Abstract
Mechanisms involving the in vitro effect of rituximab in cells from 55 patients with B-cell lymphoproliferative disorders were investigated. No cytotoxic effect was observed when cells were incubated with rituximab alone, but in the presence of human AB serum rituximab induced complement-dependent cell death (R-CDC). A cytotoxic effect was observed in cells from 9 of 33 patients with B-cell chronic lymphocytic leukemia, 16 of 16 patients with mantle-cell lymphoma, 4 of 4 patients with follicular lymphoma, and 2 of 2 patients with hairy-cell leukemia. R-CDC was observed in cells from patients expressing more than 50 × 103 CD20 molecules per cell, and directly correlated with the number of CD20 molecules per cell. Preincubation with anti-CD59 increased the cytotoxic effect of rituximab and sensitized cells from nonsensitive cases. Neither cleavage of poly-ADP ribose polymerase (PARP) nor activation of caspase-3 was observed in R-CDC. In addition, no cells with a hypodiploid DNA content were detected and R-CDC was not prevented by a broad-spectrum caspase inhibitor, suggesting a caspase-independent mechanism. Incubation with rituximab in the presence of AB serum induced a rapid and intense production of reactive oxygen species (ROS). R-CDC was blocked by the incubation of cells with N-acetyl-L-cysteine (NAC) or Tiron, 2 ROS scavengers, indicating that the cytotoxic effect was due to the generation of superoxide (O) radicals. In conclusion, the results of the present study suggest that CD20, CD59, and complement have a role in the in vitro cytotoxic effect of rituximab, which is mediated by a caspase-independent process that involves ROS generation.
Introduction
Rituximab is a chimeric monoclonal antibody directed against CD20, an antigen present both on normal B lymphocytes and on cells from most B-cell lymphoproliferative disorders.1 Rituximab is currently employed in the treatment of follicular lymphoma (FL) either alone2,3 or in combination with chemotherapy.4 Moreover, there is an increasing interest in using rituximab in other CD20+ B-cell lymphoproliferative disorders, such as mantle-cell lymphoma (MCL) or B-cell chronic lymphocytic leukemia (B-CLL).5 6 A direct relationship between the clinical efficacy and the intensity of CD20 expression has been proposed, suggesting that patients with high CD20 expression are more likely to respond to rituximab.
The signaling pathways involved in the effect of rituximab are not clearly established. Recently, complement-mediated cell lysis has been proposed as the major and most-efficient effector mechanism of rituximab.1,7 However, the signal transduction pathways activated by complement, which precede cell death, have not been fully determined. Moreover, in vitro studies performed in cell lines suggest that rituximab binding to CD20 could induce apoptosis, mainly through caspase activation,8 but other pathways, such as activation of the Src-family kinases9 and antibody-dependent cell-mediated cytotoxicity (ADCC),1 7have also been described.
Complement-mediated cell lysis involves a cascade activation of proteins leading to the formation of the membrane attack complex, which produces a direct lysis of the target cells. This lysis is regulated by membrane-bound regulatory proteins, among which CD55 and CD59 seem to be the most important. CD55 binds to C3b and C4b and accelerates the decay of C3 and C5 convertases, whereas CD59 binds to C8 and C9 and prevents pore formation by the membrane attack complex,10the final step of complement lysis. According to recent studies, these proteins could be implicated in the cytotoxic effect of rituximab.7 11
The aims of this study were to correlate the cytotoxic effect of rituximab with CD20 expression in a variety of CD20+ B-cell lymphoid malignancies, and to analyze the signaling pathways involved in complement-dependent cell death (CDC), focusing on complement regulatory proteins.
Patients, materials, and methods
Patients
Fifty-five patients (35 men and 20 women) with a median age of 64 years (range, 32 to 88 years) who were diagnosed with B-cell chronic lymphoproliferative disorders (33 B-CLL, 16 MCL, 4 FL, and 2 hairy-cell leukemia [HCL]) were included in the study. The diagnosis was established according to the World Health Organization classification.12 Patients with B-CLL entering this study were selected according to CD20 expression in conventional flow cytometry. All patients were informed of the investigational nature of this study and informed consent was obtained from each patient in accordance with Hospital Clı́nic ethical committee guidelines.
Reagents
Rituximab was obtained from Roche (Hertfordshire, United Kingdom). 4,5-dihydroxy-1,3-benzene disulfonic (Tiron) was obtained from Sigma Chemical (St Louis, MO). N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (Z-VAD.fmk) was obtained from Bachem (Bubendorf, Switzerland). N-acetyl-L-cysteine (NAC) and PP2 were purchased from Calbiochem-Novabiochem (La Jolla, CA). Antibodies against CD20 (Leu 16), CD19-phycoerythrin (PE) (Leu12), and CD3-PE (Leu 4) were obtained from Becton Dickinson (San Jose, CA); against CD59 (clone MEM 43) from CLB (Amsterdam, The Netherlands); and against CD55 (clone Bric216) from Serotec (Oxford, United Kingdom). Antibodies against CD59 (clone 193-27) and CD55 (clone 143-30) were kindly provided by Dr R. Vilella from the Immunology Department of the Hospital Clinic, Barcelona, Spain.
Isolation of cells
Mononuclear cells were isolated from peripheral blood samples by centrifugation on a Ficoll/Hypaque (Seromed, Berlin, Germany) gradient and cryopreserved in liquid nitrogen in the presence of 10% dimethyl sulfoxide (DMSO). Cells from 5 cases (1 B-CLL, 3 MCL, and 1 FL) were obtained from lymph node biopsy or spleen after repetitive infiltration with RPMI 1640 culture medium (GibcoBRL, Paisley, Scotland).
Cell culture
Lymphocytes were cultured immediately after thawing at a concentration of 5 × 106 cells/mL in RPMI 1640 culture medium supplemented with 10% heat inactivated fetal calf serum (BioWhittaker, Verviers, Belgium), 2 mM glutamine, and 0.04 mg/mL gentamicin at 37°C in a humidified atmosphere containing 5% carbon dioxide. Factors were added at the beginning of the culture.
Analysis of cell viability by annexin binding
Exposure of phosphatidylserine residues was quantified by surface annexin V staining as previously described.13Briefly, cells were washed in binding buffer (10 mM HEPES, pH 7.4, 2.5 mM CaCl2, 140 mM NaCl), resuspended in 200 μL and incubated with 0.5 μg/mL annexin V–fluorescein isothiocyanate (FITC) (Bender Medsystems, Vienna, Austria) for 15 minutes in the dark. Cells were washed again and resuspended in binding buffer. A quantity of 5 μg/mL propidium iodide (PI) (Sigma Chemical) was added to each sample prior to flow cytometric analysis (FACScan; Becton Dickinson). Ten thousand cells were acquired per sample using CELLquest software and data were analyzed with the Paint-a-gate Pro software (Becton Dickinson). All experiments were performed in duplicate. In some experiments, cells were labeled simultaneously with annexin V–FITC and anti–CD3-PE, in order to separately analyze the cytotoxic effect on B and T lymphocytes.
Propidium iodide DNA staining
Quantification of apoptosis by PI staining and fluorescence-activated cell sorting (FACS) analysis was performed as previously described.14 Briefly, cells were harvested and fixed in 70% ethanol. Cells were centrifuged, washed in phosphate-buffered saline (PBS) and resuspended in 0.5 mL PBS containing PI (5 μg/mL) and RNase (100μg/mL) (Boehringer Mannheim, Mannheim, Germany). Tubes were incubated for 30 minutes at 37°C and placed at 4°C in the dark overnight prior to flow cytometry analysis.
Assessment of mitochondrial transmembrane potential and reactive oxygen species production
Changes in mitochondrial transmembrane potential (ΔΨm) were evaluated by staining with 1 nM 3,3′-dihexyloxacarbocyanine iodide (DiOC6[3]; Molecular Probes, Eugene, OR). Reactive oxygen species (ROS) production was determined by staining with 2 μM dihydroethidine (DHE; Molecular Probes). Cells were incubated with the dyes for 15 minutes at 37°C, washed, resuspended in PBS and analyzed by flow cytometry. A decrease in the signal of DiOC6[3] (FL1) was indicative of abnormalities in ΔΨm and appearance of FL2 signal was indicative of ROS production. Ten thousand cells were acquired in a FACScan flow cytometer. All experiments were performed in duplicate.
Kinetic studies of ROS generation
Cells were preincubated for 5 minutes with 50 μg/mL rituximab, and cell acquisition in a FACScan was started after incubation with DHE for one minute. AB serum was added after the first minute of cell acquisition and ROS production was recorded for 15 minutes. Kinetics of ROS generation were analyzed using the free software WinMDI 2.8 version (http://archive.uwcm.ac.uk/uwcm/hg/hoy/software.html).
Flow cytometric detection of the active form of caspase-3
Cells were fixed and permeabilized using the Cytofix/Cytoperm kit (Pharmingen, San Diego, CA) for 20 minutes at 4°C, pelleted and washed with Perm/Wash buffer (Pharmingen). Cells were then stained with the polyclonal antibody against the active form of caspase-3 (Pharmingen) (0.25 μg/L × 106 cells) for 20 minutes at room temperature, washed in Perm/Wash buffer, stained with goat anti–rabbit-FITC (SuperTechs, Bethesda, MD), and analyzed in a FACScan.
Quantification of CD20, CD55, and CD59 membrane proteins
Cells from patients or healthy donors (n = 19) were incubated with saturating amounts of monoclonal antibodies against CD20 (Leu16), CD55 (143-30), and CD59 (MEM 43) for 45 minutes at 4°C, washed twice in PBS and incubated with goat anti–mouse-FITC (Dako, Globstrub, Denmark) in the same conditions. The beads of QIFKIT (Dako) were processed in parallel following the manufacturer's recommendations. The mean FL1 channel for every bead population was used to calculate a standard curve and the number of molecules per cell was obtained by interpolation of the mean FL1 channel value for each patient. To quantify the number of complement regulatory proteins on B cells, samples stained with CD55 and CD59 were subsequently incubated with normal mouse serum (Dako) for 5 minutes, followed by CD19-PE monoclonal antibody.
Western blot
Cells were lysed in 80 mM Tris HCl pH 6.8, 2% sodium dodecyl sulfate (SDS), 10% glycerol, 0.1 M dithiothreitol (DTT) and equal amounts of protein were separated by electrophoresis on 12% polyacrylamide gel and transferred to Immobilon-P (Millipore, Bedford, MA) membranes. The membranes were incubated with polyclonal antibody against poly-ADP ribose polymerase (PARP) (Boehringer Mannheim). Antibody binding was detected using a secondary antibody (mouse anti–rabbit immunoglobulin; Dako) conjugated to horseradish peroxidase and an enhanced chemiluminiscence (ECL) detection kit (Amersham, Buckinghamshire, United Kingdom).
Statistical analysis
A case was considered sensitive to rituximab when cell death assessed by exposure of phosphatidylserine residues was more than or equal to 15% of cell death observed in control cells. Comparison of CD20, CD55, and CD59 cell levels between healthy donors and patients, and between sensitive and nonsensitive cases were performed using the nonparametric Mann-Whitney test. The effect of blockage with anti-CD55 and/or anti-CD59 was analyzed by means of linear regression models with random effects using a panel data structure, adjusted by normalized values of CD55 and CD59 molecules per cell, with STATA Statistical Software, Release 6.0 (Stata, College Station, TX).
Results
Rituximab induces cell death of tumor B cells in the presence of complement
To analyze the in vitro effect of rituximab on the viability of tumor cells from patients with B-cell lymphoproliferative disorders, a dose-response study was first performed in cells from 3 patients. No cytotoxic effect was observed when cells were incubated with rituximab alone (with doses ranging from 10 to 100 μg/mL) for increasing periods of time (up to 5 days). However, when normal human AB serum (AB) was added as a source of complement, rituximab produced a cytotoxic effect after 24 hours of incubation. This effect was dose dependent for both rituximab and AB (with quantities ranging from 1% to 40%) and could be detected using doses as low as 10 μg/mL rituximab and 1% AB. A discriminant cytotoxic effect was observed with 50 μg/mL rituximab in the presence of 10% AB. Thus, this was the combination used in all subsequent experiments.
The cytotoxic effect of rituximab in the presence of AB (rituximab+AB) was characterized by a reduction in the forward scatter (FSC) and an increase in the side scatter (SSC), indicative of cell shrinkage (Figure 1A), as well as an exposure of phosphatidylserine residues, as shown by the increase in annexin V–labeled cells (Figure 1B), and loss of ΔΨm, determined by staining with DiOC6[3] (Figure 1C).
Cell death induction by the combination of rituximab and normal human AB serum.
Cells from a representative CD20++ patient with follicular lymphoma (FL) were incubated in the presence or absence of 50 μg/mL rituximab and 10% AB (R+AB) for 24 hours and analyzed by flow cytometry. (A) Flow cytometric plots showing FSC/SSC pattern. (B) Flow cytometric plots showing annexin V binding. (C) Histogram showing mitochondrial transmembrane potential after staining with DiOC6[3].
Cell death induction by the combination of rituximab and normal human AB serum.
Cells from a representative CD20++ patient with follicular lymphoma (FL) were incubated in the presence or absence of 50 μg/mL rituximab and 10% AB (R+AB) for 24 hours and analyzed by flow cytometry. (A) Flow cytometric plots showing FSC/SSC pattern. (B) Flow cytometric plots showing annexin V binding. (C) Histogram showing mitochondrial transmembrane potential after staining with DiOC6[3].
Inactivation of the complement by heating AB at 56°C for 30 minutes completely abolished the cytotoxic effect of rituximab. Incubation of cells with AB alone did not induce cell death but, to the contrary, produced a protective effect when compared with cells incubated with medium alone (data not shown).
The in vitro effect of rituximab+AB was analyzed on cells from 55 patients. In the above-mentioned conditions, R-CDC was observed in cells from 31 of the 55 patients (56%). The distribution of R-CDC among the different lymphoproliferative disorders was as follows: 9 of 33 in B-CLL (27%), 16 of 16 in MCL (100%), 4 of 4 in FL (100%), and 2 of 2 in HCL (100%).
R-CDC was not observed on CD3+ cells from 20 cases as assessed by multiparametric flow cytometry analysis (data not shown), with this indicating that the cytotoxic effect of rituximab was restricted to B cells.
R-CDC depends on CD20 expression
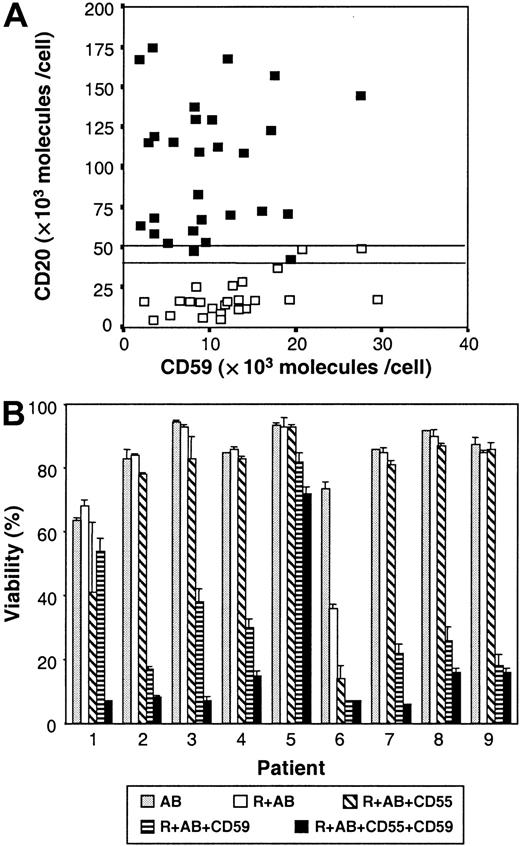
In order to analyze the differences between cases that are sensitive (cytotoxicity ≥ 15%) and nonsensitive (cytotoxicity < 15%) to rituximab+AB, we quantified the expression of the CD20 antigen. As shown in Figure 2A, a significant difference (P < .0001) was found between the CD20 mean values of sensitive cases (100.7 ± 41 × 103molecules per cell; n = 31) and nonsensitive cases (18.7 ± 12 × 103 molecules per cell; n = 24). All the cases expressing more than or equal to 50 × 103molecules of the CD20 antigen on the cell membrane surface (CD20++) (n = 29) showed R-CDC, whereas no cytotoxic effect was observed in cases with CD20 levels less than 40 × 103 molecules per cell (CD20dim) (n = 22). Interestingly, a direct correlation was found between R-CDC and the number of CD20 molecules per cell (r = 0.8,P < .0001) (Figure 2B).
Relationship between CD20 expression and R-CDC.
(A) Histograms of the mean values of the number of CD20 molecules per cell according to the sensitivity to rituximab+AB. (B) Correlation between the number of CD20 molecules per cell and the percentage of R-CDC obtained, in cells from nonsensitive (▪) and sensitive (■) patients.
Relationship between CD20 expression and R-CDC.
(A) Histograms of the mean values of the number of CD20 molecules per cell according to the sensitivity to rituximab+AB. (B) Correlation between the number of CD20 molecules per cell and the percentage of R-CDC obtained, in cells from nonsensitive (▪) and sensitive (■) patients.
No modifications were observed in the FSC/SSC pattern or in annexin V binding when cells from 4 CD20dim cases were incubated with rituximab+AB for longer periods of time (up to 5 days) (data not shown).
As shown in Table 1, the number of CD20 molecules was significantly lower in B-CLL cells than in normal B cells, whereas no difference was observed in the other types of B-lymphoproliferative disorders analyzed. Of note, cells from nonsensitive B-CLL cases had significantly lower CD20 levels than cells from sensitive B-CLL cases.
Quantification of the expression of CD20, CD55, and CD59 in cells from patients with B-lymphoproliferative disorders and healthy donors
| . | n . | Molecules/cell (× 103) . | ||
|---|---|---|---|---|
| CD20 . | CD55 . | CD59 . | ||
| B-CLL | 33 | 31.9 ± 26* | 9.7 ± 4* | 12.1 ± 6 |
| Nonsensitive | 24 | 18.7 ± 12* | 9.4 ± 5* | 12.8 ± 7 |
| Sensitive1-160 | 9 | 67.0 ± 20 | 10.4 ± 4* | 10.3 ± 6 |
| MCL | 16 | 113.2 ± 39 | 11.9 ± 5 | 9.0 ± 6 |
| FL | 4 | 96.0 ± 38 | 10.3 ± 4 | 16.5 ± 10 |
| HCL | 2 | 162.2 ± 7 | 2.1 ± 1 | 9.8 ± 11 |
| Healthy donors | 19 | 94.9 ± 37 | 13.6 ± 29 | 12.2 ± 4 |
| . | n . | Molecules/cell (× 103) . | ||
|---|---|---|---|---|
| CD20 . | CD55 . | CD59 . | ||
| B-CLL | 33 | 31.9 ± 26* | 9.7 ± 4* | 12.1 ± 6 |
| Nonsensitive | 24 | 18.7 ± 12* | 9.4 ± 5* | 12.8 ± 7 |
| Sensitive1-160 | 9 | 67.0 ± 20 | 10.4 ± 4* | 10.3 ± 6 |
| MCL | 16 | 113.2 ± 39 | 11.9 ± 5 | 9.0 ± 6 |
| FL | 4 | 96.0 ± 38 | 10.3 ± 4 | 16.5 ± 10 |
| HCL | 2 | 162.2 ± 7 | 2.1 ± 1 | 9.8 ± 11 |
| Healthy donors | 19 | 94.9 ± 37 | 13.6 ± 29 | 12.2 ± 4 |
P < .02 versus healthy donors.
P < .001 sensitive B-CLL versus nonsensitive B-CLL.
Regulation of R-CDC by CD55 and CD59
To study the role of the complement in R-CDC, we quantified the expression of CD55 and CD59, 2 complement regulatory proteins. The number of CD55 molecules per cell was significantly lower in B-CLL and HCL cells than in normal B cells, whereas no difference was observed in other B-lymphoproliferative disorders (Table 1). Regarding the number of CD59 molecules, only the subgroup of sensitive B-CLL cells expressed lower CD59 molecules per cell than normal B cells. No correlation was found between R-CDC and the number of CD55 or CD59 molecules on the cell surface. Among 4 B-CLL samples that expressed between 40 × 103 molecules per cell and 50 × 103CD20 molecules per cell (CD20+), only 2 of them were sensitive to rituximab. Interestingly, these 2 cases expressed lower CD59 levels than the 2 nonsensitive cases (Figure3A).
Role of CD59 expression in R-CDC.
(A) Expression of CD20 and CD59 in cells from nonsensitive (■) and sensitive (▪) patients. (B) Effect of CD55 and CD59 blockage on R-CDC. Cells from 9 B-CLL patients were incubated for 24 hours with 50 μg/mL rituximab and 10% AB (R+AB) alone or combined with anti-CD55 and/or anti-CD59. Cell viability was determined by annexin V binding as described in “Patients, materials, and methods.”
Role of CD59 expression in R-CDC.
(A) Expression of CD20 and CD59 in cells from nonsensitive (■) and sensitive (▪) patients. (B) Effect of CD55 and CD59 blockage on R-CDC. Cells from 9 B-CLL patients were incubated for 24 hours with 50 μg/mL rituximab and 10% AB (R+AB) alone or combined with anti-CD55 and/or anti-CD59. Cell viability was determined by annexin V binding as described in “Patients, materials, and methods.”
To better understand the role of CD55 and CD59, the effect of rituximab+AB was analyzed in the presence of anti-CD59 and/or anti-CD55 in cells from 9 B-CLL patients (Figure 3B). No cytotoxic effect was observed when cells were incubated with these monoclonal antibodies alone, or combined with AB, indicating that they cannot activate the complement by themselves. The statistical analysis of the mean values obtained for these 9 patients showed that the viability of cells incubated with rituximab+AB was 80 ± 7% (95% confidence interval [CI]: 67-93). The addition of anti-CD59 produced a significant decrease in cell viability by 48 units (95% CI: 38-57;P < .001), whereas addition of anti-CD55 only produced a decrease of 8 units (95% CI: 0-17;P = .078). The combination of the 2 monoclonal antibodies produced an additional decrease in cell viability of 7.4 units (95% CI: −5.7-20.5), this not being significantly different from the decrease observed with CD59 alone (P = .269). Finally, the cytotoxic effect produced when blocking with anti-CD59 directly correlated with the number of CD59 molecules per cell (P < .001).
Thus, in B-cell lymphoproliferative disorders, the cytotoxic effect of rituximab is mediated, at least in part, by the complement and is regulated by the number of CD20 and CD59 molecules per cell.
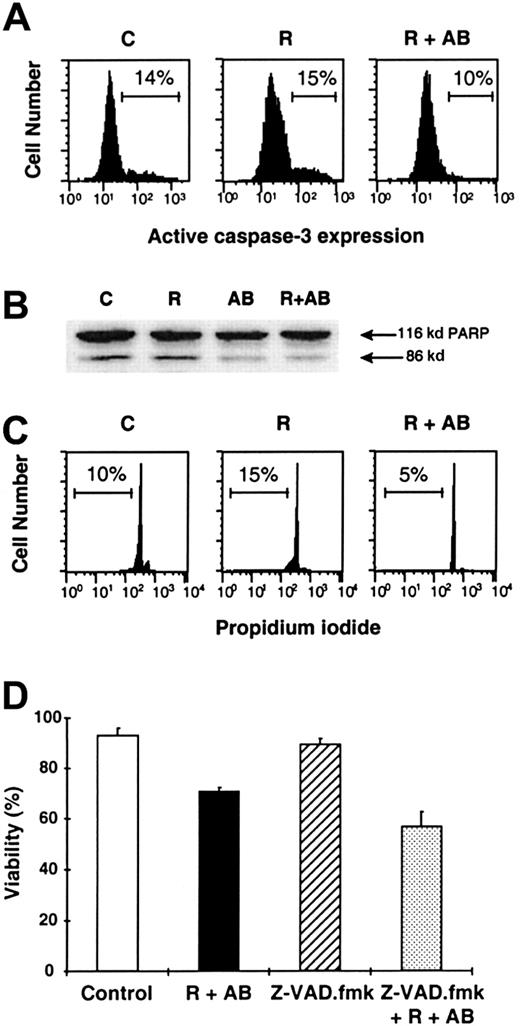
Induction of R-CDC in CD20++ cells is not mediated by caspase activation and does not induce DNA cleavage
We investigated the implication of caspases in R-CDC in 4 CD20++ cases (3 B-CLL and 1 MCL). Incubation of cells for 24 hours with rituximab+AB did not activate caspase-3, as determined by the absence of the active form of this protease by flow cytometry (Figure 4A). Furthermore, R-CDC was not associated with caspase-mediated nuclear features of apoptosis such as PARP proteolysis or DNA cleavage (appearance of the hypodiploid DNA peak) after 48 hours of incubation (Figure 4B,C). Finally, addition of 200 μM Z-VAD.fmk, a broad-spectrum caspase inhibitor, did not prevent phosphatydilserine exposure, cell shrinkage, and loss of ΔΨm induced by rituximab+AB (Figure 4D). To discard the possibility that caspase activation occurred at earlier time points, we analyzed caspase-3 activation in 3 B-CLL sensitive cases following treatment with rituximab+AB for 1, 2, and 4 hours. No evidence of caspase activation was observed, whereas cells at these time points showed the typical features of cell death (data not shown). All these results indicate that the mechanism involved in R-CDC may occur in the absence of caspase activation.
Complement-mediated R-CDC is not mediated by caspase activation.
Cells of a representative CD20++ patient with B-CLL were incubated in medium alone (C), or with 50 μg/mL rituximab in the absence (R) or presence (R+AB) of 10% AB serum. (A) Analysis by flow cytometry of the active form of caspase-3 after 24 hours of incubation. (B) Analysis of PARP cleavage by Western blot after 48 hours of incubation. (C) Analysis of DNA content by staining with propidium iodide after 48 hours of incubation. (D) Effect of 200 μM Z-VAD.fmk on cell viability after 24 hours of incubation. Z-VAD.fmk was preincubated for 1 hour prior to the addition of the indicated factors. Cell viability was quantified by exposure of phosphatidylserine residues.
Complement-mediated R-CDC is not mediated by caspase activation.
Cells of a representative CD20++ patient with B-CLL were incubated in medium alone (C), or with 50 μg/mL rituximab in the absence (R) or presence (R+AB) of 10% AB serum. (A) Analysis by flow cytometry of the active form of caspase-3 after 24 hours of incubation. (B) Analysis of PARP cleavage by Western blot after 48 hours of incubation. (C) Analysis of DNA content by staining with propidium iodide after 48 hours of incubation. (D) Effect of 200 μM Z-VAD.fmk on cell viability after 24 hours of incubation. Z-VAD.fmk was preincubated for 1 hour prior to the addition of the indicated factors. Cell viability was quantified by exposure of phosphatidylserine residues.
R-CDC induces production of ROS
Since the cytotoxic effect of rituximab+AB was accompanied by a loss in ΔΨm, we tested whether this phenomenon was associated with the production of ROS. As seen in Figure5A, incubation for 24 hours of CD20++ cells from a representative MCL patient with rituximab+AB induced the production of ROS, as assessed by staining with DHE, a compound converted by O to highly fluorescent ethidium. Similar results were obtained in all the CD20++ cases sensitive to rituximab, with no differences according to the histology of the tumor.
ROS production induced by rituximab+AB and effect of ROS scavengers.
(A) Cells from a representative CD20++ patient with MCL were incubated for 24 hours in the presence (dashed line) or absence (solid line) of 50 μg/mL rituximab and 10% AB serum (R+AB) and stained with DHE. (B) Cells from a representative CD20++patient with B-CLL were incubated for 20 hours with ROS scavengers (NAC, 25 mM; Tiron, 5 mM) in the absence or presence of rituximab and AB (R+AB). ROS scavengers were added one hour prior to the addition of rituximab and AB serum. Cell viability was determined by staining with annexin V binding. * Indicates P < .05. (C) The effect of ROS scavengers on ΔΨm and ROS generation was quantified after 20 hours by dual staining with DIOC6[3] and DHE.
ROS production induced by rituximab+AB and effect of ROS scavengers.
(A) Cells from a representative CD20++ patient with MCL were incubated for 24 hours in the presence (dashed line) or absence (solid line) of 50 μg/mL rituximab and 10% AB serum (R+AB) and stained with DHE. (B) Cells from a representative CD20++patient with B-CLL were incubated for 20 hours with ROS scavengers (NAC, 25 mM; Tiron, 5 mM) in the absence or presence of rituximab and AB (R+AB). ROS scavengers were added one hour prior to the addition of rituximab and AB serum. Cell viability was determined by staining with annexin V binding. * Indicates P < .05. (C) The effect of ROS scavengers on ΔΨm and ROS generation was quantified after 20 hours by dual staining with DIOC6[3] and DHE.
To determine the implication of ROS in R-CDC, we incubated cells with rituximab+AB in the presence of 2 ROS scavengers. As shown in Figure5B, incubation of cells from a representative CD20++patient with Tiron, a pharmacologic cell-permeable scavenger of O, or the antioxidant NAC completely abolished the generation of ROS and reverted R-CDC, as analyzed by exposure of phosphatidylserine residues, changes in FSC/SSC pattern, and loss of ΔΨm. Similar results were obtained in cells from 5 additional patients. Moreover, we observed a dose-dependent inhibitory effect of Tiron (from 5 mM to 15 mM) and NAC (from 25 mM to 100 mM) that correlated with the number of CD20 molecules per cell (data not shown).
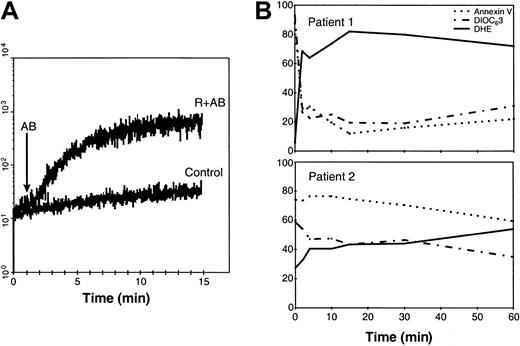
Finally, in order to establish the sequence of cellular changes induced by rituximab+AB, the kinetics of R-CDC were investigated in 4 CD20++ cases (1 B-CLL, 1 FL, 2 MCL). Addition of AB in cells preincubated with rituximab resulted in a rapid and intense detection of ROS, with maximal levels being achieved in less than 15 minutes (Figure 6A). As seen in Figure6B, ROS production was accompanied by loss in Δψm, which was detected as early as 2 minutes after the addition of AB. Exposure of phosphatidylserine residues was detected simultaneously to these changes in cells from CD20++ patients with high expression of CD20 molecules (Figure 6B, upper panel), but in cells from CD20++ patients with lower numbers of CD20 molecules (Figure 6B, lower panel), annexin V binding was observed later.
Kinetics of R-CDC–induced ROS production, loss of Δψm, and phosphatidylserine exposure.
(A) The kinetics of ROS generation were analyzed in cells from a patient with MCL with CD20++ expression (119 × 103 CD20 molecules/cell) as described in “Patients, materials, and methods.” (B) Time-course of ROS production (solid line), loss of ΔΨm (dashed line), and annexin V exposure (dotted line) in cells from a patient with MCL with very high levels of CD20 (137 × 103 molecules/cell) (top panel) and a CD20++ patient with B-CLL with high levels of CD20 (70 × 103 molecules/cell) (lower panel).
Kinetics of R-CDC–induced ROS production, loss of Δψm, and phosphatidylserine exposure.
(A) The kinetics of ROS generation were analyzed in cells from a patient with MCL with CD20++ expression (119 × 103 CD20 molecules/cell) as described in “Patients, materials, and methods.” (B) Time-course of ROS production (solid line), loss of ΔΨm (dashed line), and annexin V exposure (dotted line) in cells from a patient with MCL with very high levels of CD20 (137 × 103 molecules/cell) (top panel) and a CD20++ patient with B-CLL with high levels of CD20 (70 × 103 molecules/cell) (lower panel).
Discussion
The results presented in this study show that the cytotoxic effect of rituximab on primary malignant B cells is, at least in part, complement mediated and that this process mainly occurs through the production of ROS. The generation of cytotoxic ROS in cells is due to the partial reduction of oxygen that leads to the generation of O. To avoid toxic cellular effects, this stable radical is enzymatically converted to other radicals such as hydroxyl (OH−) and hydrogen peroxide (H2O2),15 or rapidly reacts with nitric oxide (NO) yielding peroxynitrite (ONOO−).16 The production of ROS has a role in signal transduction and has been associated with many forms of apoptosis17 as well as with the cell death that occurs in cerebrovascular accidents and neurodegenerative diseases.18-22
In the present study, we found a rapid and selective generation of ROS after the addition of rituximab and AB serum, since only specific scavengers of O, such as Tiron, and NAC completely reverted the production of ROS and all changes associated with cell death. Other ROS scavengers selective for H2O2(glutathiome [GSH], catalase) and ONOO−(L-NAME, uric acid) produced a partial or no reversion on R-CDC (data not shown). Interestingly, the specific involvement of O has been recently described in the cytotoxic effect of estrogen derivates in B-CLL cells23 and in the apoptosis of rat mesangial cells treated with tumor necrosis factor-α.24
Consistent with previous reports,11 rituximab alone was unable to induce a cytotoxic effect on primary malignant B cells, the addition of a source of complement being necessary to obtain such an effect. Another mechanism that has been involved in the cytotoxic effect of rituximab is ADCC.1,7 However, a previous report comparing specific rituximab-induced ADCC and CDC in several cell lines showed that CDC is more effective than ADCC.7 Although CDC could be the major mechanism accounting for the elimination of circulating B lymphocytes following rituximab infusion, ADCC may contribute to the elimination of B cells in tissues where interaction between target and effector cells is higher.
Incubation of cells with rituximab and AB serum induced the typical cytoplasmic features of apoptosis: flow cytometric changes in FSC and SSC indicative of cell shrinkage, exposure of phosphatidylserine residues, and decrease in Δψm. Neither caspase activation nor caspase-mediated nuclear features of apoptosis were observed and R-CDC was not blocked by the presence of the caspase inhibitor Z-VAD.fmk, indicating that R-CDC observed in primary malignant B cells occurred in the absence of caspase activation. A caspase-independent cell death has been described for treatment with several monoclonal antibodies,25,26 and particularly, in B-CLL cells by CD47 ligation.27 There are no extensive analyses of the pathways accounting for this sort of cell death but, interestingly, in some studies cell death was associated with ROS production and loss of ΔΨm,25,28as we have observed in R-CDC. In addition, it has been described that caspases are inhibited by oxidants29,30 because, presumably, when the level of cytosolic ROS becomes too high, the essential cysteine in the active center of the caspases becomes oxidized or blocked.31 32
It has been described that rituximab induces a caspase-dependent apoptosis.8,9,33 However, in all these studies in vitro crosslinking of rituximab with secondary antibodies was performed. The production of antibodies against rituximab has not been described in patients treated with rituximab; therefore, this mechanism is unlikely to occur in vivo.2 Nevertheless, preliminary studies have suggested that caspases may be activated following in vivo infusion of rituximab,34 therefore additional mechanisms could be involved in in vivo caspase activation. Although it has been described that some anti-CD20 antibodies can promote the activation of the Src-family kinases,9 no implication of these kinases has been observed in our study. In fact, incubation with a selective inhibitor of this family of kinases (PP2) did not prevent R-CDC in cells from 4 of our cases (data not shown). Altogether, it is likely that many different pathways of cell death both dependent and independent of apoptosis could mediate the elimination of tumoral B cells in vivo.
We have observed that R-CDC directly correlates with the expression of the CD20 antigen in malignant B cells and a certain amount of antigen is required to trigger R-CDC. Therefore, the clinical differences in responses among patients with different types of B-lymphoproliferative disorders could be related, at least in part, to CD20 expression. Another factor influencing the response to rituximab is complement regulatory proteins. The blockage of CD59 with specific antibodies sensitized cells to rituximab, indicating that CD59, the protein controlling the membrane-attack complex, may have an important role in R-CDC. Similar results have been obtained in previous studies,7 in contrast to others that proposed a major role for CD55.11 These differences could be related to the different clones of monoclonal antibodies used to block cellular antigens.
The present results, obtained in primary malignant B cells, can serve as the basis for new therapeutic approaches that may improve the efficacy of rituximab in lymphoid malignancies. In this regard, it has been suggested that the up-regulation of CD20 on malignant B cells could enhance the effect of rituximab.35-37 Additional strategies could involve the control of complement regulatory proteins on the surface of neoplastic B cells either by using bispecific monoclonal antibodies against both CD20 and CD59, to avoid side effects of a wide CD59 blockage, or by down-regulating the expression of CD59 with pharmacologic agents.38 39
In conclusion, this report provides evidence that CD20, CD59, and complement contribute to the in vitro cytotoxic effect of rituximab, with this being mediated by a caspase-independent process that involves ROS generation and loss of mitochondrial transmembrane potential. These results may be useful to establish a theoretical basis to improve the efficacy of therapy with rituximab.
We thank L. Quintó from the Unidad de Epidemiologı́a y Bioestadı́stica, Hospital Clı́nic, for his help in statistical analysis.
Supported in part by research fellowships from the Instituto de Salud Carlos III (B.B.) and Fondo de Investigaciones Sanitarias (FIS) (S.M.); FIS grant numbers 98/0996, 99/0189, and 00/0946; José Carreras International Foundation Against Leukemia (EM/P-01 and CR/P-00); Roche España; and the Asociación Española Contra el Cáncer, Barcelona, Spain.
Roche Spain provides funds for research activities of the Institute of Hematology and Oncology.
B.B and N.V. contributed equally to this study. D.C. and E.M. share the senior authorship of this paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Emili Montserrat, Institute of Hematology and Oncology, Department of Hematology, Hospital Clı́nic, Villarroel 170, 08036 Barcelona, Spain; e-mail: emontse@clinic.ub.es.

![Fig. 1. Cell death induction by the combination of rituximab and normal human AB serum. / Cells from a representative CD20++ patient with follicular lymphoma (FL) were incubated in the presence or absence of 50 μg/mL rituximab and 10% AB (R+AB) for 24 hours and analyzed by flow cytometry. (A) Flow cytometric plots showing FSC/SSC pattern. (B) Flow cytometric plots showing annexin V binding. (C) Histogram showing mitochondrial transmembrane potential after staining with DiOC6[3].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/9/10.1182_blood.v98.9.2771/4/m_h82111711001.jpeg?Expires=1769140540&Signature=ahjfoaQnivypFYqftej8E71DwX3ogBZhtfq~1UCROR3nRIdYAmpYukWcoIiPbirXs9Tds45hK9Smof2eg-6sVOiX~AIZ4DV15H-oN2RTo20~-MSwIFeikLvvvpAIcexhjfz04GbaHsEAfHieHGdWdVQSphZZQcw~eDFd48apCYkHC5YvLhHfQhsxOKBoLpwiJOjGRSLA80aZK4wTxwRkHTvYbl1rOM1BSIyau1KtembK0Jtyk6zTRW0LkWCDgaoNSwpzWgzYNjU7xZz9uo7hoRhzTYbY055HFbhSI1Wb3HuD1Hn9T7IVUTHB39YfVy2UO1A6weAJuXMQRxXhgfrYjQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 5. ROS production induced by rituximab+AB and effect of ROS scavengers. / (A) Cells from a representative CD20++ patient with MCL were incubated for 24 hours in the presence (dashed line) or absence (solid line) of 50 μg/mL rituximab and 10% AB serum (R+AB) and stained with DHE. (B) Cells from a representative CD20++patient with B-CLL were incubated for 20 hours with ROS scavengers (NAC, 25 mM; Tiron, 5 mM) in the absence or presence of rituximab and AB (R+AB). ROS scavengers were added one hour prior to the addition of rituximab and AB serum. Cell viability was determined by staining with annexin V binding. * Indicates P < .05. (C) The effect of ROS scavengers on ΔΨm and ROS generation was quantified after 20 hours by dual staining with DIOC6[3] and DHE.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/9/10.1182_blood.v98.9.2771/4/m_h82111711005.jpeg?Expires=1769140540&Signature=mtya3ZbtKujB6TozAtZaQRImyJHEu1hfTrnS4QPySUTshrXXCJGTZYbddThqQT0hXDe2rYaq74Wxi2gvtm85MiE5pokonxpTZqu03vhiYuDA5R3TXVM0uzGEqYOOyBxqQe56iBrKcAg6s8zVDcqliJiDw~G48NzOzOikS9OiZxUAjrbcK5j1j2ixkqvV6Swe7~eMw~6PSEQXZ9jOybF-8wo2uDJWQ13H1kJsr-I9XDzOyLmofRVMBwqjbp2EvKUt~hhYzH1GTsoP0xeLqBNNMIlisPu45MyXHNtjEiaJVQ1tLQJat8OTQeTh~MrcpmILVbazHgruUxYNF4HpxzbDHg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal