Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) is a 130-kd transmembrane glycoprotein and a member of the growing family of receptors with immunoreceptor tyrosine-based inhibitory motifs (ITIMs). PECAM-1 is expressed on platelets, certain T cells, monocytes, neutrophils, and vascular endothelial cells and is involved in a range of cellular processes, though the role of PECAM-1 in platelets is unclear. Cross-linking of PECAM-1 results in phosphorylation of the ITIM allowing the recruitment of signaling proteins that bind by way of Src-homology domain 2 interactions. Proteins that have been implicated in the negative regulation of cellular activation by ITIM-bearing receptors include the tyrosine phosphatases SHP-1 and SHP-2. Tyrosine phosphorylation of immunoreceptor tyrosine-based activatory motif (ITAM)–bearing receptors such as the collagen receptor GPVI-Fc receptor γ-chain complex on platelets leads to activation. Increasing evidence suggests that ITIM- and ITAM-containing receptors may act antagonistically when expressed on the same cell. In this study it is demonstrated that cross-linking PECAM-1 inhibits the aggregation and secretion of platelets in response to collagen and the GPVI-selective agonist convulxin. In these experiments thrombin-mediated platelet aggregation and secretion were also reduced, albeit to a lesser degree than for collagen, suggesting that PECAM-1 function may not be restricted to the inhibition of ITAM-containing receptor pathways. PECAM-1 activation also inhibited platelet protein tyrosine phosphorylation stimulated by convulxin and thrombin; this was accompanied by inhibition of the mobilization of calcium from intracellular stores. These data suggest that PECAM-1 may play a role in the regulation of platelet function in vivo.
Introduction
Platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) is a 130-kd membrane-spanning glycoprotein whose expression is restricted to several hematopoietic cell types including platelets, monocytes, neutrophils, certain T lymphocytes, and vascular endothelial cells.1-4 The functions of PECAM-1 are diverse and include angiogenesis,5 vasculogenesis,6integrin regulation,7,8 transendothelial migration of leukocytes,9-11 and T- and B-cell antigen receptor function,12,13 though the role of this molecule in platelets is unclear. When PECAM-1 was cloned, it was assigned to the family of cell adhesion molecules on the basis of structural similarities.4 PECAM-1 is involved in adhesion, though much attention has been directed recently to studying its ability to participate in signal transduction. The cytoplasmic tail of PECAM-1 contains a conserved motif called an immunoreceptor tyrosine-based inhibitory motif (ITIM), which underlies its signaling properties and is shared by a growing family of inhibitory receptors. These include immunoglobulin G (IgG) receptor FcγRIIB, killer inhibitory receptors, and signal regulatory proteins, but PECAM-1 is the only ITIM-bearing receptor reported to be expressed in platelets.14,15Therefore, it has been proposed that PECAM-1 be assigned to the immunoglobulin–ITIM family of receptors.14
The ligand-binding properties of PECAM-1 are complex. It has the capacity for homophilic interactions3,16 and heterophilic interactions with a number of molecules that include integrin αvβ3 and CD38.17,18 PECAM-1 becomes phosphorylated on tyrosine residues in response to a variety of stimuli that include PECAM-1 cross-linking,19 activation of the high-affinity receptor for IgE (FcεRI),20 shear stress,21 and oxidative stress.22Furthermore, we have recently reported that platelet activation through the collagen receptor glycoprotein VI (GPVI) and through thrombin receptors results in PECAM-1 tyrosine phosphorylation and that this is not dependent on platelet aggregation and secretion,23though tyrosine phosphorylation is enhanced by aggregation.24 The tyrosine residues that become phosphorylated in PECAM-1 have been mapped and fall within the ITIM.25 Phosphorylated ITIMs recruit signaling molecules, such as the tyrosine phosphatases SHP-1 and SHP-2, which bind to the motif through Src-homology 2 domain interactions. Indeed, both SHP-1 and SHP-2 have been shown to associate with tyrosine-phosphorylated PECAM-1,24-26 and PECAM-1 ITIM phosphopeptides activate these phosphatases in vitro.19,25,26 Generally, these protein tyrosine phosphatases exhibit inhibitory effects by counteracting tyrosine kinase–dependent pathways, though SHP-2 has been shown to positively regulate growth factor receptor signaling.27
Immunoreceptor tyrosine-based activatory motif (ITAM)–bearing receptors have a critical place in the regulation of platelet function.28 Indeed, the collagen receptor GPVI-FcR γ-chain complex signals through an ITAM on the cytoplasmic tail of the FcR γ-chain.29-33 Several studies in other cell systems have provided evidence of an antagonistic relationship between ITAM- and ITIM-containing receptors when they are expressed on the same cell. An example of this is the receptor for IgG FcγRIIB (ITIM), which negatively regulates cell activation stimulated by FcγRIIA (ITAM).34
In this study we examined the effect of PECAM-1 signaling on the activation of human platelets. We demonstrate that the activation of PECAM-1 signaling by antibody-mediated cross-linking results in the inhibition of collagen-mediated activation; similar results were obtained using a GPVI-selective agonist convulxin (Cvx). Furthermore, we present evidence to indicate that the inhibitory functions of PECAM-1 may not be restricted to the inhibition of ITAM-containing receptor signaling pathways because thrombin-stimulated activation was also inhibited. Inhibition of platelet activation is accompanied by a concomitant inhibition of platelet protein tyrosine phosphorylation and decreased levels of calcium mobilization from intracellular stores. Our data support the notion that PECAM-1 signaling may be involved in the regulation of platelet function in vivo.
Materials and methods
Materials
Horm-Chemie collagen (collagen fibers from equine tendons) was purchased from Nycomed (Munich, Germany). Cvx was purified from the venom of the rattlesnake (Crotalus durissus terrificus), as described previously.35 Thromboxane mimetic U46619 and adenosine diphosphate (ADP) was purchased from Sigma (Poole, United Kingdom). Antiphosphotyrosine monoclonal antibody (mAb) (4G10) was obtained from Upstate Biotechnology (TCS Biologicals, Buckinghamshire, United Kingdom). Anti–PECAM-1 antibodies were obtained as follows: monoclonal antibody HC1/6, from Serotec (Oxford, United Kingdom); polyclonal anti–PECAM-1 (C-20) and monoclonal antibody AB468, from Autogen Bioclear (Wiltshire, United Kingdom); and monoclonal antibody PECAM-1.3, kindly provided by Professor Peter Newman (The Blood Center of Southeastern Wisconsin, Milwaukee). Control mouse IgG1was purchased from Sigma (Poole, United Kingdom). Monoclonal antibody IV.3 was purified from hybridoma cell culture medium, and F(ab′)2 fragments were generated by pepsin digestion using reagents purchased from Pierce (Perbio Scientific, Chester, United Kingdom). Horseradish peroxidase–conjugated secondary antibodies and the enhanced chemiluminescence detection system were purchased from Amersham Pharmacia Biotech (Buckinghamshire, United Kingdom). Fura-2am was from Molecular Probes (Cambridge Bioscience, United Kingdom). All other reagents were from previously described sources.23 32
Preparation and stimulation of platelets
Human platelets from drug-free donors were prepared on the day of the experiment by differential centrifugation, as described previously,32 and suspended in modified Tyrode-HEPES buffer (134 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM HEPES, 5 mM glucose, 1 mM MgCl2, pH 7.3) to a density of 2 × 108cells/mL. For protein precipitation experiments, platelets were resuspended at 8 × 108 cells/mL in buffer containing 1 mM EGTA to prevent aggregation. Stimulation of platelets (450 μL) with collagen, Cvx, and thrombin (delivered in 50 μL) was performed at 37°C in an optical platelet aggregometer (Chrono-log, Havertown, PA) with continuous stirring (1200 rpm). PECAM-1 activation was stimulated by incubation with anti–PECAM-1 antibodies (AB468, 1 μg/mL) or PECAM 1.3, 10 μM (μg/mL)) for 5 minutes, followed by incubation for 90 seconds with F(ab′)2fragments of anti-IgG secondary antibodies (30 μg/mL) to cross-link. Monoclonal antibody AB468 was generated against full-length PECAM-1 ectodomain, and the precise binding epitope has not been mapped. The binding epitope of PECAM 1.3 has been mapped to immunoglobulin domain 1.12 Control experiments were performed using an irrelevant isotype-matched antibody in place of AB468 or PECAM 1.3 and were used at the same concentration. In some experiments, the low-affinity receptor for IgG was blocked by incubation with a saturating concentration (1 μg/mL) of F(ab′)2 fragments of mAb IV.3 for 5 minutes. Saturating concentrations of mAb IV.3 antibody were established by determining the concentration of F(ab′)2 fragment that completely inhibited subsequent whole IgG–mediated FcγRIIA cross-linking and platelet activation (as described previously36). Platelet aggregation was determined by optical aggregometry.
Immunoprecipitation studies
Platelet stimulation was terminated by the addition of an equal volume of ice-cold lysis buffer (2% (vol/vol) Nonidet P40, 20 mM Tris, 300 mM NaCl, 10 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 2 mM Na3VO4, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 1 μg/mL pepstatin A, pH 7.3). Detergent-insoluble debris was removed, and the lysates were precleared by mixing with protein A–Sepharose for 1 hour at 4°C (20 μL of a 50% (wt/vol) suspension of protein A–Sepharose in Tris-buffered saline–Tween (TBS-T; 20 mM Tris, 137 mM NaCl, 0.1% (vol/vol) Tween 20, pH 7.6)). Protein A–Sepharose was removed from the lysates before the addition of anti–PECAM-1 antibody (HC1/6, 1 μg). After rotation at 4°C for 1 hour, 0.5 μL secondary antiserum was added (rabbit anti–mouse IgG) and mixed for a further 30 minutes. Protein A–Sepharose suspension (25 μL) was added to each sample, and mixing continued for 1 hour before the Sepharose pellet was washed in lysis buffer; this was followed by a wash with TBS-T and by the addition of Laemmli sample-treatment buffer. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions using 10% gels and were transferred to polyvinylidene difluoride membranes by semidry Western blotting.
Immunoblotting
Membranes were blocked by incubation in 10% (wt/vol) bovine serum albumin dissolved in TBS-T. Primary and secondary antibodies were diluted in TBS-T containing 2% (wt/vol) bovine serum albumin and were incubated with membranes for 1 hour at room temperature. Blots were washed for 2 hours in TBS-T after incubation with antibodies for 1 hour at room temperature and were developed using an enhanced chemiluminescence detection system. Primary antibodies were used at a concentration of 1 μg/mL (antiphosphotyrosine, 4G10; anti-PECAM-1, C-20), and horseradish peroxidase–conjugated secondary antibodies were diluted at 1:10 000.
5-Hydroxytryptamine secretion assay
Platelets were loaded with [3H]5-hydroxytryptamine (5-HT) by incubation with 0.5 μCi/mL (18.5 kBq) platelet-rich plasma for 1 hour at 37°C. Platelets were prepared from the platelet-rich plasma as described above. Stimulation of platelets was terminated by the addition of an equal volume of 6% glutaraldehyde and microcentrifugation, and the level of [3H]5-HT release into the supernatant was determined by scintillation spectrometry. [3H]5-HT release was expressed as a percentage of the total tissue content after subtraction of release under basal conditions.
Measurement of [Ca++]i by spectrofluorometry
Washed human platelets (prepared as above) were incubated at 2 × 109 cells/mL in calcium-free Tyrode HEPES buffer with 3 μM Fura-2 am for 45 minutes. Platelets were washed once and resuspended at 2 × 108 cells/mL in modified Tyrode HEPES buffer. Stimulation of platelets (450 μL) in the presence of 2 mM EGTA with Cvx and thrombin (delivered in 50 μL) was performed with constant stirring at 37°C in a luminescence spectrophotometer (LS-50B; Perkin-Elmer) with excitation wavelengths of 340 nm and 380 nm. Fluorescence emission was measured at a wavelength of 510 nm. Where required, PECAM-1 was cross-linked before stimulation with agonist as described above. The ratio of emission values (excitation at 340/380 nm) was calculated and converted to calcium concentration using FLWinLab software (Perkin-Elmer) using the equation [Ca++]i = Kd × (R − Rmin)/(Rmax − R) × SFB, where R is emission ratio value (340/380 nm). Rmax, the maximum 340/380 ratio, was determined by lysing platelets with 25 μM digitonin in the presence of 1 mM CaCl2. The Rmin 340:380 nm ratio was obtained by adding 2 mM EGTA.Kd was the dissociation constant of the Fura-2/Ca++ complex (224 nM), and SFB was the fluorescence ratio at 340/380nm Rmin and Rmax).
Statistical analysis
Determination of statistical significance was performed using the Student paired t test. Results are expressed as mean ± SEM.
Results
Cross-linking PECAM-1 inhibits collagen-stimulated platelet aggregation
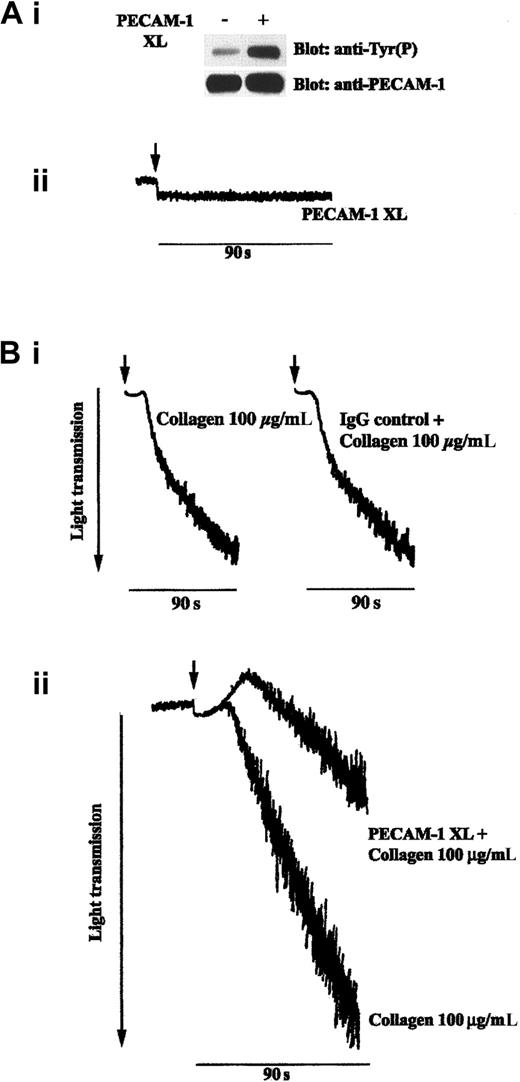
An antagonistic relationship has been reported between ITIM- and ITAM-containing receptors when they are expressed in the same cell.12,13,34 Because the platelet collagen receptor GPVI signals through an ITAM on the FcRγ-chain with which it is associated, we investigated the effect of PECAM-1 signaling on platelet activation with collagen. PECAM-1 was activated by incubation with antibodies specific for the ectodomain of PECAM-1 (AB468 (Figure1) or PECAM1.3, not shown) and was cross-linked with a secondary antibody, (Fab′)2 fragment. Consistent with findings from other reports,19 this resulted in increased tyrosine phosphorylation of the protein and did not result in the stimulation of platelet aggregation (Figure 1A). Tyrosine phosphorylation of PECAM-1 was maintained on cross-linking in the presence of EGTA (1 mM), RGDS peptide (0.5 mM), and γ-chain peptide of fibrinogen (100 μM), added separately or together (not shown). This, together with fact that these experiments were performed on washed platelets, indicates that the tyrosine phosphorylation of PECAM-1 on cross-linking is not dependent on integrin αIIbβ3 engagement. The effect of PECAM-1 cross-linking for 90 seconds before stimulation with collagen was found to have a marked inhibitory effect on collagen-stimulated platelet aggregation. At lower concentrations of collagen (eg, 10 μg/mL), cross-linking of PECAM-1 before agonist addition completely abolished aggregation (data not shown). Figure 1Bii shows the marked inhibitory effect of PECAM-1 cross-linking on a high concentration of collagen (100 μg/mL). The use of an isotype-matched IgG control and cross-linker F(ab′)2 had no effect on PECAM-1 tyrosine phosphorylation (not shown) and collagen-stimulated platelet aggregation (Figure 1Bi). Results are representative of 3 separate experiments. Similar results were obtained using the alternative anti-PECAM-1 antibody, PECAM 1.3. In some experiments, the low-affinity receptor for IgG FcγRIIA was blocked before PECAM-1 cross-linking and agonist stimulation using a saturating concentration of F(ab′)2 fragments of mAb IV.3. The inhibitory effect of PECAM-1 cross-linking was unaltered under these conditions, indicating that the inhibitory effect of PECAM-1 using antibodies was not caused by the activation of FcγRIIA. This result was not surprising given that the activation of FcγRIIA by cross-linking with antibodies results in platelet activation.36
Cross-linking of PECAM-1 inhibits collagen-stimulated platelet aggregation.
(A) PECAM-1 cross-linking on platelet surfaces results in its tyrosine phosphorylation and does not stimulate platelet aggregation. (i) PECAM-1 was immunoprecipitated from washed human platelets under resting conditions or after PECAM-1 cross-linking. Proteins were separated by SDS-PAGE and immunoblotted to detect phosphotyrosine residues (upper panel). Immunoprecipitation was verified by reprobing for PECAM-1 (lower panel). (ii) PECAM-1 was cross-linked on washed platelets (as described in “Materials and methods”), and aggregation was monitored using optical aggregometry. (B) (i) Platelets were incubated with isotype-matched control IgG for 5 minutes before the addition of F(ab′)2 cross-linker for 90 seconds and then were stimulated with collagen (100 μg/mL). Aggregation was monitored using optical aggregometry. (ii) Cross-linking of PECAM-1 inhibits collagen-stimulated platelet aggregation. Platelets were stimulated with collagen (100 μg/mL) for 90 seconds with and without first cross-linking PECAM-1 and platelet aggregation monitored by optical aggregometry. Data are representative of 3 separate experiments. Tyr(P), tyrosine phosphorylation; PECAM-1 XL, PECAM-1 cross-linking.
Cross-linking of PECAM-1 inhibits collagen-stimulated platelet aggregation.
(A) PECAM-1 cross-linking on platelet surfaces results in its tyrosine phosphorylation and does not stimulate platelet aggregation. (i) PECAM-1 was immunoprecipitated from washed human platelets under resting conditions or after PECAM-1 cross-linking. Proteins were separated by SDS-PAGE and immunoblotted to detect phosphotyrosine residues (upper panel). Immunoprecipitation was verified by reprobing for PECAM-1 (lower panel). (ii) PECAM-1 was cross-linked on washed platelets (as described in “Materials and methods”), and aggregation was monitored using optical aggregometry. (B) (i) Platelets were incubated with isotype-matched control IgG for 5 minutes before the addition of F(ab′)2 cross-linker for 90 seconds and then were stimulated with collagen (100 μg/mL). Aggregation was monitored using optical aggregometry. (ii) Cross-linking of PECAM-1 inhibits collagen-stimulated platelet aggregation. Platelets were stimulated with collagen (100 μg/mL) for 90 seconds with and without first cross-linking PECAM-1 and platelet aggregation monitored by optical aggregometry. Data are representative of 3 separate experiments. Tyr(P), tyrosine phosphorylation; PECAM-1 XL, PECAM-1 cross-linking.
PECAM-1 cross-linking inhibits GPVI- and thrombin receptor-mediated platelet aggregation
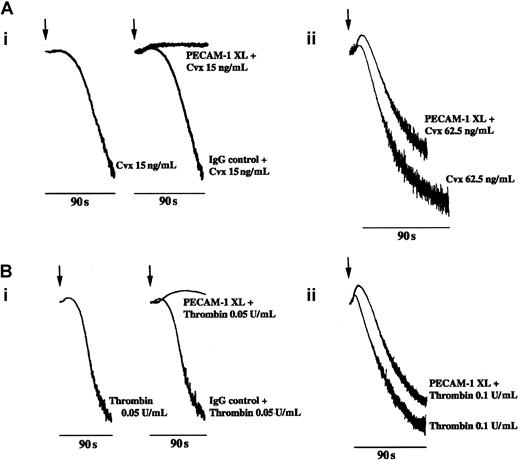
Given the marked effect of PECAM-1 signaling on collagen-mediated platelet aggregation, we investigated whether this was restricted to GPVI-mediated signaling. GPVI was stimulated using the selective agonist Cvx, a protein purified from the venom of the rattlesnake,C durissus terrificus. Aggregation stimulated with 15 ng/mL Cvx was completely inhibited by prior activation of PECAM-1 (Figure2Ai), and a partial inhibitory effect was observed at higher concentrations of Cvx (31.25 and 62.5 ng/mL; not shown and Figure 2Aii, respectively). Similar results were observed on the stimulation of platelets with the G protein–coupled receptor agonist thrombin. Complete inhibition of aggregation at 90-second stimulation was observed at a thrombin concentration of 0.05 U/mL (Figure 2Bi) and a partial effect at 0.1 U/mL (Figure 2Bii). The use of an isotype-matched IgG control and cross-linker F(ab′)2 had no effect on Cvx- or thrombin-stimulated platelet aggregation (Figure 2Ai-Bi). No inhibitory effect of PECAM-1 activation was observed at higher concentrations of thrombin (eg, 0.5 and 1, U/mL; data not shown). Results are representative of 5 separate experiments. Similar results were obtained using the alternative anti–PECAM-1 antibody PECAM 1.3 and when FcγRIIA was blocked before PECAM-1 cross-linking.
PECAM-1 cross-linking inhibits GPVI- and thrombin receptor–stimulated platelet aggregation.
(A) Platelets were stimulated with Cvx at (i) 15 ng/mL and (ii) 62.5 ng/mL with and without first cross-linking PECAM-1, and aggregation responses were monitored by optical aggregation (arrow indicates the addition of Cvx). (B) Platelets were stimulated with thrombin at (i) 0.05 U/mL and (ii) 0.1U/mL with and without prior cross-linking of PECAM-1 (as described in “Materials and methods”). Platelet aggregation was monitored by optical aggregometry (arrow indicates the addition of thrombin). Treatment of platelets with isotype-matched control IgG before stimulation with Cvx (15 ng/mL) and thrombin (0.05 U/mL) is shown in A(i) and B(i), respectively. Data are representative of 3 separate experiments. PECAM-1 XL, PECAM-1 cross-linking.
PECAM-1 cross-linking inhibits GPVI- and thrombin receptor–stimulated platelet aggregation.
(A) Platelets were stimulated with Cvx at (i) 15 ng/mL and (ii) 62.5 ng/mL with and without first cross-linking PECAM-1, and aggregation responses were monitored by optical aggregation (arrow indicates the addition of Cvx). (B) Platelets were stimulated with thrombin at (i) 0.05 U/mL and (ii) 0.1U/mL with and without prior cross-linking of PECAM-1 (as described in “Materials and methods”). Platelet aggregation was monitored by optical aggregometry (arrow indicates the addition of thrombin). Treatment of platelets with isotype-matched control IgG before stimulation with Cvx (15 ng/mL) and thrombin (0.05 U/mL) is shown in A(i) and B(i), respectively. Data are representative of 3 separate experiments. PECAM-1 XL, PECAM-1 cross-linking.
Platelet secretion is inhibited by PECAM-1 signaling
Platelet activation is accompanied by secretion from dense granules. Dense granule secretion was assessed by measuring the release of [3H]5-HT from preloaded washed platelets. Figure3 shows the results of experiments to determine the effect of PECAM-1 cross-linking on [3H]5-HT secretion. A significant reduction in secretion was observed in platelets in which PECAM-1 was activated before stimulation with Cvx (81.9% ± 2.9% to 39.8% ± 4.1%;P = .02; n = 3) or thrombin (70.9% ± 4.6% to 37.0% ± 8.0%; P = .01; n = 3). The use of an isotype-matched IgG control and cross-linker F(ab′)2 had no effect on Cvx- or thrombin-stimulated dense granule secretion (not shown). Experiments performed in the presence of mAb IV.3 to block the Fc receptor FcγRIIA produced similar results.
Platelet-dense granule secretion is inhibited by PECAM-1 signaling.
Platelets were loaded with [3H]5-HT before stimulation with Cvx (62.5 ng/mL) or thrombin (0.1 U/mL). Where required, PECAM-1 was cross-linked an agonist was added (as described in “Materials and methods”). Secretion of [3H]5-HT into cell medium was measured using scintillation spectrometry. [3H]5-HT release is expressed as a percentage of total tissue content after subtraction of basal secretion values. Results represent mean ± SE (n = 3). Student t test was used to compare PECAM-1 cross-linked and non–cross-linked sample for statistical significance. *P < .05. PECAM-1 XL, PECAM-1 cross-linking
Platelet-dense granule secretion is inhibited by PECAM-1 signaling.
Platelets were loaded with [3H]5-HT before stimulation with Cvx (62.5 ng/mL) or thrombin (0.1 U/mL). Where required, PECAM-1 was cross-linked an agonist was added (as described in “Materials and methods”). Secretion of [3H]5-HT into cell medium was measured using scintillation spectrometry. [3H]5-HT release is expressed as a percentage of total tissue content after subtraction of basal secretion values. Results represent mean ± SE (n = 3). Student t test was used to compare PECAM-1 cross-linked and non–cross-linked sample for statistical significance. *P < .05. PECAM-1 XL, PECAM-1 cross-linking
PECAM-1 inhibits platelet protein tyrosine phosphorylation
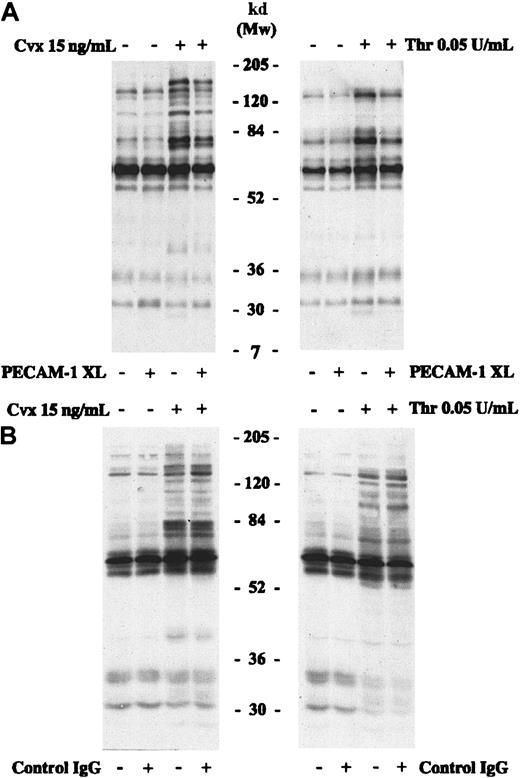
The effect of PECAM-1 cross-linking on GPVI- and thrombin receptor–stimulated signaling was investigated. Platelets were stimulated with Cvx (15 ng/mL) or thrombin (0.05 U/mL) with or without prior cross-linking of PECAM-1 for 90 seconds. Whole-cell protein tyrosine phosphorylation levels were determined by immunoblotting. Cross-linking PECAM-1 alone had no effect on basal platelet tyrosine phosphorylation levels (Figure 4). Stimulation with Cvx (15 ng/mL) or thrombin (0.05 U/mL) caused an increase in the level of tyrosine phosphorylation of a broad range of proteins, consistent with previous reports.32 37 In samples in which PECAM-1 signaling was stimulated by cross-linking before incubation with Cvx or thrombin, total tyrosine phosphorylation was reduced (Figure 4). The use of an isotype-matched IgG control and cross-linker F(ab′)2 had no detectable effect on Cvx- or thrombin-stimulated total tyrosine phosphorylation levels.
PECAM-1 signaling inhibits platelet protein tyrosine phosphorylation.
Platelet lysates were prepared in Laemmli buffer from nonstimulated platelets (stirred with buffer alone) and platelets stimulated for 90 seconds with Cvx (15 ng/mL) or thrombin (0.05 U/mL). Before stimulation, PECAM-1 was cross-linked in some samples (A) or platelets were incubated with isotype-matched control IgG and cross-linker F(ab′)2 (B). Proteins were separated by SDS-PAGE under reducing conditions and immunoblotted to detect protein tyrosine phosphorylation. PECAM-1 XL, PECAM-1 cross-linking
PECAM-1 signaling inhibits platelet protein tyrosine phosphorylation.
Platelet lysates were prepared in Laemmli buffer from nonstimulated platelets (stirred with buffer alone) and platelets stimulated for 90 seconds with Cvx (15 ng/mL) or thrombin (0.05 U/mL). Before stimulation, PECAM-1 was cross-linked in some samples (A) or platelets were incubated with isotype-matched control IgG and cross-linker F(ab′)2 (B). Proteins were separated by SDS-PAGE under reducing conditions and immunoblotted to detect protein tyrosine phosphorylation. PECAM-1 XL, PECAM-1 cross-linking
PECAM-1 inhibits the mobilization of calcium from intracellular stores
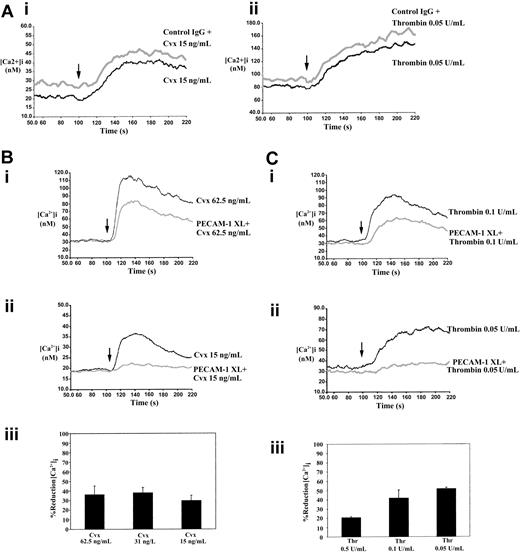
Stimulation of the collagen receptor GPVI and thrombin receptors leads to rapid intracellular mobilization of calcium, an effect that is essential for secretion and aggregation.38 Intracellular calcium levels were measured fluorometrically using the calcium-sensitive dye Fura-2 am. Experiments were performed in the presence of 2 mM EGTA to prevent the entry of extracellular calcium. Stimulation of platelets with Cvx and thrombin resulted in a rapid increase in the levels of intracellular calcium that declined over a period of approximately 5 minutes (Figure5). Incubation of platelets with control antibody and cross-linker F(ab′)2 caused no change in Cvx- and thrombin-stimulated intracellular calcium mobilization (Figure 5A). Cross-linking of PECAM-1 for 90 seconds before incubation with Cvx (62.5 ng/mL) or thrombin (0.1 U/mL) resulted in a markedly reduced level of calcium mobilization (Figure 5Bi-Ci). At the lower concentrations of agonists used (Cvx, 15 ng/mL; thrombin, 0.05 U/mL), calcium mobilization was almost abolished (Figure 5Bii-Cii). The effect of PECAM-1 cross-linking on the reduction of peak intracellular calcium levels for a range of agonist concentrations is shown in Figure 5Biii-Ciii. A reduction of at least 50% in calcium mobilization was observed at all concentrations of Cvx and thrombin tested. Similar results were obtained using the alternative anti–PECAM-1 antibody, PECAM 1.3. Furthermore, experiments performed in the absence of extracellular EGTA indicate that PECAM-1 cross-linking does not inhibit agonist-induced influx of calcium (data not shown).
PECAM-1 cross-linking inhibits the mobilization of calcium from intracellular stores.
Fura-2 am–loaded human platelets were stimulated with either Cvx or thrombin, and the mobilization of calcium was measured fluorometrically as described in “Materials and methods” (arrow indicates the addition of agonist). (A) Treatment of platelets with control IgG and cross-linker F(ab′)2 has no effect on Cvx- and thrombin-stimulated calcium mobilization. (B) (i, ii) Representative calcium responses for stimulation with Cvx at 62.5 ng/mL and 15 ng/mL, respectively, with and without first cross-linking PECAM-1. Traces are representative of 3 separate experiments. (iii) PECAM-1–induced percentage reduction in peak levels of intracellular calcium stimulated by 62.5, 31.25, and 15 ng/mL Cvx. Data represent mean ± SE (n = 3). C (i, ii) Representative calcium responses for stimulation with thrombin at 0.1 U/mL and 0.05 U/mL, respectively, with and without first cross-linking PECAM-1. Traces are representative of 3 separate experiments. (iii) PECAM-1–induced percentage reduction in peak levels of intracellular calcium stimulated by 0.5, 0.1, and 0.05 U/mL thrombin. Data represent mean ± SE (n = 3). PECAM-1 XL, PECAM-1 cross-linking.
PECAM-1 cross-linking inhibits the mobilization of calcium from intracellular stores.
Fura-2 am–loaded human platelets were stimulated with either Cvx or thrombin, and the mobilization of calcium was measured fluorometrically as described in “Materials and methods” (arrow indicates the addition of agonist). (A) Treatment of platelets with control IgG and cross-linker F(ab′)2 has no effect on Cvx- and thrombin-stimulated calcium mobilization. (B) (i, ii) Representative calcium responses for stimulation with Cvx at 62.5 ng/mL and 15 ng/mL, respectively, with and without first cross-linking PECAM-1. Traces are representative of 3 separate experiments. (iii) PECAM-1–induced percentage reduction in peak levels of intracellular calcium stimulated by 62.5, 31.25, and 15 ng/mL Cvx. Data represent mean ± SE (n = 3). C (i, ii) Representative calcium responses for stimulation with thrombin at 0.1 U/mL and 0.05 U/mL, respectively, with and without first cross-linking PECAM-1. Traces are representative of 3 separate experiments. (iii) PECAM-1–induced percentage reduction in peak levels of intracellular calcium stimulated by 0.5, 0.1, and 0.05 U/mL thrombin. Data represent mean ± SE (n = 3). PECAM-1 XL, PECAM-1 cross-linking.
Discussion
Rapid and complete activation of platelets at sites of tissue damage is ensured through numerous positive feedback pathways, mainly through the actions of mediators such as thromboxane A2 and ADP that are released from activated platelets. The existence of such a rapid and reactive system emphasizes the need for effective regulation of platelet function to prevent disorders such as thrombosis and hemorrhage. Platelet reactivity is a controlled balance between positive and negative regulatory factors and signaling mechanisms. Much attention has recently been focused on the identification of the receptors and signaling pathways that lead to platelet activation, particularly on exposure to collagen,30,31,33thrombin,39 and ADP.40 Negative regulatory mechanisms in platelets are less well understood. The effects of endothelium-derived prostacyclin and nitric oxide are recognized for their roles in inhibiting platelet activation in healthy blood vessels. These molecules inhibit activation through cyclic adenosine monophosphate– and cyclic guanosine monophosphate–dependent signaling mechanisms, respectively. We report here on a third negative regulation system that is mediated through a cell-surface ITIM-bearing adhesion receptor, PECAM-1.
Platelet collagen receptor GPVI signals through an ITAM present on the cytoplasmic tail of the FcRγ-chain with which it is associated. The basis for the hypothesis that PECAM-1 may be a negative regulator of platelet activation was the presence of an ITIM because in some contexts ITAM- and ITIM-containing receptors have been reported to function in an antagonistic fashion.34 Indeed, the activation of PECAM-1 by cross-linking has been reported to inhibit signaling by T-cell and B-cell antigen receptors, each of which signals through an ITAM.12,13 We have reported previously that activation of GPVI or thrombin receptors on platelets leads to tyrosine phosphorylation of PECAM-1 in an integrin-independent manner,23 and other reports suggest that thrombin receptor activatory peptide-mediated PECAM-1 tyrosine phosphorylation is enhanced by integrin engagement.24 In addition, platelet activation with Cvx or thrombin results in increased cell surface exposure of PECAM-1.23 If PECAM-1 signaling is inhibitory in platelets, this may form part of a negative feedback pathway.
PECAM-1 was stimulated through cross-linking using antibodies directed to the extracellular domain of the receptor. This strategy was chosen as the most specific manner through which to activate PECAM-1, and activation was confirmed because cross-linking stimulated its tyrosine phosphorylation. Confusion surrounds the natural endogenous ligand for PECAM-1 on platelets in vivo. As described earlier, PECAM-1 has been shown to participate in homophilic and heterophilic ligand binding. One would anticipate that results similar to those described here would be obtained using the recombinant extracellular domain of PECAM-1 as ligand; however, results of such experiments may be complicated by the ability of PECAM-1 to bind to other cell surface proteins. As has been reported previously, PECAM-1 cross-linking stimulates tyrosine phosphorylation (Figure 1) and association of SHP-2 (not shown) but did not in itself cause platelet activation. Furthermore, tyrosine phosphorylation of PECAM-1 on cross-linking appeared not to be dependent on integrin αIIbβ3 engagement, though we cannot rule out the possibility that in vivo it may enhance this effect. Cross-linking PECAM-1 for 90 seconds before stimulation with collagen caused the inhibition of platelet aggregation. At lower concentrations of collagen, aggregation was inhibited completely, but even at high concentrations of collagen (100 μg/mL) PECAM-1 activation caused a substantial inhibition of aggregation. Because collagen is also able to bind other receptors on the platelet, including the integrin α2β1, we examined the effect of PECAM-1 cross-linking on GPVI-mediated platelet aggregation using the specific agonist Cvx. Similar results were obtained, with complete inhibition of aggregation at lower concentrations of agonist (15 ng/mL) and partial effects at higher concentrations (62.5 ng/mL). This suggests that PECAM-1 is a potent inhibitor of GPVI-mediated (ITAM) platelet activation. This is consistent with the findings of Patil et al,41 who have recently reported that PECAM-1–deficient mouse platelets are hyperresponsive to collagen and display enhanced collagen adhesion.
Experiments were conducted to determine whether the inhibitory effect of PECAM-1 is restricted to signaling through ITAM-containing receptors. On the contrary, PECAM-1 cross-linking was also found to inhibit thrombin-stimulated platelet aggregation. It is not possible to compare directly the potency of 2 different agonists that mediate their effects through different receptors and signaling pathways. However, no inhibitory effect of PECAM-1 was observed at moderate concentrations of thrombin (0.5 and 1 U/mL; results not shown), where dramatic levels of inhibition were observed at high concentrations of collagen (100 μg/mL). PECAM-1–mediated inhibition of thrombin-stimulated aggregation was observed only at lower thrombin concentrations (complete inhibition with 0.05 U/mL and slight inhibition at 0.1 U/mL). This suggests that PECAM-1 activation inhibits thrombin-stimulated platelet aggregation less efficiently than it does collagen-stimulated aggregation, consistent with the general inhibitory effect on platelet aggregation by an antibody bound to PECAM-1 reported by Wu et al.42 We also examined the effect of PECAM-1 cross-linking on platelet aggregation stimulated by other G protein–coupled receptor agonists. Aggregation in response to low concentrations of the thromboxane mimetic U46619 was also reduced by PECAM-1 signaling (M.C. and J.M.G., unpublished results, May 2001). In addition, preliminary work suggests that ADP-induced platelet aggregation at low agonist concentrations may also be affected. This is consistent with PECAM-1 performing a negative regulatory role in the control of platelet activation stimulated by ITAM- and non–ITAM-containing receptor agonists. Platelet responses that are not dependent ITAM-bearing– or G protein–coupled receptors—for example, ristocetin-induced agglutination—are not altered by PECAM-1 signaling (results not shown). In contrast to our results, thrombin- and ADP-stimulated platelet aggregation has been reported to be normal in PECAM-1–deficient mouse platelets.11,41 In these 2 reports, the effect of removing the negative regulator from platelets was investigated, whereas we examined the consequences of stimulating the receptor to a high level. Our approach was more likely to reveal more modest inhibitory effects, such as those observed for thrombin, through which no change was observed in removing PECAM-1 from the cells. Patil et al41 have reported recently that PECAM-1–deficient platelets are hyperresponsive to collagen and collagen-related peptide (CRP), but not to thrombin.41 In assays of platelet aggregation and secretion, hyperresponsiveness was almost completely lost at high concentrations of collagen and CRP (10 μg/mL and 5 μg/mL, respectively). The concentrations of thrombin used in their experiments were 0.1 U/mL and 1 U/mL. At these concentrations the results obtained appeared similar to those seen at high concentrations of collagen or CRP, particularly on aggregation traces. This suggests that hyperresponsiveness to thrombin might have been masked because of the relatively high concentrations used. Indeed, in our experiments in which PECAM-1 was activated by cross-linking, the inhibition of thrombin-stimulated responses were modest where 0.1 U/mL of thrombin was used, and no inhibition was observed at higher concentrations. We suggest that closer examination of responses in PECAM-1–deficient platelets to lower concentrations of thrombin, ADP, and U46619 may clarify this matter. The observation that ITAM-mediated signaling is inhibited to a higher degree than non-ITAM–mediated signaling is further supported by experiments in platelets in which PECAM-1 was coligated with FcγRIIA (an ITAM-containing IgG receptor). Coligation resulted in the inhibition of FcγRIIA-mediated platelet aggregation and intracellular calcium mobilization stimulated by receptor cross-linking (M.C. and J.M.G., unpublished data, December 2000). Platelet secretion in response to Cvx and thrombin was also inhibited to a significant level by PECAM-1 cross-linking. It is unclear whether the inhibition of platelet aggregation after PECAM-1 cross-linking was a consequence of reduced integrin αIIbβ3 regulation or reduced secretion of fibrinogen from α-granules (these experiments were performed on washed platelets; therefore, for aggregation, fibrinogen must be secreted). The inhibitory effects of PECAM-1 on stimulation with agonists—such as the collagen Cvx, thrombin, and the thromboxane mimetic U46619—suggest that PECAM-1 may inhibit primary signaling events and secondary stimulation by factors released by activated platelets. Indeed, Nieswandt et al43 have recently reported cross-talk between GPVI and Gi-coupled receptors for ADP and adrenaline. It is possible that the inhibition of receptors that cross-talk with GPVI-mediated signaling may contribute to the inhibitory actions of PECAM-1 on collagen- and Cvx-stimulated activation. This may also explain why the inhibitory functions of PECAM-1 on GPVI-mediated activation are more effective than on thrombin-mediated activation.
Having established that PECAM-1 cross-linking inhibits platelet function, we examined the effect of this on some aspects of signal transduction. Platelet activation by the collagen receptor GPVI is dependent on tyrosine kinases and consequently is associated with the rapid tyrosine phosphorylation of a wide variety of platelet proteins. Stimulation with thrombin results in protein tyrosine phosphorylation, but to a lesser degree. In this study we have demonstrated that cross-linking PECAM-1, which in itself does not alter whole-cell protein tyrosine phosphorylation levels, inhibits substantially the level of tyrosine phosphorylation induced by subsequent stimulation with Cvx or thrombin. This is consistent with the reduction observed in aggregation and secretion. The identities of the phosphoproteins whose phosphorylation is reduced on PECAM-1 cross-linking is under investigation. It has been reported that PECAM-1 stimulation by cross-linking inhibits the mobilization of calcium from intracellular stores by the T-cell antigen receptor.12 A recent report suggests that a similar effect is seen with B-cell antigen receptors.13 Indeed, all the observations presented in this report have been reproduced in platelets using one of the anti–PECAM-1 antibodies used in the above 2 studies (PECAM 1.3). Curiously, cross-linking PECAM-1 on endothelial cells has been reported to stimulate increases in intracellular calcium concentration.44 In the data presented here, we demonstrate that underlying the PECAM-1–mediated inhibition of platelet activation is a significant level of inhibition of calcium release from intracellular stores. As seen with aggregation assays, at lower concentrations of Cvx and thrombin (15 ng/mL and 0.05 U/mL, respectively), PECAM-1 signaling inhibits release almost completely, whereas a partial effect is observed at higher agonist concentrations. Calcium mobilization is stimulated through the intracellular generation of inositol 1,4,5-trisphosphate from phosphatidylinositol 4,5-bisphosphate by phospholipase C (PLC). It is well established that in platelets, stimulation with collagen leads to phosphorylation and activation of PLCγ2 isoform and that thrombin signaling regulates PLCβ.45,46 47 We have begun to investigate the signaling that links PECAM-1 activation with the inhibition of calcium mobilization generated by the collagen- and thrombin-receptor signaling pathways by examining the production of inositol phosphates. PECAM-1 cross-linking results in a substantial and significant reduction in total inositol phosphates stimulated by Cvx and thrombin (M.C. and J.M.G., unpublished data, May 2001). This suggests that PECAM-1 exerts its effects on collagen- and thrombin-stimulated signaling either upstream of the PLC isoforms or on these enzymes themselves. Much work must be done, however, to establish how PECAM-1 signaling exerts its effects on different platelet activation signaling pathways.
This report illustrates that PECAM-1, an ITIM-containing receptor, is capable of regulating platelet function. However, its role in vivo remains to be established. It is possible that this molecule provides a mechanism to prevent platelet activation on collision of platelets with healthy endothelium and collisions with other platelets. PECAM-1 signaling may result in a negative feedback on platelet activation pathways and thereby set the threshold stimulation level for platelet activation. PECAM-1 ligation on platelet contact, and between platelets and healthy endothelium, may also be instrumental in limiting the size of a thrombus formation through inactivating platelets on the thrombus periphery. A recent report by Vollmar et al45 using an in vivo thrombosis model in PECAM-1 null mice suggests that PECAM-1 has no role in vascular thrombosis. This is surprising given the in vitro data presented here and ex vivo studies on PECAM-1–deficient platelets.41 This may be explained by the redundancy in activation systems through which platelets may be stimulated given that we show here that thrombin-induced platelet activation is less sensitive to inhibition by PECAM-1. It is also highly likely that additional ITIM-containing receptors are expressed on the platelet, resulting in redundancy in inhibitory regulation systems. The above report does not address the effect of PECAM-1 deficiency on larger arterial vessels in which shear forces are likely to reach higher levels and platelet thrombi may be encountered. This is a complex area, further complicated by the potential roles of PECAM-1 in endothelial cell function, and it requires greater investigation.
In conclusion, this report provides evidence for an extension of the known roles of ITIM receptors in the negative regulation of nonimmune cell function. In addition, in this study we have begun to explain at the cell signaling level the inhibitory effects of PECAM-1 on platelet activation. Future work is required to determine precisely how PECAM-1 inhibits the signaling pathways activated by multiple platelet agonists.
We thank Prof Peter Newman for providing the antibody PECAM 1.3 and for valuable discussion regarding this study. We also thank Dr Fiona Barry and Dr Peter Jordan for their advice in the preparation of this report and Tanya Sage for expert technical assistance.
Supported by funding from the Biotechnology and Biological Sciences Research Council, the Medical Research Council, and the University of Reading Research Endowment Trust Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jonathan Gibbins, Cardiovascular Research Group, School of Animal and Microbial Sciences, University of Reading, Whiteknights, Reading RG6 6AJ, United Kingdom; e-mail:j.m.gibbins@reading.ac.uk.

![Fig. 3. Platelet-dense granule secretion is inhibited by PECAM-1 signaling. / Platelets were loaded with [3H]5-HT before stimulation with Cvx (62.5 ng/mL) or thrombin (0.1 U/mL). Where required, PECAM-1 was cross-linked an agonist was added (as described in “Materials and methods”). Secretion of [3H]5-HT into cell medium was measured using scintillation spectrometry. [3H]5-HT release is expressed as a percentage of total tissue content after subtraction of basal secretion values. Results represent mean ± SE (n = 3). Student t test was used to compare PECAM-1 cross-linked and non–cross-linked sample for statistical significance. *P < .05. PECAM-1 XL, PECAM-1 cross-linking](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/1/10.1182_blood.v99.1.137/6/m_h80121926003.jpeg?Expires=1769240243&Signature=x2U0~tLF0VfrFlPFEeuTjcwp4TWQJpnHCtRqzouWcxw-9dd7rVE7Rf~h5i841wyoe-Yez2QZRdk2inWUDAtnZsV8CFCakxfzDXWYATf9ReLB9POxtO1vbuEF~hy2-A1qV7WwOtufvGAoOTDxFAltn4VMaLAXqGDS40-NS26pjXBkGRDd1Ppb4KmJinwm~sJhvSPOOkW1BEMJ6Ccl5kQ~~p3wjGO8G~ZNWdf~Sld38IZ0xpN03BGcQKcz5ILPa2lzYmjrvZVe0rB-lO4JcL4QiyQxU2c~6Qmi0iyqc19GYkjVHO84K9joXHMxLAS~PVmAnDRG5NfWBgLH5JEy-L149g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal