When interleukin-2 (IL-2) was added to immature, low-ploidy (greater than 80% 2N+4N) megakaryocytes generated in IL-3 and stem cell factor (SCF)–containing liquid cultures of blood mononuclear cells highly enriched in hematopoietic progenitors, a 2- to 6-fold increase in the absolute number of polyploid (more than 8N) megakaryocytes was noted. This effect was found to be indirect and was mediated through natural killer (NK) cells that constitute the major lymphoid cell contaminating day 6 megakaryocyte cell populations. IL-2 had no effect on megakaryocytes generated from CD34+ cells stimulated with IL-3 and SCF. However, medium conditioned by IL-2–stimulated, but not resting, NK cells (NKCM) contained a trypsin-sensitive factor capable of increasing 2- to 5-fold the number of polyploid megakaryocytes generated in vitro from IL-3 and SCF-stimulated CD34+ cells. The activity in NKCM was dose dependent and could not be neutralized by an excess of antibodies to IL-6, IL-11, leukemia inhibitory factor (LIF), gp130, stromal cell derived factor-1a (SDF-1a), and thrombopoietin (TPO). Addition of IL-11, but not TPO, to NKCM-containing cultures resulted in further augmentation of polyploidy, with the generation of 50% to 70% polyploid megakaryocytes with a modal ploidy of 16N. This factor is distinct from TPO because it induces endomitosis in IL-3–generated megakaryocytes in vitro, whereas TPO does not, and its activity on megakaryocyte ploidy is not altered by optimal concentrations of TPO. In addition, no message for TPO is detectable in IL-2–stimulated NK cells by reverse transcription–polymerase chain reaction. These findings indicate that IL-2–stimulated NK cells produce a novel peptide, distinct from TPO, IL-6, IL-11, LIF, other gp130-associated interleukins, and SDF1a, that can induce in vitro endomitosis in immature human megakaryocytes in the presence of IL-3 and SCF.
Introduction
Polyploidy is a unique feature of bone marrow megakaryocytes. Terminal differentiation of megakaryocytes includes the development of polyploid cells through the process of endomitosis. Although the need for megakaryocytes to achieve polyploid status is poorly understood, it seems related to their ability to produce platelets because the modal ploidy of marrow megakaryocytes increases in states of accelerated thrombopoiesis and decreases in states of suppressed thrombopoiesis.1,2 Humoral factors that may influence the ploidy of megakaryocytes are only partially known. Among them, thrombopoietin has been shown to be capable of inducing endomitosis in vitro in serum-containing cultures of unseparated whole marrow murine cells,3 but its activity on endomitosis is limited in serum-free cultures of purified human CD34+cells.4 5
During studies on the effects of various known growth factors, interleukins, and cytokines on the ploidy of human blood CFUMEG–derived megakaryocytes in vitro, it was noted than when interleukin-2 (IL-2) was added to cultures containing optimal concentrations of IL-3 and stem cell factor (SCF), the ploidy of generated megakaryocytes was shifted toward higher classes. This observation led to further experiments, described herein, by which a unique soluble peptide was identified that is secreted by IL-2–stimulated natural killer cells and is capable of inducing endomitosis in human megakaryocytes generated in vitro under the stimulation of IL-3 and SCF.
Materials and methods
Cell separation and cell cultures
The effect of various factors on endomitosis was tested on immature human megakaryocytes generated in vitro from peripheral blood mononuclear cells of healthy volunteers.6 The studies were approved by the McGuire Research Institute Institutional Review Board. Approximately 300 mL peripheral blood was collected in heparin, and the light-density mononuclear cells were separated by centrifugation over Ficoll-Hypaque (density, 1.077) for 20 minutes at 700g at room temperature. Platelets were then removed by 2 sequential centrifugations over Ficoll-Hypaque (density, 1.044) at 600g for 10 minutes. T lymphocytes and Fcγ-bearing cells were removed by rosetting with sheep erythrocytes and with sheep erythrocytes coated with goat anti-sheep red blood cell immunoglobulin (Ig)G.7 Residual adherent cells and clumps of platelets were removed by adherence to plastic. Remaining cells were further enriched in hematopoietic progenitors by negative panning on dishes coated with soybean agglutinin (SBA) at 4°C for 40 minutes.8 Nonadherent SBA-negative cells were then coated with a mixture of monoclonal antibodies containing 1 to 2 μg from each antibody per 107 cells of anti-CD2, -CD4, -CD11b, and -CD20. After removing the unbound antibody through washings, the cells were incubated at 4°C for 45 minutes in plastic tissue culture dishes that had been coated with affinity-purified goat anti-murine IgG.7 Antibody-negative, nonadherent cells were then collected and plated in liquid medium at a concentration of 2.5 × 105 cells/mL. The culture medium consisted of Iscoves modified Dulbecco medium (IMDM) containing 5% human plasma supplemented with 150 μg/mL human iron-saturated transferrin, 10 μg/mL human insulin, 2 mg/mL bovine serum albumin, 1 μg/mL soybean lipids, 2 mM L-glutamine, 1 mM sodium pyruvate 1000, 1 × 10−5 M β-mercaptoethanol, 2000 U/mL penicillin, and 10 μg/mL gentamicin. IL-3 and SCF were added at a concentration of 200 U/mL and 100 ng/mL, respectively. All other factors when used were added to cultures at concentrations designated in specific experiments.
In a number of experiments in which CD34+ cells were used for the generation of immature day 6 megakaryocytes in vitro, cells were isolated from light-density mononuclear blood cells by anti-CD34–coated magnetic beads (Dynabeads, Dynal, Oslo, Norway) according to the manufacturer's protocol. They were cultured in medium as already described for 12 to 14 days at 37°C in a highly humidified 5% CO2 atmosphere. They were then collected, counted, and analyzed for CD41+ cells and distribution into ploidy classes by flow cytometry.
Natural killer (NK) lymphocytes were isolated from peripheral blood mononuclear cells by density-gradient centrifugation over Percoll (density, 1.066)9 followed by depletion of T lymphocytes either by sheep red cell rosetting or by anti-CD3–coated magnetic beads. In other experiments, NK cells were isolated by anti-CD56–coated magnetic beads. After overnight incubation in IMDM with 10% autologous serum and 4 nM (ng/mL) IL-2, were separated from the beads and depleted of T lymphocytes with anti-CD3–coated magnetic beads. Medium conditioned by NK lymphocytes was prepared by culturing NK cells at a concentration of 5 × 104/mL in IMDM with 5% human serum in the presence of 10 ng/mL IL-2 for 3 to 7 days at 37°C in a 5% CO2highly humidified atmosphere.
Growth factors and antibodies
All growth factors, cytokines, and antibodies used in the described experiments were purchased from R&D Systems (Minneapolis, MN), and all antibodies for cell isolation or flow cytometry were from Becton Dickinson unless otherwise indicated.
Measurement of megakaryocyte ploidy
Measurement of DNA content of CFUMEG-derived cells was performed by 2-color flow cytometry.10 Cells from liquid cultures were removed on day 12 to 14 and were stained with fluorescein isothiocyanate (FITC)–conjugated anti-CD41 (Gen Trak, Plymouth Meeting, PA) or isotype control murine IgG1 (DAKO, Carpinteria, CA). After washing, the cells were fixed in 70% cold methanol for 30 minutes at 4°C, washed again, and treated at 37°C for 30 minutes with DNase-free RNase (Cellular DNA-Flow Cytometric Analysis Reagent Set; Boehringer Mannheim, Indianapolis, IN). After staining with propidium iodide (50 μg/mL), nuclear ploidy was measured by a FACScan (Becton Dickinson) flow cytometer. Fluorescence intensity from FITC and propidium iodide was analyzed using logarithmic display. Megakaryocytes were selected on the basis of specific membrane immunofluorescence. A threshold for GPIIb/IIIa expression (CD41) was determined using the mouse isotype control. A fluorescence gate containing 2% or less of the isotype control-positive cells was established, and the propidium iodide staining of cells within this gate was used to analyze ploidy. Ploidy distribution was determined by setting markers at the nadirs between peaks at approximately 50-channel intervals. At least 10 000 CD41+ cells were analyzed per experiment. All data were stored in list mode and then analyzed using FACScan Research Software. Results were expressed as the mean ± SEM of data obtained from 3 or more experiments. Statistical significance of ploidy distribution was determined using the χ2 test.
Reverse transcription–polymerase chain reaction for thrombopoietin and SDF1a
Total NK RNA was prepared with the use of guanidinium salts,11 and mRNA was purified by oligo-dT–coated magnetic beads (Dynal). NK mRNA (200 ng) and an equal amount of human liver mRNA (Clontech) were subjected to reverse transcription–polymerase chain reaction (RT-PCR). Reverse transcription was performed using the SuperScript Preamplification kit (BRL) with both oligo-dT12-18 and random hexamers according to the manufacturer's protocol. For amplification of thrombopoietin (TPO) message, the products of this reaction were subjected to PCR using two 20-mers as primers corresponding to positions 301 to 320 (5′ primer) and 551 to 570 (3′ primer) of the TPO complementary DNA (cDNA) sequence and Taq polymerase. The 270-bp sequence is part of the TPO cDNA that encodes for the active part of the TPO protein. As a control for the integrity of mRNA and the absence of inhibitors, a 5′ and a 3′ 20-mer of a 1300-bp sequence of the transferrin receptor (rTF Amplimer; Clontech) were used. After 30 cycles, the PCR products were analyzed by agarose gel electrophoresis.
For amplification of SDF1a message, the products of reverse transcription were subjected to PCR using 2 oligodeoxyribonucleotide primers corresponding to positions 160 to 184 (5′ primer) and 559 to 533 (3′ primer) of the human SDF1a cDNA (Maxim Biotech, San Francisco, CA) in the presence of Taq polymerase. The 400-bp sequence is part of human SDF1a cDNA encoding active protein. As a control for the integrity of mRNA and the absence of inhibitors, a 5′ and a 3′ 20-mer of a 329-bp sequence of the enolase cDNA were used. As a positive control, 400-bp human SDF1a cDNA (Maxim Biotech) was used. After 30 cycles, the PCR products were analyzed by agarose gel electrophoresis.
Results
Interleukin-2 induces endomitosis in immature megakaryocytes generated from blood CFUMEG in vitro
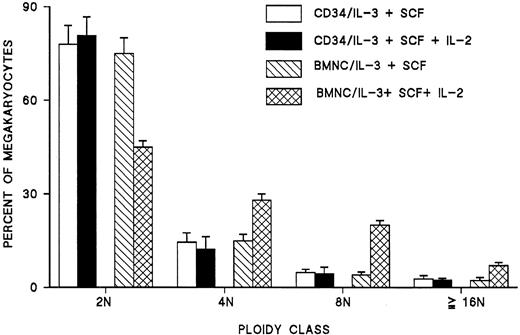
When IL-2 was added to cultures of peripheral blood mononuclear cells enriched in hematopoietic progenitors containing optimal concentrations of IL-3 and SCF, the ploidy of generated megakaryocytes was shifted to higher classes (Figure 1). Among 12 experiments, the mean proportion of megakaryocytes greater than or equal to 8N increased from 26% ± 5% to 45% ± 4% (P < .0001), and the mean megakaryocyte ploidy increased from 3.3 ± 0.3N to 4.9 ± 0.2N (P < .0001). A typical histogram of megakaryocyte ploidy by flow cytometry of CD41+ cells from cultures without or with IL-2 is shown in Figure 2. Addition of IL-2 to cultures did not affect in any consistent way the number of megakaryocytes generated in vitro from blood CFUMEG.
Effect of addition of IL-2 (2 ng/mL) to day 6 cultures of peripheral blood mononuclear cells highly enriched in hematopoietic progenitors on the ploidy of CFU-MK derived megakaryocytes.
Each point represents the mean ± SEM from 12 experiments.
Effect of addition of IL-2 (2 ng/mL) to day 6 cultures of peripheral blood mononuclear cells highly enriched in hematopoietic progenitors on the ploidy of CFU-MK derived megakaryocytes.
Each point represents the mean ± SEM from 12 experiments.
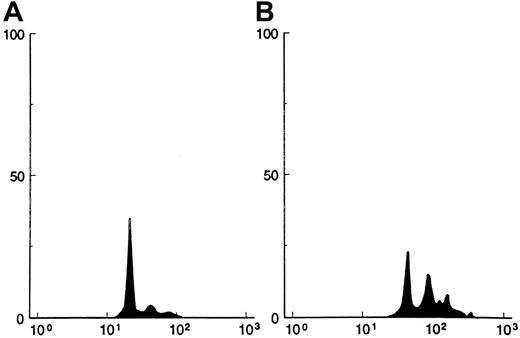
Histogram of megakaryocyte ploidy by flow cytometry from a representative experiment.
The horizontal axis represents the intensity of fluorescence from propidium iodide expressed in a logarithmic scale, and the vertical axis represents the number of CD41+ cells. (A) Histogram from cultures without IL-2. (B) Histogram from cultures with IL-2 (2 ng/mL). In this experiment, the ploidy distribution in 2N, 4N, 8N, and more than 8N was 77%, 15%, 6.5%, and 1.5%, respectively, for panel A and 38%, 33%, 22%, and 7%, respectively, for panel B.
Histogram of megakaryocyte ploidy by flow cytometry from a representative experiment.
The horizontal axis represents the intensity of fluorescence from propidium iodide expressed in a logarithmic scale, and the vertical axis represents the number of CD41+ cells. (A) Histogram from cultures without IL-2. (B) Histogram from cultures with IL-2 (2 ng/mL). In this experiment, the ploidy distribution in 2N, 4N, 8N, and more than 8N was 77%, 15%, 6.5%, and 1.5%, respectively, for panel A and 38%, 33%, 22%, and 7%, respectively, for panel B.
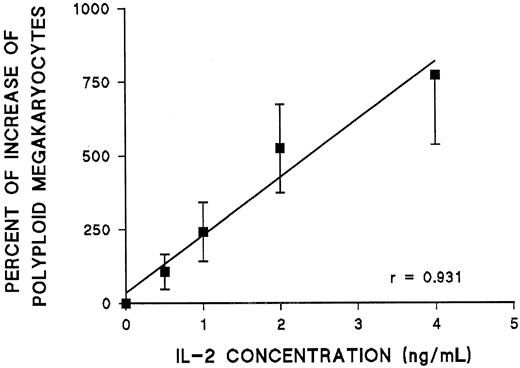
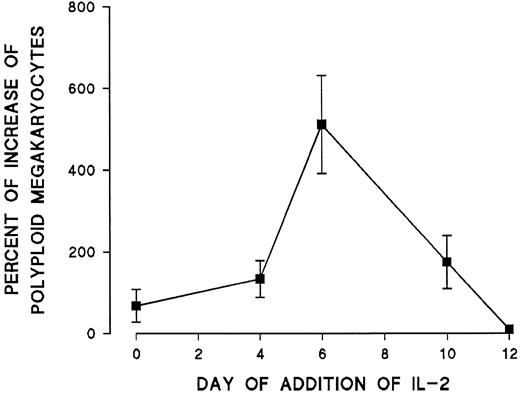
The effect of IL-2 on endomitosis was dose dependent between 0.5 and 4 ng/mL (Figure 3). Further increases in IL-2 concentration did not result in any additional increase of polyploid megakaryocytes (more than 8N) in vitro. Because previous work in this laboratory indicated that most endomitotic events occur between days 6 and 10 in culture,6 time-course experiments were performed in which IL-2 was added to the cultures on different days. The effect of IL-2 was mostly pronounced when IL-2 was added on day 6 of cultures. Less pronounced effects could be seen when IL-2 was added earlier, and its effect on ploidy of megakaryocytes disappeared on day 10 (Figure 4). In all subsequent experiments, IL-2 was added to the culture medium on day 6.
Effect of increasing concentrations of IL-2 added to day 6 cultures on the ploidy of CFU-MK–derived megakaryocytes.
For each concentration of IL-2, the percentage increase in the absolute number of polyploid megakaryocytes (more than 4N) was calculated in comparison with cultures without IL-2. Each point represents the mean ± SEM from 3 experiments.
Effect of increasing concentrations of IL-2 added to day 6 cultures on the ploidy of CFU-MK–derived megakaryocytes.
For each concentration of IL-2, the percentage increase in the absolute number of polyploid megakaryocytes (more than 4N) was calculated in comparison with cultures without IL-2. Each point represents the mean ± SEM from 3 experiments.
Effect of addition of IL-2 (2 ng/mL) at different times to cultures of blood mononuclear cells enriched in progenitors.
Percentage increase of the polyploid megakaryocytes (more than 4N) was calculated in comparison with cultures that received no IL-2. Each point represents the mean ± SEM from 3 experiments.
Effect of addition of IL-2 (2 ng/mL) at different times to cultures of blood mononuclear cells enriched in progenitors.
Percentage increase of the polyploid megakaryocytes (more than 4N) was calculated in comparison with cultures that received no IL-2. Each point represents the mean ± SEM from 3 experiments.
Effect of IL-2 on megakaryocyte endomitosis is mediated by natural killer cells
Day 6 megakaryocytes, the cells most responsive to IL-2, were found to have no receptors for IL-2 as tested by FACS using CD41-phycoerythrin and FITC-conjugated CD25 or CD122 for the IL-2 receptor α and β, respectively. Adding IL-2 to CD34+cells failed to cause a shifting of megakaryocyte ploidy in vitro (Figure 5). These experiments were interpreted as evidence that the effect of IL-2 on developing megakaryocytes is indirect.
Effects of IL-2 on the polyploidy of CFU-MK–derived megakaryocytes in blood CD34+ cell cultures as opposed to cultures of blood mononuclear cells highly enriched in hematopoietic progenitors.
The effect of IL-2 on megakaryocyte ploidy cannot be detected in cultures of CD34+ cells. Each point represents the mean ± SEM of 4 experiments.
Effects of IL-2 on the polyploidy of CFU-MK–derived megakaryocytes in blood CD34+ cell cultures as opposed to cultures of blood mononuclear cells highly enriched in hematopoietic progenitors.
The effect of IL-2 on megakaryocyte ploidy cannot be detected in cultures of CD34+ cells. Each point represents the mean ± SEM of 4 experiments.
To identify the nature of the accessory cell(s) responsible for the IL-2 effect, we analyzed by FACS and a battery of monoclonal antibodies the cell population from day 6 cultures. Seventy-five percent to 90% of the cells expressed CD33, of which 15% to 27% were positive for CD41 and the remaining for CD13. Of the remaining nonmyeloid cells, T and B cells (CD3 and CD19) made up 2% to 3% of the total cells; the remaining were CD56+, CD11+, and CD16+. Thus, the major type of accessory cells in day 6 cultures were phenotypically NK cells. It seems that the use of CD2 and CD11 for the removal of T lymphocytes, monocytes, and NK cells during the process of enrichment of peripheral blood mononuclear cells in hematopoietic progenitors by negative panning was not sufficient for the effective removal of NK cells.
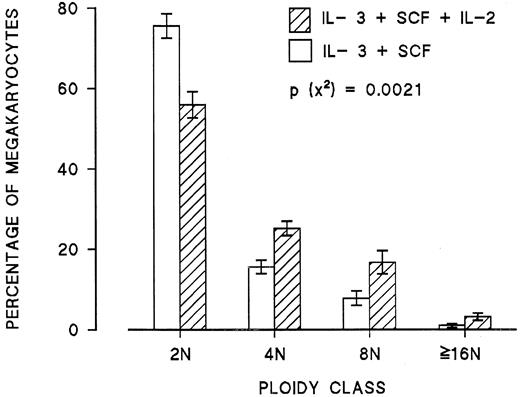
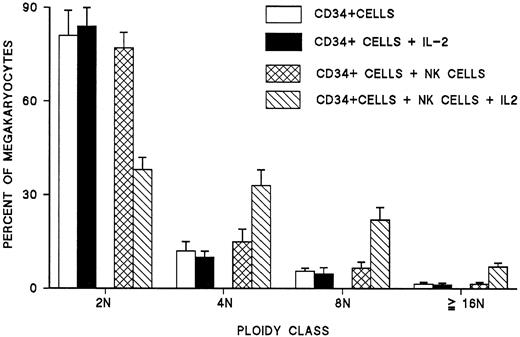
The role of NK cells was further confirmed by purifying and culturing them in the presence of IL-2. After 7 to 10 days, more than 85% of cultured cells expressed CD16 and CD56, and almost 95% of them exhibited the morphology of large granular lymphocytes with blastic nuclei. When these cells were washed and added to autologous CD34+ cells grown in the presence of IL-3 and SCF, no statistically significant change in the ploidy of megakaryocytes was noted. However, when IL-2 was added to these cultures, a significant increase in the ploidy of megakaryocytes was observed as compared with cultures of CD34+ cells alone (Figure6). Similar experiments performed by coculturing purified autologous T lymphocytes or monocytes with CD34+ cells with or without IL-2 showed no effect on the ploidy of day 6 megakaryocytes (data not shown). These experiments confirmed the role of NK cells as mediators of IL-2 effects on megakaryocyte ploidy.
Effect of addition of NK cells and IL-2 either alone or in combination on the ploidy of megakaryocytes generated from CD34+ blood cells under the stimulation of IL-3 and SCF.
Each point represents the mean ± SEM of 3 experiments run in quadruplicate.
Effect of addition of NK cells and IL-2 either alone or in combination on the ploidy of megakaryocytes generated from CD34+ blood cells under the stimulation of IL-3 and SCF.
Each point represents the mean ± SEM of 3 experiments run in quadruplicate.
Interleukin-2–stimulated natural killer cells release a soluble peptide responsible for induction of endomitosis
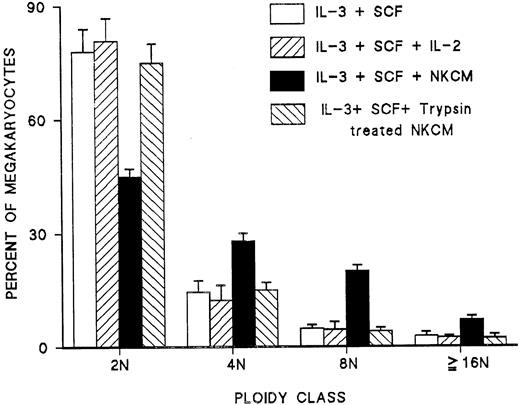
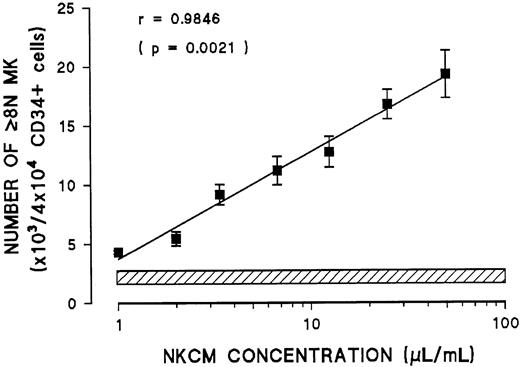
Addressing whether NK cells release a soluble factor that mediates the effect of IL-2 or whether they require direct cell-to-cell interaction with day 6 megakaryocytes, conditioned medium from NK cells (NKCM) was tested on blood CD34+ cells grown for 6 days under the stimulation of IL-3 and SCF. This day 6 cell population was tested by FACS and was found to contain no CD56+ cells. Addition of NKCM, but not IL-2 alone, to day 6 megakaryocytes resulted in the induction of endomitosis and the generation of polyploid megakaryocytes after 6 days in culture (Figure7). Furthermore, the effect of NKCM on the number of polyploid megakaryocytes was dose dependent (Figure8). Medium conditioned by purified T cells or monocytes stimulated with IL-2 had no effect on the ploidy of day 6 megakaryocytes (data not shown). These experiments demonstrated that the effect of IL-2 by NK cells is mediated through a soluble factor released in the conditioned medium.
Effect of medium conditioned by NK cells stimulated with IL-2 (NKCM) on the polyploidy of megakaryocytes derived from blood CD34+ cells stimulated with IL-3 and SCF.
IL-2 by itself had no effect. The activity in the NKCM that induced endomitosis was abolished by pretreatment of NKCM with trypsin immobilized to agarose. Each point represents the mean ± SEM from triplicate cultures of 3 experiments.
Effect of medium conditioned by NK cells stimulated with IL-2 (NKCM) on the polyploidy of megakaryocytes derived from blood CD34+ cells stimulated with IL-3 and SCF.
IL-2 by itself had no effect. The activity in the NKCM that induced endomitosis was abolished by pretreatment of NKCM with trypsin immobilized to agarose. Each point represents the mean ± SEM from triplicate cultures of 3 experiments.
Effect of increasing concentration of medium conditioned by NK cells stimulated with IL-2 (NKCM) on the number of polyploid megakaryocytes (more than 4N) generated in vitro from blood CD34+ cells stimulated with IL-3 and SCF.
Hatched line represents the range of polyploid megakaryocytes generated in the absence of NKCM. Each point represents the mean ± SEM of quadruplicate cultures in 3 experiments.
Effect of increasing concentration of medium conditioned by NK cells stimulated with IL-2 (NKCM) on the number of polyploid megakaryocytes (more than 4N) generated in vitro from blood CD34+ cells stimulated with IL-3 and SCF.
Hatched line represents the range of polyploid megakaryocytes generated in the absence of NKCM. Each point represents the mean ± SEM of quadruplicate cultures in 3 experiments.
To characterize the chemical nature of this factor, NKCM was treated with trypsin immobilized on agarose in the presence of 1 mM EDTA for 1 hour at 37°C; the beads were then removed by centrifugation, and the NKCM was dialyzed in IMDM, filtered, and tested on day 6 immature megakaryocyte derived from CD34+ blood cells. Control medium was exposed to plain agarose beads and treated through the same procedures. Exposure of conditioned medium to trypsin resulted in complete loss of the activity of the soluble factor inducing megakaryocyte endomitosis compared with control-conditioned medium (Figure 7), indicating that the factor responsible for this activity is a peptide released in the culture medium.
NK-released peptide is a novel lymphokine distinct from IL-6, IL-11, LIF, other gp130-related interleukins, and SDF1a
Because the activation of NK cells is known to result in the release of a variety of growth factors and interleukins9 12 in subsequent experiments we investigated the effect of IL-1, -4, -6, -7, -8, -9, -10, -11, -12, tumor necrosis factor-α, interferon (IFN)α, MIP1a, and granulocyte macrophage–colony-stimulating factor (GM-CSF), G-CSF, and M-CSF on the ploidy of megakaryocytes generated from CD34+ blood cells compared with NKCM. No one of these factors was found to be able to induce endomitosis with shifting of the megakaryocyte ploidy, as was invariably noted with NKCM (data not shown). Therefore, the effect of NKCM could not be attributed to the presence of either of these factors in the conditioned medium.
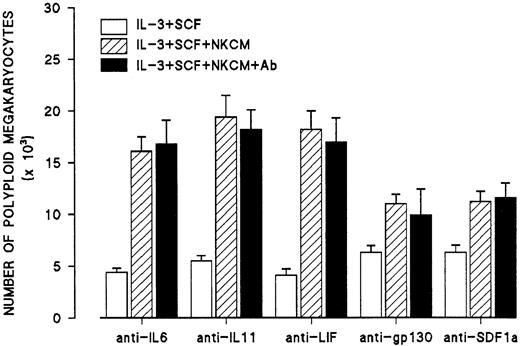
Because IL-6, IL-11, and leukemia inhibitory factor (LIF) have been proposed as factors promoting maturation and endomitosis in megakaryocytes, additional experiments were performed in which NKCM was incubated with an excess of neutralizing antibodies to IL-6, IL-11, and LIF at 37°C for 1 hour and then were added to day 6 megakaryocytes. The concentration of neutralizing antibodies in these experiments was capable of inhibiting 5 to 10 times the amounts of IL-6, IL-11, or LIF that have been reported to increase the percentage of megakaryocytes in a single ploidy class. In these experiments, the shifting of megakaryocyte ploidy toward higher classes was not affected by any of the neutralizing antibodies (Figure 9). Similar experiments were performed using an antibody to gp-130 to exclude the possibility that the effects of NKCM were mediated through a combination of gp130-related interleukins or a gp130-related interleukin other than IL-6, IL-11, and LIF (Figure 9). In addition, since a recent report indicated that human stromal cell derived factor-1a (SDF-1a) increases ploidy of human megakaryocytes in vitro,13 similar experiments were performed using a neutralizing antibody to human SDF1a (Figure 9). Furthermore, mRNA from IL-2–activated NK cells was subjected to RT-PCR for the amplification of human SDF1a message showing that activated NK cells do not express SDF1a (Figure 10).
Effects of antibodies to IL-6, IL-11, LIF, gp130, and SDF1a on the endomitosis-inducing activity in NKCM stimulated with IL-2.
Control cultures (second bars, IL-3 + SCF + NKCM) contain mouse IgG isotype control. Each point represents the mean ± SEM from quadruplicate cultures of 2 experiments.
Effects of antibodies to IL-6, IL-11, LIF, gp130, and SDF1a on the endomitosis-inducing activity in NKCM stimulated with IL-2.
Control cultures (second bars, IL-3 + SCF + NKCM) contain mouse IgG isotype control. Each point represents the mean ± SEM from quadruplicate cultures of 2 experiments.
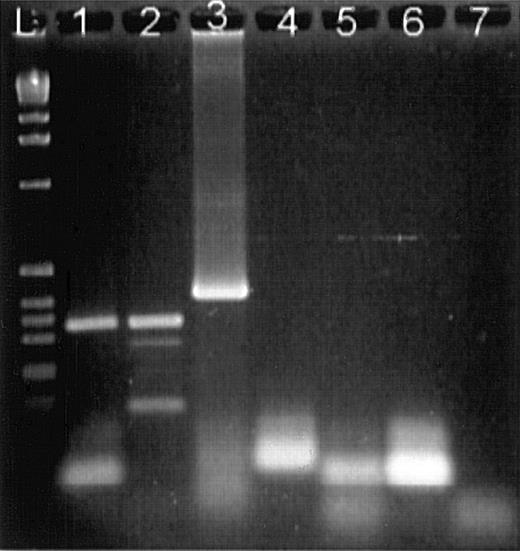
Agarose gel electrophoresis of RT-PCR products stained with ethidium bromide.
(L) DNA ladder. (1) Bone marrow mononuclear cell mRNA with enolase primers showing an expected product of 329 bp. (2) mRNA from IL-2–activated NK cells with enolase primers. (3) Positive control of a 400-bp sequence of human SDF1a amplified with SDF1a primers. (4) mRNA from IL-2–activated NK cells with SDF1a primers showing an absence of SDF1a message. (5) Negative control, mRNA from IL-2–activated NK cells, reverse transcriptase was omitted. (6) Negative control, mRNA from activated NK cells was omitted. (7) Negative control, mRNA from activated NK cells, Taq polymerase omitted.
Agarose gel electrophoresis of RT-PCR products stained with ethidium bromide.
(L) DNA ladder. (1) Bone marrow mononuclear cell mRNA with enolase primers showing an expected product of 329 bp. (2) mRNA from IL-2–activated NK cells with enolase primers. (3) Positive control of a 400-bp sequence of human SDF1a amplified with SDF1a primers. (4) mRNA from IL-2–activated NK cells with SDF1a primers showing an absence of SDF1a message. (5) Negative control, mRNA from IL-2–activated NK cells, reverse transcriptase was omitted. (6) Negative control, mRNA from activated NK cells was omitted. (7) Negative control, mRNA from activated NK cells, Taq polymerase omitted.
These data indicated that NK cells activated by IL-2 release a soluble peptide that is functionally a novel lymphokine different from GM-CSF, M-CSF, G-CSF, erythropoietin, SCF, IL-1, -4, -6, -7 to -12, IFNα, IFNγ, MIP1a, and transforming growth factor-β and that is immunologically distinct from IL-6, IL-11, LIF, other gp130-related interleukins, and human SDF1a and that activated NK cells do not express SDF1a.
Natural killer cell–released peptide is distinct from thrombopoietin
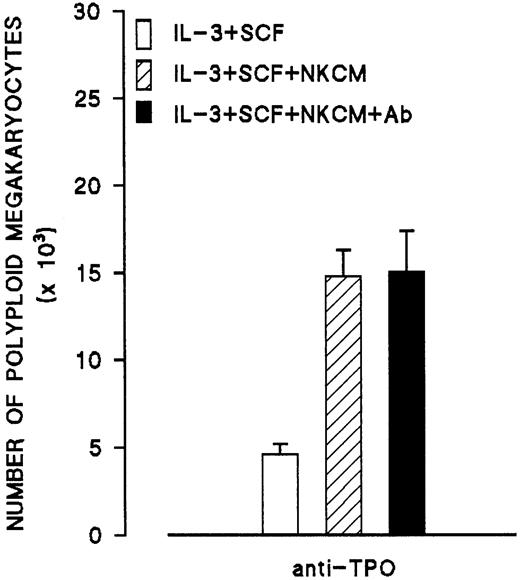
To investigate whether the peptide produced by NK cells is TPO, NKCM was incubated with an excess amount of anti-TPO antibody (nonneutralizing) followed by incubation with protein G bound to agarose. After removal of the agarose beads and dialysis, the treated NKCM was tested on day 6 megakaryocytes generated from CD34+ cells. Treatment of NKCM with anti-TPO had no effect on the increase of ploidy of megakaryocytes, indicating that the peptide in NKCM is immunologically distinct from TPO (Figure11).
Effects of antibody to TPO on the endomitosis-inducing activity in medium conditioned by IL-2–activated NK cells (NKCM).
Control cultures (second bar) contain nonimmune mouse IgG isotype. Each point represents the mean ± SEM of 2 experiments run in quadruplicates.
Effects of antibody to TPO on the endomitosis-inducing activity in medium conditioned by IL-2–activated NK cells (NKCM).
Control cultures (second bar) contain nonimmune mouse IgG isotype. Each point represents the mean ± SEM of 2 experiments run in quadruplicates.
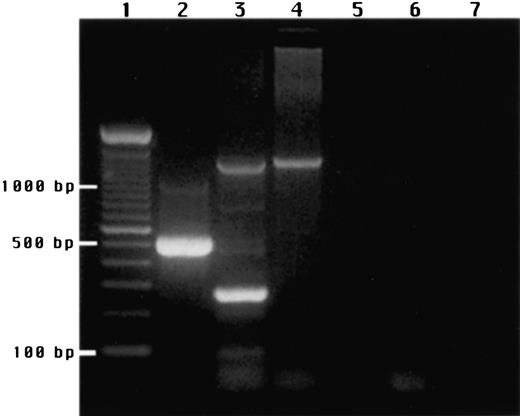
In addition, NK cells were purified and cultured with IL-2, and their mRNA was isolated and subjected to RT-PCR for the detection of TPO message. Both the TPO 270-bp sequence and the 1300-bp rTF sequence were amplified from liver mRNA, but only the rTF sequence was amplified from NK mRNA, indicating lack of expression of the TPO gene (Figure 12). Increasing the PCR cycles to 35 and 40 resulted in a stronger signal for rTF, but the 270-bp TPO sequence could not be amplified. Electrophoresed PCR products were further analyzed by Southern blotting and hybridization with a32P end-labeled 21-oligomer complementary to the 335- to 355-bp sequence of the TPO cDNA. After autoradiography, only a single band was detected in the lane containing the RT-PCR products from liver mRNA (corresponding to 270-bp band on agarose gel), but no signal was detected from NK cell mRNA (data not shown). Consequently, the activity in NKCM responsible for the induction of endomitosis cannot be attributed to TPO production by IL-2–stimulated NK cells.
Agarose gel electrophoresis of RT-PCR products stained with ethidium bromide.
(1) DNA ladder. (2) RT control. (3) Human liver mRNA. (4) mRNA from NK cells simulated with IL-2. (5) Negative control, NK mRNA, RT omitted. (6) Negative control, NK mRNA, Taq polymerase omitted. (7) Negative control, NK mRNA omitted. The 2 products seen with human liver mRNA correspond to a 1300-bp band derived from the transferrin receptor and a 270-bp band from TPO. NK mRNA gave a single product derived from the transferrin receptor but no TPO-derived product.
Agarose gel electrophoresis of RT-PCR products stained with ethidium bromide.
(1) DNA ladder. (2) RT control. (3) Human liver mRNA. (4) mRNA from NK cells simulated with IL-2. (5) Negative control, NK mRNA, RT omitted. (6) Negative control, NK mRNA, Taq polymerase omitted. (7) Negative control, NK mRNA omitted. The 2 products seen with human liver mRNA correspond to a 1300-bp band derived from the transferrin receptor and a 270-bp band from TPO. NK mRNA gave a single product derived from the transferrin receptor but no TPO-derived product.
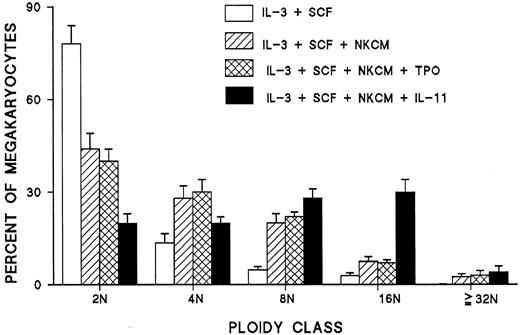
NK-related peptide synergizes with IL-11 in promoting endomitosis
In additional experiments, the effects of TPO and IL-11 on the ploidy of megakaryocytes generated from CD34+ cells were studied in NKCM-containing cultures. In comparison to NKCM, TPO was found to have no additive effect on the ploidy of megakaryocyte in the presence of IL-3. However, a synergy was noted between IL-11 and NKCM that resulted in the generation of polyploid megakaryocyte in vitro with a modal ploidy of 16N, a pattern similar to that found in normal bone marrow megakaryocyte (Figure13).
Synergistic effect of IL-11 and NK-released peptide on the ploidy of megakaryocytes derived from blood CD34+ cells under stimulation with IL-3 and SCF and lack of an additive or synergistic effect of TPO added to NKCM.
Each point represents the mean ± SEM of quadruplicate cultures of 2 experiments.
Synergistic effect of IL-11 and NK-released peptide on the ploidy of megakaryocytes derived from blood CD34+ cells under stimulation with IL-3 and SCF and lack of an additive or synergistic effect of TPO added to NKCM.
Each point represents the mean ± SEM of quadruplicate cultures of 2 experiments.
Discussion
Considering the ploidy distribution of megakaryocytes as an index of human megakaryocyte maturation, it has been well established that IL-3, though the most potent stimulator of megakaryocyte colony formation in vitro, does not promote their terminal differentiation into polyploid cells.6,14,15 Support for this view is also provided by electron microscopy studies of megakaryocytes from IL-3–stimulated cultures showing that these cells are relatively immature and have only a few α-granules and a poorly developed demarcation membrane system.14,15 In contrast to IL-3, TPO, the major humoral regulator of thrombopoiesis in vivo,16 is a weak stimulator of megakaryocyte colony formation in vitro17 but is capable of inducing endomitosis and terminal differentiation of CFUMEG-derived megakaryocytes.17,18 When these 2 factors are combined, an additive effect is noted as far as megakaryocyte colony formation is concerned, but the endomitosis-promoting effect of TPO is severely inhibited.4,5 19
To identify factors affecting endomitosis by CFUMEG-generated megakaryocytes under the stimulation of IL-3, we performed a large number of experiments using known recombinant factors, interleukins, and cytokines, either alone or in additive combinations. In these experiments we noted that only combinations of factors containing IL-2 promoted megakaryocyte endomitosis. The experiments described in this report demonstrate that IL-2 can induce endomitosis in megakaryocytes generated from blood CFUMEG under the stimulation by IL-3 and SCF. This effect of IL-2 is indirect and is mediated through NK cells that release a peptide-inducing endomitosis in immature day 6 megakaryocytes. Unstimulated NK cells did not promote endomitosis. The peptide released by IL-2–stimulated NK cells is functionally distinct from all known and tested growth factors and interleukins and is immunologically distinct from IL-6, IL-11, LIF, other gp130-related interleukins, SDF1a, and TPO. In addition, either SDF1a or TPO cannot be held responsible for the observed effect because NK cells were shown to lack SDF1a and TPO mRNA by RT-PCR. Thus, it seems that this NK-released peptide is a novel factor capable of inducing endomitosis in megakaryocytes generated from CFUMEG stimulated by IL-3 and SCF, conditions under which TPO has only minimal or no effect on endomitosis.4,5 19
Because IL-2 is the inducer of endomitosis-promoting NK cell–related peptide, one may assume that in vivo administration of IL-2 may result in enhanced thrombopoiesis. However, clinical experience with IL-2 indicates the opposite. Thrombocytopenia is a recognized hematologic side effect of IL-2 administration in high doses.20,21This is not a paradox between in vitro and in vivo results because IL-2 administered to humans induces the release of multiple factors including transforming growth factor β,22 a known potent suppressor of megakaryopoiesis and endomitosis,23,24 an inducer of coagulopathy20 and abnormal platelet–endothelium interactions25 that may explain the thrombocytopenia regardless of any possible positive direct effect on thrombopoiesis. The effects of IL-2 administration on megakaryocyte ploidy have not yet been studied.
The presence of thrombopoietic factors other than TPO has been suggested by a number of observations. Mice deficient in c-mpl are capable of maintaining 15% of normal platelet count despite a complete block in the action of TPO.26 In these mice residual thrombopoiesis is independent of IL-3, IL-6, IL-11, and LIF.27,28 The factor(s) responsible for maintaining residual thrombopoiesis remains unknown. In addition, in reactive thrombocytosis there is no correlation between circulating platelets and serum TPO levels, a substantial number of patients have near normal serum TPO levels, and no correlation can be established between platelet count and circulating IL-6, IL-11, or LIF serum levels.29-33
The association of NK cells with megakaryopoiesis has been previously noted. Mice injected with an anti-NK monoclonal antibody showed an almost complete abolishment of CFUMEG cycling in the marrow, whereas an increase was noted in the cycling of marrow erythroid burst-forming units (BFU-E).34 Natural killer cells have been found to enhance megakaryopoiesis in vitro,35 and CAMPATH-1G activated NK cells and their supernatant have been reported to augment BFUMEG and CFUMEG formation by canine aplastic anemia plasma.36 In addition, activated NK cells have been shown to promote granulocytic and megakaryocytic reconstitution after syngeneic bone marrow transplantation in mice.37The current findings lend further support to older observations that NK cells may play an important role in modulating thrombopoiesis in vitro and in vivo. It is premature to hypothesize on the role these peptides may have in vivo, but because it is a product of NK cells involved in infectious and inflammatory conditions, it is tempting to postulate a possible role for it in reactive thrombocytosis. The spectrum of the activities of this NK-released peptide and its action(s) in vivo cannot be predicted until its cDNA is isolated and expressed and the appropriate experiments are completed.
Supported by a Merit Review Grant from the Research Service of the Department of Veterans Affairs and the McGuire Research Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Emmanuel N. Dessypris, Division of Hematology/Oncology (111K), Hunter Holmes McGuire Veterans Affairs Medical Center, 1201 Broad Rock Blvd, Richmond, VA 23249; e-mail:edessypr@hsc.vcu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal