulated by receptor-mediated uptake by platelets. Inflammatory thrombocytosis is thought to be related to increased interleukin-6 (IL-6) levels. To address whether IL-6 might act through TPO to increase platelet counts, TPO was neutralized in vivo in C57BL/10 mice treated with IL-6, and hepatic TPO mRNA expression and TPO plasma levels were studied. Transcriptional regulation of TPO mRNA was studied in the hepatoblastoma cell line HepG2. Furthermore, TPO plasma levels were determined in IL-6–treated cancer patients. It is shown that IL-6–induced thrombocytosis in C57BL/10 mice is accompanied by enhanced hepatic TPO mRNA expression and elevated TPO plasma levels. Administration of IL-6 to cancer patients results in a corresponding increase in TPO plasma levels. IL-6 enhances TPO mRNA transcription in HepG2 cells. IL-6–induced thrombocytosis can be abrogated by neutralization of TPO, suggesting that IL-6 induces thrombocytosis through TPO. A novel pathway of TPO regulation by the inflammatory mediator IL-6 is proposed, indicating that the number of platelets by themselves might not be the sole determinant of circulating TPO levels and thus of thrombopoiesis. This regulatory pathway might be of relevance for the understanding of reactive thrombocytosis.
Introduction
Thrombocytosis can be classified into primary and secondary forms. Whereas primary thrombocytosis is observed in myeloproliferative syndromes, secondary or reactive thrombocytosis is noted in numerous clinical situations,1 especially in association with inflammatory states of either infectious or noninfectious origin such as trauma and malignancy.2 3 The extent to which mediators of the immune or hematopoietic system are involved in the regulation of the circulating platelet count in these conditions is insufficiently understood.
Interleukin-6 (IL-6) plays a prominent role in inflammatory and neoplastic diseases.4-6 Accordingly, mice deficient in IL-6 show a severely impaired acute-phase response.7Administration of IL-6 to humans has been associated with an increase in circulating platelet counts.8-14 Furthermore, serum levels of IL-6 were significantly higher in patients with reactive thrombocytosis than in control patients.15 16 Whether the thrombopoietic effect of IL-6 in vivo is caused by direct stimulation of hematopoietic progenitor cells or is indirectly mediated is unknown.
Thrombopoietin (TPO), the ligand of the c-mplproto-oncogene, is the primary regulator of proliferation and differentiation of megakaryocyte progenitors.17-23 Mice rendered deficient in TPO or TPO receptor by gene targeting show severe thrombocytopenia, with platelet counts reduced by approximately 90%.22,24 Treatment of mice, nonhuman primates, and humans with recombinant TPO or recombinant megakaryocyte growth and development factor, which constitutes a truncated, biologically active form of TPO, results in significant increases in platelet counts.22,25-27 Receptor-mediated uptake of constitutively synthesized TPO by platelets is recognized as the predominant regulatory mechanism of TPO plasma levels and subsequent platelet production.28-30 Because inflammatory states and malignant diseases are often associated with elevated IL-6 levels and thrombocytosis, we hypothesized that these effects might be mediated through an IL-6–induced increase in TPO levels.
Patients, materials, and methods
Interleukin-6 clinical trial
Patients with advanced malignancies who participated in this study were treated at the New England Medical Center (Boston, MA) in accordance with phase 1 and 2 trials that were developed by the Cytokine Working Group. Eligible patients had measurable or evaluable, locally advanced or metastatic cancer that progressed or failed to respond to available curative or palliative therapy. Any prior therapy was permitted if completed more than 4 weeks before IL-6 therapy (6 weeks for nitrosoureas or biologicals). Patients received 30 mg/kg per day IL-6 (Novartis, East Hanover, NJ) intravenously for 5 consecutive days as a 120-hour continuous infusion. Blood samples were collected immediately before and 1, 2, 4, 8, 24, 48, 72, 96, and 120 hours after the start of therapy. Blood samples were collected in EDTA-containing tubes. Samples were centrifuged within 20 minutes of venipuncture at 2000g for 10 minutes, and the plasma was stored at −70°C until assayed. The clinical study was approved by the Human Investigation Review Committee at the New England Medical Center–Tufts University School of Medicine (Boston, MA), and all study participants gave their written, informed consent.
Mice
Male C57BL/10 mice 10 to 12 weeks old, each weighing 25 to 30 g, were obtained from Harlan-Winkelmann (Borchen, Germany). Animals were housed under standard conditions at the animal center of the University of Innsbruck and were fed with rat chow and water ad libitum before and after treatment. All procedures were carried out in adherence with the Principles of Laboratory Animal Care and the Guide to the Care and Use of Laboratory Animals (National Institutes of Health publication no. 86-23; revised 1985). Mice received 1 μg mIL-6 (dissolved in 150 μL phosphate-buffered saline (PBS)–0.5% bovine serum albumin (BSA); endotoxin level 0.18 EU/μg; Peprotech, London, United Kingdom) intraperitoneally (i.p.) every 12 hours for 6 consecutive days. Control mice were injected with 150 μL PBS–0.5% BSA. In neutralization experiments, mice additionally received 500 μg affinity-purified rabbit anti-TPO polyclonal Ab (pAb; Biodesign, Kennebunk, ME) in 500 μL PBS–0.5% BSA i.p. on day 1 concomitantly with the first dose of mIL-6. The respective control mice received 500 μg rabbit immunoglobulin G (rIgG; Sigma, Vienna, Austria). Mouse blood was obtained by retro-orbital bleeding. One hundred microliters venous blood was drawn and collected in EDTA-containing tubes before the first administration of mIL-6 on day 1 and then on days 3, 5, 7, 9, and 11. For the study of TPO expression in the liver, mice received 4 doses of 1 μg mIL-6 or PBS–0.5% BSA i.p. every 12 hours. After another 12 hours, animals were killed and total RNA was extracted from the liver as described below.
Cell culture
The hepatoma cell line HepG2 was obtained from the American Type Culture Collection (ATCC, Rockville, MD). Cells were grown in 75-cm2 cell culture flasks and were split once weekly. New medium was added twice weekly. The culture medium used was RPMI 1640 (Schoeller Pharma, Vienna, Austria) supplemented with 10% heat-inactivated (30 minutes, 56°C) fetal calf serum (Gibco Life Technologies, Vienna, Austria), penicillin, and streptomycin (both from Gibco). For Northern blot analysis, cells were harvested, seeded in 6-well plates, grown for 2 days, subsequently intensely washed with PBS, and further cultured for the indicated period in RPMI 1640 with various concentrations of human (hu) IL-6. For nuclear run-off transcription assays, confluent HepG2 cells were stimulated with 1 ng/mL huIL-6 in 75-cm2 tissue culture flasks for 24 hours. Nuclei were then prepared as described below.
Thrombopoietin enzyme-linked immunosorbent assay
Human TPO was detected with a sandwich enzyme-linked immunosorbent assay (ELISA) system, which uses chimeric mpl-IgG for capture and biotinylated rabbit anti-TPO antibody for detection.31 The lower detection limit of the assay was 80 pg/mL. Murine TPO was detected by a commercially available ELISA system (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The lower detection limit of this assay was 20 pg/mL.
Northern blot analysis
Total RNA was isolated from adherent HepG2 cells by the guanidinium isothiocyanate phenol chloroform extraction method (RNA Clean; Hybaid-AGS, Heidelberg, Germany), and 10 μg RNA was gel-electrophoresed and blotted onto nylon membranes as described.32 For the detection of mRNA in C57BL/10 mice, livers were homogenized and total RNA was extracted by the guanidinium–isothiocyanate phenol chloroform extraction method (RNA Clean; Hybaid-AGS). By subjecting total RNA to poly-T–coated polystyrene-latex particles according to manufacturer's instructions (Qiagen, Hilden, Germany), poly-A+ messenger RNA was purified, and 0.5 μg mRNA was gel-electrophoresed and blotted onto nylon membranes. Murine and human TPO cDNAs were obtained by specific reverse transcription–polymerase chain reaction (RT-PCR). RT was performed from mRNA of mouse liver and HepG2 cells, respectively, by Superscript II reverse transcriptase (Gibco) using a random hexanucleotide mix (Roche, Basel, Switzerland). Murine TPO cDNA PCR was carried out with Hot Star Taq polymerase (Qiagen) using 10 mM dNTPs (Amresco, Solon, OH), 15 pM each sense and antisense primer in 30 cycles of 95°C, 55°C, and 72°C for 1 minute each in a total volume of 50 μL. Human TPO cDNA PCR was carried out identically at 95°C, 59°C, and 72°C. Primers were as follows: human TPO, 5′ TCT GCT GGA GGG AGT GAT GG and 3′ GTG GGC AAG GTG GGT GGA AG; murine TPO, 5′ CGG ACC TGT GAA TGG AAC TC and 3′ GCT AGC TGC TCT GAT GAA TA. Specific PCR products were purified using NucleoSpin Extract (Macherey-Nagel, Düren, Germany). The probes were radioactively labeled with [32P]dCTP using the random primed labeling method according to the manufacturer's (Roche) instructions and hybridized as described.32 Control hybridizations were performed with β-actin to ensure equal loading of RNA.
Nuclear run-off transcription
Nuclei of HepG2 cells left untreated or incubated with 1 ng/mL huIL-6 were prepared after 24 hours of stimulation. Purification of nuclei and in vitro transcription were performed as described.33 Briefly, nuclei were isolated from 1 × 107 cells with NP-40 lysis buffer containing 10 mM Tris-HCl, pH 7.4, 10 mM NaCl, 3 mM MgCl2, and 0.5% NP-40 and were stored in 50 mM Tris-HCl, pH 8.3, 40% glycerol, 5 mM MgCl2, and 5 mM EDTA. For in vitro transcription, α[32P]UTP (3.7 MBq [100 μCi]/1 × 107nuclei) was used. cDNAs for huTPO and β-actin were prepared as described above and were spotted onto Duralon-UV nylon membranes (Stratagene, La Jolla, CA) using a dot blot (500 ng/dot) apparatus and bound by UV cross-linking. Freshly transcribed32P-labeled RNA was hybridized to membranes for 24 hours at 65°C, applying equal amounts of TCA-precipitable counts. Filters were then washed twice in 2 × SSC at 65°C for 30 minutes, treated with RNase A, washed in 2 × SSC, and exposed to storage phosphor screens for 24 hours. Scanning of screens was performed with a Cyclone PhosphorImager (Packard Instrument, Meriden, CT). Individual band intensities were quantified with Optiquant software (Packard Instrument) and were expressed in arbitrary units as counts × mm−2 [huTPO]/counts × mm−2[β-actin].
Statistical analysis
Differences in TPO plasma levels between the various time points were tested with the Mann-Whitney U test. Platelet data were analyzed by analysis of variance, and significance within groups was subsequently assessed by paired Student t test. Data are presented as mean ± SEM.
Results
IL-6 administration increases TPO plasma levels in patients with cancer
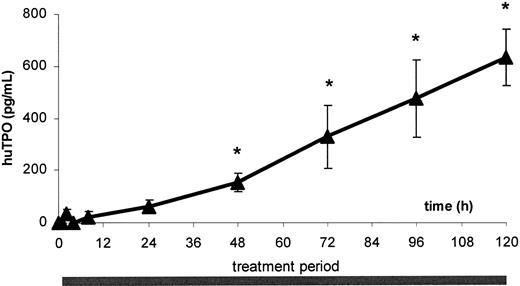
Six patients treated with IL-6 were studied. Pretreatment TPO levels in these patients were below the detection limit of the assay in all 6 patients. TPO levels showed a slight but significant increase within 48 hours of initiating therapy (152 ± 35 pg/mL;P < .05 compared with pretreatment levels). Thereafter, levels increased progressively throughout the treatment period without ever reaching a plateau and were highest on day 5 of therapy (630 ± 110 pg/mL; P < .001 compared with pretreatment levels [Figure 1]).
Plasma TPO levels in IL-6–treated cancer patients.
Circulating TPO levels in patients during 5-day continuous IL-6 infusion. Patients received 30 mg/kg per day rhIL-6. Venous blood was drawn, and plasma was analyzed for huTPO synthesis by specific ELISA. Data are expressed as mean ± SEM for n = 6. *P < .05 compared to baseline levels.
Plasma TPO levels in IL-6–treated cancer patients.
Circulating TPO levels in patients during 5-day continuous IL-6 infusion. Patients received 30 mg/kg per day rhIL-6. Venous blood was drawn, and plasma was analyzed for huTPO synthesis by specific ELISA. Data are expressed as mean ± SEM for n = 6. *P < .05 compared to baseline levels.
Interleukin-6 induces TPO mRNA and protein synthesis in C57BL/10 mice
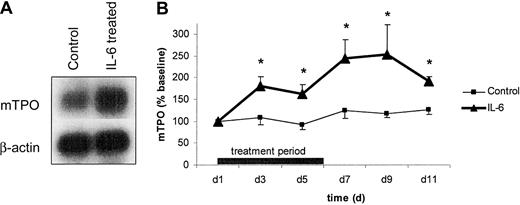
As outlined in Figure 2A, 4 injections of 1 μg mIL-6 given at 12-hour intervals resulted in enhanced TPO mRNA expression in the liver when compared to control animals (treated with PBS–0.5% BSA). In subsequent experiments, mice received 1 μg mIL-6 twice daily for 6 consecutive days. Plasma TPO was determined before the first mIL-6 administration (day 1) and on days 3, 5, 7, 9, and 11. Plasma TPO levels increased by day 3 and peaked on day 9 with a more than 2.5-fold increase compared to baseline (Figure 2B). On day 11, TPO levels started to decrease as shown in Figure 2B. Control mice receiving PBS–0.5% BSA did not show any significant change in TPO levels throughout the experiment (Figure2B).
TPO mRNA expression and TPO plasma levels in IL-6–treated mice.
(A) To evaluate the in vivo effect of IL-6 on TPO mRNA, mice were treated with 4 doses of 1 μg mIL-6 (IL-6) or PBS–0.5% BSA (Control) at 12-hour intervals. Twelve hours after the last dose, messenger RNA was prepared from the liver, gel-electrophoresed, blotted onto nylon membranes, and hybridized with radioactively labeled mTPO and β-actin cDNA. The figure is representative of 3 independent experiments. (B) Plasma TPO in IL-6–treated mice. Mice were treated with 1 μg mIL-6 (▴) or PBS–0.5% BSA (■) at 12-hour intervals for 6 consecutive days. Venous blood was drawn, and plasma was analyzed for mTPO synthesis by specific ELISA. Data are presented as percentage change compared to baseline (control group, 734 ± 90 pg/mL; IL-6 group, 571 ± 77 pg/mL) for n = 6 per group. *P < .05 compared to baseline.
TPO mRNA expression and TPO plasma levels in IL-6–treated mice.
(A) To evaluate the in vivo effect of IL-6 on TPO mRNA, mice were treated with 4 doses of 1 μg mIL-6 (IL-6) or PBS–0.5% BSA (Control) at 12-hour intervals. Twelve hours after the last dose, messenger RNA was prepared from the liver, gel-electrophoresed, blotted onto nylon membranes, and hybridized with radioactively labeled mTPO and β-actin cDNA. The figure is representative of 3 independent experiments. (B) Plasma TPO in IL-6–treated mice. Mice were treated with 1 μg mIL-6 (▴) or PBS–0.5% BSA (■) at 12-hour intervals for 6 consecutive days. Venous blood was drawn, and plasma was analyzed for mTPO synthesis by specific ELISA. Data are presented as percentage change compared to baseline (control group, 734 ± 90 pg/mL; IL-6 group, 571 ± 77 pg/mL) for n = 6 per group. *P < .05 compared to baseline.
Interleukin-6 induces TPO mRNA expression in HepG2 cells
As depicted in Figure 3A, incubation of the hepatoblastoma cell line HepG2 with various concentrations of mIL-6 for 12 hours led to a dose-dependent increase in TPO mRNA steady-state levels as determined by Northern hybridization. To test the extent to which this increase in TPO mRNA steady-state levels resulted from transcriptional induction, nuclear run-off transcription assays were performed. HepG2 cells were either left untreated or were stimulated with 1 ng/mL huIL-6 for 24 hours. Subsequently, nuclei were prepared, and in vitro transcription was performed with [32P]UTP. Radioactively labeled RNA was allowed to hybridize to huTPO cDNAs blotted onto nylon membranes. Hybridization to the housekeeping gene β-actin was performed for standardization. As depicted in Figure 3B, stimulation with IL-6 resulted in a modest increase in TPO mRNA transcription.
TPO mRNA expression in IL-6–treated HepG2 cells.
(A) To determine the effect of IL-6 on TPO mRNA levels in vitro, HepG2 cells were cultured with increasing concentrations of huIL-6 for 12 hours, total RNA extracted, gel-electrophoresed, blotted onto nylon membranes, and hybridized with radioactively labeled huTPO and β-actin cDNA. The figure is representative of 3 independent experiments. (B) Nuclear run-off transcription in IL-6–treated HepG2 cells. Nuclei of HepG2 cells cultured with huIL-6 (1 ng/mL) or left untreated were prepared after 24 hours of stimulation. Purification of nuclei and in vitro transcription were performed as described in “Materials and methods.” The left panel shows a representative hybridization. Densitometric evaluation of the respective experiment is presented in the right panel in arbitrary units as counts × mm−2 [huTPO]/counts × mm−2[β-actin].
TPO mRNA expression in IL-6–treated HepG2 cells.
(A) To determine the effect of IL-6 on TPO mRNA levels in vitro, HepG2 cells were cultured with increasing concentrations of huIL-6 for 12 hours, total RNA extracted, gel-electrophoresed, blotted onto nylon membranes, and hybridized with radioactively labeled huTPO and β-actin cDNA. The figure is representative of 3 independent experiments. (B) Nuclear run-off transcription in IL-6–treated HepG2 cells. Nuclei of HepG2 cells cultured with huIL-6 (1 ng/mL) or left untreated were prepared after 24 hours of stimulation. Purification of nuclei and in vitro transcription were performed as described in “Materials and methods.” The left panel shows a representative hybridization. Densitometric evaluation of the respective experiment is presented in the right panel in arbitrary units as counts × mm−2 [huTPO]/counts × mm−2[β-actin].
Interleukin-6 administration results in a TPO-dependent increase in platelet counts in C57BL/10 mice
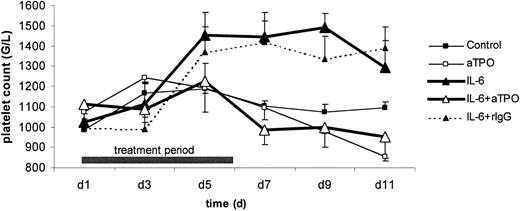
Figure 4 shows platelet counts during the course of IL-6 treatment in mice. Twice daily mIL-6 administration for 6 consecutive days resulted in a continuous increase in circulating platelet counts throughout the studied time period, with a maximum increase of 46% on day 9 (1.492 ± 70 G/L [day 9] vs 1.025 ± 80 G/L [day 1]; P < .05). Twice daily PBS–0.5% BSA administration to control mice had no significant effect (Figure 4). Neutralization of TPO by injection of rabbit anti-TPO pAb before mIL-6 administration abolished the IL-6–induced increase in platelet counts and, in fact, resulted in a slight decrease compared to baseline values (Figure 4). In control experiments, the administration of irrelevant rabbit immunoglobulin (rIgG) instead of anti-TPO pAb to mIL-6–treated mice showed an increase in platelets similar to that in mice treated with mIL-6 alone (Figure 4). Administration of anti-TPO pAb before a 6-day course of PBS–0.5% BSA resulted in a slight progressive decrease in platelet counts, as depicted in Figure4.
Platelet counts in C57BL/10 mice.
Mice were treated with 1 μg mIL-6 (▴) or PBS–0.5% BSA (■) every 12 hours for 6 consecutive days. Additionally, mice were injected with 500 μg rabbit anti-TPO pAb (open symbols) or irrelevant control rabbit IgG (filled symbols, dashed line). Venous blood was drawn on day 1 (pretreatment) and every second day thereafter and was analyzed for platelet counts. Data are expressed as mean ± SEM for n = 3 per group. The increase in platelet counts in mIL-6–treated animals was significant (P < .05), as were the differences between mIL-6 and mIL-6 plus anti-TPO pAb-treated mice (P < .05).
Platelet counts in C57BL/10 mice.
Mice were treated with 1 μg mIL-6 (▴) or PBS–0.5% BSA (■) every 12 hours for 6 consecutive days. Additionally, mice were injected with 500 μg rabbit anti-TPO pAb (open symbols) or irrelevant control rabbit IgG (filled symbols, dashed line). Venous blood was drawn on day 1 (pretreatment) and every second day thereafter and was analyzed for platelet counts. Data are expressed as mean ± SEM for n = 3 per group. The increase in platelet counts in mIL-6–treated animals was significant (P < .05), as were the differences between mIL-6 and mIL-6 plus anti-TPO pAb-treated mice (P < .05).
Discussion
The regulation of TPO as the predominant determinant of platelet counts has become a topic of recent research interest. It is believed that TPO plasma levels are dependent on the rate of platelet–megakaryocyte TPO receptor-mediated uptake and destruction.28-30,34 When platelet levels are high, an increased amount of TPO is taken up by platelets and megakaryocytes, resulting in a decrease in circulating levels of this cytokine, thereby limiting megakaryocyte production.28-30 For various thrombocytopenic disorders, an inverse correlation between platelet counts and plasma TPO levels has been observed.31Furthermore, an inverse correlation between platelet counts and TPO mRNA levels in the bone marrow of mice has been reported, yet no such regulation of TPO mRNA levels was noted in the liver and kidneys.34,35 This suggests that TPO might additionally be regulated at the transcriptional level by feedback control with some kind of sensing mechanism for circulating platelets.22,34,35 Platelet-derived transforming growth factor TGF-β1 has been suggested as such a feedback regulator of TPO synthesis in the bone marrow.36 In any case, the circulating platelet count is regarded as the sole determinant of TPO level.23 TPO mRNA expression has been reported in the liver, kidney, bone marrow, and spleen.37The liver has been shown to be the primary site of constitutive TPO production by tissue-specific knockout experiments.38Recently, this view has been further substantiated by linking insufficient TPO production to thrombocytopenia in end-stage liver disease and its reversal by orthotopic liver transplantation.39
In this paper, we suggest a novel pathway of TPO regulation that might be operative in inflammatory conditions. We demonstrate that the administration of IL-6 to C57BL/10 mice results in increased TPO mRNA steady-state levels in the liver, accompanied by increased TPO plasma levels. Furthermore, IL-6 administration results in a substantial elevation in platelet counts. Neutralization of TPO by i.p. injection of anti-TPO pAb abrogates platelet elevation in IL-6–treated mice, suggesting that this property of IL-6 might be mediated through the induction of TPO.
From an experimental point of view, several issues should be addressed regarding the experimental protocol chosen: Alternatively to the pathway proposed, TPO neutralization could result in a developmental block of megakaryocyte progenitors, leading to a reduction in the number of megakaryocytes IL-6 could directly act on. To exclude this possibility, we enumerated megakaryocytes in the bone marrow on day 11 after anti-TPO pAb administration and found no significant decrease but did find a slight increase of megakaryocytes (data not shown). Another issue regards repeated phlebotomy performed in our protocol, which might be interpreted as ongoing bleeding—a known stimulus of thrombocytosis. It is unlikely that the latter contributed to thrombocytosis in our model. First, because all treatment groups were phlebotomized in parallel, one would expect a rise in the control group as well, which was not the case. Second, the increase in liver TPO mRNA steady-state levels after IL-6 administration was noted in mice that were not phlebotomized at all. Third, an increase in TPO mRNA upon bleeding would be expected in the bone marrow36 because no such regulation has so far been observed in the liver.
In apparent contrast to our data are findings published by Carver-Moore et al,40 who demonstrated megakaryopoietic activity of IL-6 in TPO and c-mpl gene knockout mice. In that study the absolute increase in platelets in IL-6–treated TPO−/−mice was equivalent to approximately 7% of the platelet count of untreated wild-type mice.40 In contrast, we demonstrated an increase in platelet counts of 46% of baseline value in IL-6–treated wild-type mice. Our data on TPO neutralization in IL-6–treated mice indicated that the latter pathway might account for most of the increase in platelets. However, it is conceivable that IL-6 could exert potent direct effects on a subpopulation of megakaryocytes—eg, those developing independently of TPO andc-mpl.40-42 In this context it should be noted that only a minority of megakaryocyte progenitor cells express IL-6 receptor. Sui et al43 recently demonstrated that soluble IL-6 receptor/IL-6 complexes associate with glycoprotein (gp) 130 expressed on CD34+ marrow progenitor cells, allowing IL-6 to act on megakaryocyte progenitors directly. In contrast, Kaushansky et al44 reported that production of murine megakaryocytes in vitro in response to IL-6 is eliminated by neutralizing the biologic activity of TPO, suggesting that the effect of IL-6 is indirect. We now demonstrate in vivo that neutralizing the biologic activity of TPO in IL-6–treated mice results in the abolishment of thrombocytosis. This effect is specific for TPO because the injection of rabbit control IgG had no effect. Thus, we provide evidence that IL-6 increases platelets primarily through the induction of TPO, whereas direct IL-6 action on megakaryocyte progenitors might account only for minor effects in vivo.
We demonstrated that IL-6 dose-dependently increases TPO mRNA steady-state levels in the hepatoblastoma cell line HepG2. This is in accordance with a recent report showing enhanced TPO mRNA expression and protein secretion in IL-6–treated HepG2 and Hep3B cells.45 We furthermore provide evidence that the increase in TPO mRNA expression might be transcriptionally regulated. However, it should be noted that the increase in TPO mRNA transcription in nuclear run-off assays is modest; thus we cannot exclude the possibility that post-transcriptional effects have some role in the enhancement of TPO mRNA steady-state levels in IL-6–stimulated HepG2.46 The binding of Ets family transcription factors to the sequence 5′-ACTTCCG-3′ in the human TPO promotor has been implicated in the expression of the TPO gene in the liver.47 IL-6 has been shown to rapidly induce DNA-binding activity to the ets motif of the junB promotor,48 49 which suggests that an Ets family transcription factor might be involved in the enhancement of TPO gene expression by IL-6.
Although frequently encountered in clinical practice,2 the exact biochemical mechanisms underlying inflammatory, autoimmune, and neoplastic thrombocytosis are unknown. Several lines of evidence support a decisive role for IL-615,50: IL-6 serum levels are elevated in patients with reactive thrombocytosis compared to levels in healthy controls15,16,51,52; IL-6 knockout mice show a severely impaired acute-phase response7; and transgenic overexpression of IL-64 and administration of recombinant IL-6 to mice,4,53 primates,14,54and humans8-14 results in an increase in the circulating platelet count. In consideration of data presented in this paper, we propose that IL-6 mediates reactive thrombocytosis primarily through TPO. Cerutti et al55 recently demonstrated that circulating TPO behaves like an acute-phase reactant in reactive conditions. They showed that following hip-replacement surgery, the increase in TPO serum levels—correlating with IL-6—preceded the peak in platelet counts by 11 days. Accordingly, Heits et al51,52 recently reported concomitant elevations of IL-6 and TPO plasma levels accompanied by elevated platelet counts in patients with inflammatory bowel disease51 and solid tumors.52 We show that the administration of IL-6 to patients with cancer results in a substantial increase in TPO plasma levels. It has previously been demonstrated that an increase in platelet counts becomes evident 10 to 14 days after the onset of IL-6 treatment.10 56
Altogether, we present a novel pathway of TPO regulation by the inflammatory mediator IL-6, indicating that the number of megakaryocytes or platelets by themselves might not be the sole determinant of circulating TPO levels and thus of megakaryopoiesis. This regulatory pathway might be of relevance for the understanding of reactive thrombocytosis.
We thank Gloria Y. Meng, Andrea Hebert, and Paul Sims from Genentech for performing the ELISA for human TPO assessment.
Supported by grant P14681 from the Austrian Science Fund (H.T.) and by grant P8833 from the Jubiläumsfonds of the Austrian National Bank (A.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Herbert Tilg, Department of Medicine, University Hospital Innsbruck, Anichstrasse 35, 6020 Innsbruck, Austria; e-mail:herbert.tilg@uibk.ac.at.

![Fig. 3. TPO mRNA expression in IL-6–treated HepG2 cells. / (A) To determine the effect of IL-6 on TPO mRNA levels in vitro, HepG2 cells were cultured with increasing concentrations of huIL-6 for 12 hours, total RNA extracted, gel-electrophoresed, blotted onto nylon membranes, and hybridized with radioactively labeled huTPO and β-actin cDNA. The figure is representative of 3 independent experiments. (B) Nuclear run-off transcription in IL-6–treated HepG2 cells. Nuclei of HepG2 cells cultured with huIL-6 (1 ng/mL) or left untreated were prepared after 24 hours of stimulation. Purification of nuclei and in vitro transcription were performed as described in “Materials and methods.” The left panel shows a representative hybridization. Densitometric evaluation of the respective experiment is presented in the right panel in arbitrary units as counts × mm−2 [huTPO]/counts × mm−2[β-actin].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/9/10.1182_blood.v98.9.2720/4/m_h82111716003.jpeg?Expires=1769094745&Signature=Ul8Sp7iYkWOK~1icWaTsyQOfuqXWq2gZJGnRXOD~rx8r7g3uTXpGcSRaEzvWHhDYeEWi4Ba7~H563gpoe-DTprsuHJdKrlEOarJ~7g4b1NMmVe3yRsT1M0dDPCk8HG155R7Lnvt4flH5bxncXthI45IU3efOWcA2sjA5C6Diqrk-Q7-z1gWd9zqFfw~HqjdDkcKR6i-oRWvtTDhvAJiDR5WB8L~nsq1B6FFtGtqeG95SVjKc0DMrdF5Ix5TQaCL-r77h0YCLBXlApfiSnb2tuEI6E6G7iq3eJHN3RWHr08p~0NOctjGRV6umOcMZlgbe4aE~3MLJKa7mACQLYZMhBg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal