Platelet glycoprotein Ib-IX-V (GPIb-IX-V) mediates adhesion to von Willebrand factor (vWF) in (patho)physiological thrombus formation. vWF binds the N-terminal 282 residues of GPIbα, consisting of an N-terminal flank (His1–Ile35), 7 leucine-rich repeats (Leu36–Ala200), a C-terminal flank (Phe201–Gly268), and a sulfated tyrosine sequence (Asp269–Glu282). By expressing canine–human chimeras of GPIbα on Chinese hamster ovary cells, binding sites for functional anti-GPIbα antibodies to individual domains were previously mapped, and it was shown that leucine-rich repeats 2 to 4 were required for optimal vWF recognition under static or flow conditions. Using novel canine–human chimeras dissecting the C-terminal flank, it is now demonstrated that (1) Phe201-Glu225 contains the epitope for AP1, an anti-GPIbα monoclonal antibody that inhibits both ristocetin- and botrocetin-dependent vWF binding; (2) VM16d, an antibody that preferentially inhibits botrocetin-dependent vWF binding, recognizes the sequence Val226-Gly268, surrounding Cys248, which forms a disulfide-bond with Cys209; (3) vWF binding to chimeric GPIbα is comparable to wild-type in 2 chimeras in which the sixth leucine-rich repeat was of the same species as the first disulfide loop (Phe201-Cys248) of the C-terminal flank, suggesting an interaction between these domains may be important for optimal vWF binding; and (4) replacing the C-terminal flank second disulfide loop (Asp249-Gly268) in human GPIbα with the corresponding canine sequence enhanced vWF binding under static and flow conditions, providing the first evidence for a gain-of-function phenotype associated with the second loop of the C-terminal flank.
Introduction
The platelet membrane glycoprotein Ib-IX-V (GPIb-IX-V) complex plays a central role in vascular biology. The interaction of GPIbα with its adhesive ligand, von Willebrand factor (vWF), in the subendothelial matrix mediates platelet adhesion at high shear in the hemostatic response to vessel wall injury.1,2In thrombosis, pathologic shear stress induces binding of platelet GPIb-IX-V to plasma vWF, initiating platelet aggregation.3More recently, GPIbα has been identified as a counter-receptor for P-selectin expressed on activated endothelial cells4,5 and for the leukocyte αMβ2 integrin, Mac-1.6 These interactions potentially mediate platelet-endothelium or platelet-leukocyte adhesion in thrombosis, inflammation, and atherogenesis. GPIb-IX-V also constitutes a binding site, and a substrate for, α-thrombin.2 Recent evidence suggests that GPIbα acts as a cofactor for thrombin-dependent cleavage of the 7-transmembrane protease-activated receptor, PAR-1, on platelets7 and that GPIbα is itself a thrombin receptor following thrombin-dependent cleavage of GPV.8 Other GPIbα ligands—factor XII and high-molecular–weight kininogen—are inhibitors of thrombin binding, and they down-regulate thrombin-dependent platelet activation.9 10
All these ligands of GPIbα—vWF, P-selectin, Mac-1, thrombin, factor XII, and high-molecular–weight kininogen—recognize the N-terminal 282 residues of GPIbα.2 The binding site for vWF comprises elements from 4 structural regions of GPIbα: the N-terminal flank (His1–Ile35), 7 leucine-rich repeats (Leu36–Ala200), a C-terminal flank (Phe201–Gly268), and a sulfated tyrosine sequence (Asp269–Glu282).2,11 Previous studies with the metalloproteinase mocarhagin, which cleaves between Glu282 and Asp283 of GPIbα, suggest that the vWF binding site is within residues 1 to 282.11 We have recently expressed on Chinese hamster ovary (CHO) cells canine–human and human–canine chimeras of GPIbα, corresponding to boundaries between these structural domains. Murine monoclonal antibodies against the N-terminal domain of human GPIbα are species-specific and do not bind canine GPIbα; canine and human amino acid sequences are only 65.2% identical in the region His-1–Glu-282.12,13 The vWF activator, ristocetin, induces binding of human vWF only to human GPIbα, not to the canine receptor.11,14 In contrast, the snake venom modulator, botrocetin, does not discriminate between the 2 species, inducing the binding of human vWF to human and canine GPIbα.11 Botrocetin-dependent vWF binding to all the GPIbα chimeras provided evidence that they retained a functional conformation. These combined studies allow detailed mapping of binding sites for anti-GPIbα monoclonal antibodies and vWF, and they suggest leucine-rich repeats 2, 3, and 4 of GPIbα are critical for vWF binding to GPIb-IX-V induced by ristocetin or under hydrodynamic flow.11
The C-terminal flank domain (Phe201-Gly268) contains 2 loops by virtue of disulfide bonds between Cys209-Cys248 and Cys211-Cys264. Several lines of evidence implicate the C-terminal flank as a critical factor in regulating vWF binding. Congenital gain-of-function mutations within the GPIbα gene (platelet-type von Willebrand disease) result in single amino acid substitutions, Gly233 to valine or Met239 to valine, within the first of the 2 disulfide loops comprising the C-terminal flank domain.15-17 Mutation of Gly233 or Met239 to valine in recombinant GPIbα, in addition to the artificial mutation of Asp235 or Lys237 to valine, also results in a gain-of-function phenotype.18,19 In contrast, mutation of Ala238 to valine results in partial loss of function.18 The anti-GPIbα monoclonal antibodies, AP1 and VM16d, which map into the C-terminal flank, also inhibit vWF binding. AP1 blocks both ristocetin- and botrocetin-induced binding and rolling of GPIb-IX–expressing cells on vWF, whereas VM16d preferentially inhibits botrocetin-dependent binding11,20 but not ristocetin-dependent binding or GPIb-IX–dependent rolling on vWF.21 In addition, both AP1 and VM16d inhibit Mac-1 binding to GPIbα.6
In this study, we have analyzed a series of canine–human chimeras of GPIbα corresponding to the replacement of specific sequences involving the C-terminal flank. These chimeras, expressed on CHO cells, enabled more precise mapping of binding sites for the functional anti-GPIbα monoclonal antibodies AP1 and VM16d, and they allowed the functional importance of each disulfide loop to be assessed regarding vWF binding to GPIbα. Combined results infer a possible interaction between the sixth leucine-rich repeat and the first disulfide loop (Phe201-Cys248), and they provide the novel observation that alterations to the second loop (Asp249-Gly268) of the C-terminal flank of GPIbα result in enhanced vWF binding.
Materials and methods
Cell lines, antibodies, and reagents
Sodium iodide I 125 was purchased from Amersham (Castle Hill, Australia). Ristocetin was purchased from Boehringer-Mannheim (Mannheim, Germany). Oligonucleotides (20-25 base pair [bp]) based on canine or human cDNA sequences12,13 were obtained from Beckman (Melbourne, Australia). Human factor VIII concentrate was a gift of the Commonwealth Serum Laboratories (Melbourne, Australia). vWF was purified from human factor VIII concentrate and radio-iodinated where appropriate using the chloramine T method, as previously described.22-24 CHO cells stably transfected with GPIbβ and GPIX (CHO βIX cells) were prepared as reported.25-27Murine monoclonal antibodies AP123,28 and VM16d,20,29 directed against the N-terminal vWF-binding domain of GPIbα, and WM23, directed against an epitope within the extracellular macroglycopeptide region of GPIbα, were purified as described in detail elsewhere.23 Botrocetin was purified as described elsewhere.11
Preparation of expression vectors for canine–human chimeras of GPIbα
Canine–human chimeras were generated using cDNA of canine13 and human12 GPIbα. The expression vector consisting of the full-length GPIbα cDNA inserted at anEcoRI site of the plasmid pDX has been described.25-27,30 The strategy for generating chimeric constructs involved polymerase chain reaction amplification of the appropriate GPIbα constructs using plaque-forming unit DNA polymerase to avoid 3′ T extension on the amplification products, blunt-end ligation of the canine and human DNA at domain junctions, and subcloning back into the human GPIbα expression plasmid using appropriate restriction sites as previously described.11Each construct was verified by sequencing.
Expression of canine–human chimeras in Chinese hamster ovary βIX cells
Canine–human GPIbα expression vectors (1-3 μg) were stably transfected into CHO βIX cells using Lipofectamine (Gibco BRL, Gaithersburg, MD) by established methods.11,25-27 CHO cells contain no endogenous GPIbα, GPIbβ, GPIX, or GPV, but cotransfection with GPIbβ and GPIX facilitates stable surface expression of GPIbα; GPV is not necessary for functional GPIbα expression.27,30 Cells expressing surface GPIbα were selected using the anti-GPIbα monoclonal antibody WM23 conjugated to magnetic beads (Dynal, Oslo, Norway) according to the manufacturer's instructions. WM23 has an epitope downstream of Glu28223present in all the chimeras.
Binding of von Willebrand factor and monoclonal antibodies to Chinese hamster ovary cells
CHO βIX cells lacking GPIbα or CHO β/IX cells cotransfected with wild-type human GPIbα or canine–human chimeras of GPIbα were assessed for the ability to bind human vWF and monoclonal antibodies against human GPIbα using previously described methods.11,25-27 30 Monoclonal antibody binding to transfected CHO cells was analyzed using fluorescein isothiocyanate–labeled secondary antibody and flow cytometry. Cells were harvested by 10-minute treatment of cultures with EDTA (0.53 mM), washed by centrifugation (10 minutes, 700g), and resuspended in 0.01 M sodium phosphate, 0.15 M sodium chloride, pH 7.4, containing 0.1% (wt/vol) bovine serum albumin (BSA) (phosphate-buffered saline [PBS]–BSA buffer). Cells were then incubated with monoclonal antibodies (5 μg/mL) for 40 minutes at 22°C, washed twice, incubated in the presence of fluorescein isothiocyanate–labeled rabbit antimouse antibody for a further 40 minutes, washed, and resuspended in PBS–BSA buffer. The geometric mean fluorescence of each sample was analyzed using a FACScan flow cytometer (Becton Dickinson, San Jose, CA), fitted with an argon-ion laser that stimulates at 488 nm and records fluorescence above 530 nm, and Cellquest software (Becton Dickinson).
For measuring vWF binding, CHO βIX cells or CHO βIX cells expressing wild-type GPIbα or chimeras were first suspended in PBS–BSA buffer to a final concentration of 5 × 106/mL. Cells were then incubated with purified125I-labeled human vWF (1 μg/mL, final concentration) and either ristocetin (0.1-2.0 mg/mL, final concentration) or botrocetin (0.1-10 μg/mL, final concentration). After 30 minutes at 22°C, cells were centrifuged through 20% (wt/vol) sucrose in PBS–BSA buffer (4 minutes, 8750g), and label associated with the pellet was counted in a γ-counter.11 27 Nonspecific binding was measured in the absence of ristocetin or botrocetin in a parallel assay.
Binding of 125I-labeled monoclonal antibodies (1 μg/mL, final concentration) to cells (5 × 106/mL) was carried out using the same method as described for vWF binding. Nonspecific binding was determined by including 100 μg/mL unlabeled antibody in a parallel assay.
Adhesion of Chinese hamster ovary cells to von Willebrand factor under flow
Cells in the flow chamber were visualized by phase-contrast videomicroscopy and were analyzed by digital image processing as previously described.11,21 31 Cells in PBS (5 × 106/mL) were passed over a glass slide coated with vWF (50 μg/mL) at a shear stress of 10 or 15 dynes/cm2. The velocity of rolling cells was calculated by overlapping sequential images snapped at 30 frames/s and determining the distance the cells rolled during the time period of the snaps. Mean velocities were determined from a minimum of 100 cells per experiment. The number of rolling cells was calculated from a single field of view and counting each cell that rolled on the vWF surface during a 4-minute flow period. Three to 5 experimental runs were performed using different populations of cells.
Results
Binding of monoclonal antibodies to GPIbα chimeras on Chinese hamster ovary cells
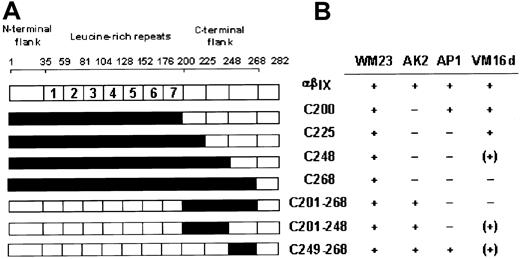
Previous studies have suggested the C-terminal flank region (Phe201-Gly268) is important in regulating vWF binding because gain-of-function mutations in platelet-type von Willebrand disease occur within this sequence.17 In addition, 2 anti-GPIbα monoclonal antibodies, AP1 and VM16d, that mapped to this region differentially inhibit vWF binding.11 21 The first series of chimeras (Figure 1A) was designed to complement 2 previously reported canine–human chimeras, C200 and C268, and to further dissect the C-terminal flank domain of GPIbα. Sequences within the pair of disulfide loops formed by the Cys209-Cys248 and Cys211-Cys264 disulfide bonds were incrementally made canine in the chimeras C225 and C248 (Figure 1A). Additional chimeras replaced human sequence with canine sequence solely within the entire C-terminal flank (C201-268), the first disulfide loop (C201-248), or the second loop (C249-268). Expression vectors for all these chimeras of GPIbα were prepared from human and canine cDNA using a blunt-end ligation method to facilitate replacement of structural domains at precise boundaries (Figure 1A). The constructs were stably transfected into CHO βIX cells (CHO βIX cells were stably transfected with GPIbβ and GPIX to facilitate GPIbα expression).
Binding of monoclonal antibodies to canine–human chimeras of GPIbα.
(A) Canine–human chimeras of GPIbα, where residue numbers correspond to amino acid sequences of human12 and canine13GPIbα. Canine sequence is represented in black. Chimeras C200 and C268 have been reported previously.11 (B) Binding of anti-GPIbα monoclonal antibodies to CHO cells expressing wild-type GPIbα or the chimeras indicated, as assessed by flow cytometry. −, no binding; (+) approximately 50% maximal binding; +, maximal binding.
Binding of monoclonal antibodies to canine–human chimeras of GPIbα.
(A) Canine–human chimeras of GPIbα, where residue numbers correspond to amino acid sequences of human12 and canine13GPIbα. Canine sequence is represented in black. Chimeras C200 and C268 have been reported previously.11 (B) Binding of anti-GPIbα monoclonal antibodies to CHO cells expressing wild-type GPIbα or the chimeras indicated, as assessed by flow cytometry. −, no binding; (+) approximately 50% maximal binding; +, maximal binding.
Epitopes for AP1 and VM16d were evaluated by flow cytometry of wild-type GPIbα- or chimeric GPIbα-expressing CHO βIX cells (Figure 1B). For each population of cells, binding of AP1 or VM16d was compared with binding of WM23, a monoclonal antibody whose epitope is downstream of Glu-28220,23 and is, therefore, present on all the chimeras (Figure 1).11 WM23 bound to cells expressing wild-type human GPIbα and all the chimeras, but not to CHO βIX cells, which lack GPIbα (data not shown). A conformation-dependent monoclonal antibody, AK2, with an epitope in the first leucine-rich repeat,11 bound to all the chimeras in which this domain contained human sequence (Figure 1B). Like wild-type human and canine GPIbα, all the chimeras bound vWF in the presence of the modulator botrocetin (see next section). Together, these results suggested the chimeric receptors were expressed in a functional form, without gross disruption of the conformation of their N-terminal domains.11
As the human GPIbα sequence was incrementally replaced by the canine sequence from the N-terminus, AP1 bound to C200 (canine sequence from the N-terminus up to 200, human sequence from 201 on) but not to C225 (Figure 1B). AP1 also bound to the C249-268 chimera, but not to the C201-268 or the C201-248 chimera, which contained canine sequence within the entire C-terminal flank or first disulfide loop (Phe201-Cys248), respectively. The epitope for AP1 was, therefore, localized to the sequence Phe201-Glu225 because AP1 bound to all the chimeras in which this sequence was human, but to none of the chimeras in which this sequence was canine.
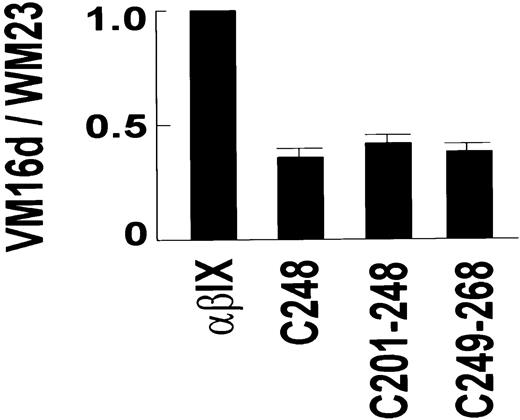
The VM16d epitope included elements from the sequence either side of Cys248, within Asn226-Gly268. VM16d bound to C200 and C225, but not to C268 (Figure 1B). However, there was partial binding to C248. Similarly, there was partial binding to C201-248 and C249-268, but no binding to C201-268. To confirm this partial reactivity of VM16d to C248, C201-248, and C249-268 observed by flow cytometry, binding of VM16d to these chimeras was also assessed using125I-labeled VM16d (Figure2). Specific binding of125I-labeled VM16d was consistent with the flow cytometry results shown in Figure 1B.
Binding of VM16d to GPIbα chimeras.
Specific binding of 125I-labeled anti-GPIbα monoclonal antibody, VM16d (1 μg/mL), to CHO βIX cells (5 × 106/mL) co-expressing wild-type human GPIbα, or canine–human chimeras of GPIbα for 30 minutes at 22°C. Binding is expressed relative to specific binding of 125I-labeled WM23 for each cell line, to account for any differences in expression of GPIbα between different populations of cells. Specific binding for each antibody was calculated by subtracting nonspecific binding measured in the presence of 100 μg/mL unlabeled antibody in a parallel assay. Error bars, ± SEM (n = 2).
Binding of VM16d to GPIbα chimeras.
Specific binding of 125I-labeled anti-GPIbα monoclonal antibody, VM16d (1 μg/mL), to CHO βIX cells (5 × 106/mL) co-expressing wild-type human GPIbα, or canine–human chimeras of GPIbα for 30 minutes at 22°C. Binding is expressed relative to specific binding of 125I-labeled WM23 for each cell line, to account for any differences in expression of GPIbα between different populations of cells. Specific binding for each antibody was calculated by subtracting nonspecific binding measured in the presence of 100 μg/mL unlabeled antibody in a parallel assay. Error bars, ± SEM (n = 2).
Binding of von Willebrand factor to canine–human chimeras of GPIbα on Chinese hamster ovary cells
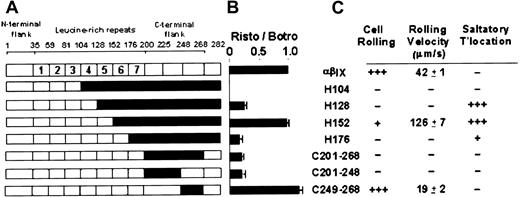
Previous analysis of recombinant GPIbα expressed on mammalian cells11,18,19 and mapping of functional anti-GPIbα antibodies such as AP1 and VM16d (refer to preceding section) has pointed to a role for the C-terminal flank region of GPIbα in regulating vWF binding. We assessed vWF binding to the canine–human chimeras involving replacement of human with canine sequence of the entire C-terminal flank (C201-268) or the first (C201-248) or second (C249-268) disulfide loops (Figure 3A). These new chimeras were compared with a series of complementary human–canine chimeras, H104, H128, H152, and H176 (incremental restoration of human sequence from canine sequence) reported previously.11
Functional analysis of human–canine and canine–human chimeras of GPIbα.
(A) Human–canine and canine–human chimeras of GPIbα, where residue numbers correspond to amino acid sequences of human12 and canine13 GPIbα. Canine sequence is represented in black. Chimeras H104, H128, H152, and H176 have been described previously.11 (B) Ristocetin-dependent vWF binding to GPIbα chimeras. Specific binding of 125I-labeled vWF (1 μg/mL) in the presence of ristocetin (1 mg/mL) to CHO βIX cells (5 × 106/mL) co-expressing wild-type human GPIbα, canine GPIbα, or canine–human chimeras of GPIbα for 30 minutes at 22°C. Binding is expressed relative to specific binding of125I-labeled vWF to each cell line in the presence of botrocetin (2.5 μg/mL) in parallel assays.11 Specific binding was calculated from total binding by subtracting nonspecific binding measured in the absence of modulator in parallel assays. Error bars, ± SEM (n = 3). (C) Adhesion of GPIbα chimera-expressing CHO cells to vWF under flow. Grades are defined as follows: +, 1% to 10% of cells; ++, 11% to 79% of cells; +++, more than 80% of cells. (Rolling velocities are ± SEM.)
Functional analysis of human–canine and canine–human chimeras of GPIbα.
(A) Human–canine and canine–human chimeras of GPIbα, where residue numbers correspond to amino acid sequences of human12 and canine13 GPIbα. Canine sequence is represented in black. Chimeras H104, H128, H152, and H176 have been described previously.11 (B) Ristocetin-dependent vWF binding to GPIbα chimeras. Specific binding of 125I-labeled vWF (1 μg/mL) in the presence of ristocetin (1 mg/mL) to CHO βIX cells (5 × 106/mL) co-expressing wild-type human GPIbα, canine GPIbα, or canine–human chimeras of GPIbα for 30 minutes at 22°C. Binding is expressed relative to specific binding of125I-labeled vWF to each cell line in the presence of botrocetin (2.5 μg/mL) in parallel assays.11 Specific binding was calculated from total binding by subtracting nonspecific binding measured in the absence of modulator in parallel assays. Error bars, ± SEM (n = 3). (C) Adhesion of GPIbα chimera-expressing CHO cells to vWF under flow. Grades are defined as follows: +, 1% to 10% of cells; ++, 11% to 79% of cells; +++, more than 80% of cells. (Rolling velocities are ± SEM.)
Consistent with our earlier results showing leucine-rich repeats 2 to 4 were required for ristocetin-dependent vWF binding,11 all chimeras that contained human sequence within repeats 2 to 4, but not H104, which contained human sequence only to the end of the third repeat, exhibited vWF binding in the presence of ristocetin (Figure3B). As expected, all chimeras bound vWF in the presence of botrocetin because botrocetin does not discriminate between vWF binding to human or canine GPIbα.11 (Cell line, % specific binding: αβIX, 100%; H104, 52% ± 3; H128, 84% ± 4; H152, 71% ± 4; H176, 34% ± 9; C201-C268, 95% ± 3; C201-C248, 126% ± 6; C249-C268, 169% ± 6). We previously showed that botrocetin-dependent binding of vWF correlates, at least within a factor of 2, with levels of binding of WM23, an anti-GPIbα monoclonal antibody with an epitope downstream of Glu282 and present in all the chimeras.11 Therefore, differences in botrocetin-induced binding to different populations of cells presumably reflect variations in levels of GPIbα surface expression. For this reason, we have shown levels of ristocetin-dependent binding of vWF relative to botrocetin-dependent binding in Figure 3B. Like H104, there was also no ristocetin-dependent vWF binding to chimeras C225 or C248 that lacked human repeats 2 to 4 (data not shown), consistent with lack of binding to C200 or C268.11
Ristocetin-dependent vWF binding to the new chimeras, C201-268 and C201-248, containing canine sequence in the entire C-terminal flank or its first disulfide loop, respectively, was less than binding to wild-type GPIbα and comparable to binding to H128 and H176 (Figure3B). However, one of the chimeras (C249-268), containing canine sequence only in the second disulfide loop (Asp249-Gly268) of the C-terminal flank, bound vWF in the presence of ristocetin to an even greater extent than wild-type GPIbα (Figure 3B). In fact, binding of vWF to chimeric GPIbα-expressing cells was comparable to binding to wild-type GPIbα only when the sixth leucine-rich repeat and the first loop of the C-terminal flank (Phe201-Cys248) is of the same species—that is, either both human (wild-type or C249-268) or both canine (H152) (Figure 3B). This implies the possibility of an interaction between sequences within the sixth leucine-rich repeat and Phe201-Cys248.
Because C249-268 bound vWF to a greater extent than wild-type receptor (Figure 3B), we tested vWF binding to this chimera over a range of ristocetin concentrations. Binding was found to be consistently enhanced (Figure 4A). In contrast, botrocetin-dependent vWF binding to C249-268 was comparable to wild-type GPIbα at all botrocetin concentrations tested (Figure 4B). This suggested the C249-268 chimera was a gain-of-function receptor, a result confirmed by measuring adhesion of this cell line to vWF under flow conditions in the absence of modulators.
Effect of ristocetin or botrocetin concentration on vWF binding to GPIbα.
Specific binding of 125I-labeled vWF (1 μg/mL) to CHO βIX cells (106/mL) expressing wild-type human GPIbα or the C249-268 chimera in the presence of ristocetin (A) or botrocetin (B) at the concentrations indicated for 30 minutes at 22°C. Specific binding was calculated from total binding by subtracting nonspecific binding measured in the absence of modulator in parallel assays.
Effect of ristocetin or botrocetin concentration on vWF binding to GPIbα.
Specific binding of 125I-labeled vWF (1 μg/mL) to CHO βIX cells (106/mL) expressing wild-type human GPIbα or the C249-268 chimera in the presence of ristocetin (A) or botrocetin (B) at the concentrations indicated for 30 minutes at 22°C. Specific binding was calculated from total binding by subtracting nonspecific binding measured in the absence of modulator in parallel assays.
Adhesion of Chinese hamster ovary cells to von Willebrand factor under flow
Under flow conditions in the absence of modulators, cells expressing C249-268, containing canine sequence only in the second disulfide loop of the C-terminal flank, rolled significantly more slowly on a vWF-coated surface than cells expressing wild-type GPIbα (Figure 5). This effect was observed at shear stress of 5 and 10 dynes/cm2 and was consistent with the gain-of-function found for ristocetin-dependent binding under static conditions described above. The interactions of other relevant chimera-expressing cells with vWF under flow are summarized in Figure3C. Compared with cells expressing wild-type GPIbα, only C249-268 and the previously described chimera, H152, containing human sequence to the end of the fifth repeat,11 rolled on vWF.
Binding of chimera-expressing CHO cells to vWF under flow.
Binding of CHO βIX cells expressing wild-type GPIbα or CHO βIX cells expressing the C249-268 chimera of GPIbα. Mean rolling velocity was derived from images snapped at 30 frames/s during a 4-minute period of perfusion at 5 or 10 dynes/cm2 over a glass surface coated with 50 μg/mL vWF. Error bars, ± SEM.
Binding of chimera-expressing CHO cells to vWF under flow.
Binding of CHO βIX cells expressing wild-type GPIbα or CHO βIX cells expressing the C249-268 chimera of GPIbα. Mean rolling velocity was derived from images snapped at 30 frames/s during a 4-minute period of perfusion at 5 or 10 dynes/cm2 over a glass surface coated with 50 μg/mL vWF. Error bars, ± SEM.
Discussion
The N-terminal 282 residues of glycoprotein Ibα (GPIbα) of the platelet membrane GPIb-IX-V complex contain the binding domain for vWF and for other ligands such as P-selectin, Mac-1, α-thrombin, factor XII, and high-molecular–weight kininogen.1-10 In the current study, we have focused on the structure–function relationships of the leucine-rich repeats and the C-terminal flank domain of GPIbα, regions previously implicated in regulating vWF binding.2 11 Based on the species specificity of binding of vWF and anti-GPIbα monoclonal antibodies to human GPIbα, we used a series of canine–human chimeras involving the C-terminal flank to examine vWF binding under static and flow conditions and to map epitopes for 2 functional antibodies that recognize this region of the receptor.
Anti-GPIbα monoclonal antibodies AP1 and VM16d were of particular interest because of their functional effect on binding of vWF and other ligands. AP1 was mapped to the sequence Phe201-Glu225, within the first disulfide loop of the C-terminal flank of GPIbα, because it specifically recognized chimeras in which this sequence was human (C200, C249-268) but not in which it was canine (C225, C248, C268, C201-268, C201-248). Previous studies showed that AP1 binding to GPIbα either was unaffected or was partially decreased (by 50% or less compared with wild-type GPIbα) by mutagenesis of individual residues between Gly233 and Met239.18 Unlike AP1, other anti-GPIbα monoclonal antibodies that completely inhibit ristocetin-dependent binding (AK2, Hip1, and 6D1) have epitopes within the first 4 leucine-rich repeats.11 AP1 also inhibits botrocetin-dependent vWF binding to GPIbα and rolling of GPIb-IX-expressing cells on vWF.21
In contrast to AP1, VM16d only inhibits botrocetin-dependent, but not ristocetin-dependent, binding of vWF to GPIbα12,20 or rolling of GPIb-IX–expressing cells on vWF.21 The epitope for VM16d was apparently composed of elements on either side of Cys248 within the sequence Asn226-Gly268 because it bound to all chimeras where this sequence was human but only to some (approximately 50% of wild-type) where either Asn226-Cys248 or Asp249-Gly268 was canine (C248, C201-248, C249-268). This result was confirmed by flow cytometric analysis and by binding of radiolabeled VM16d to chimera-expressing cells. There was no binding when Asn226-Cys248 and Asp249-Gly268 sequences were canine (C268, C201-268). The partial VM16d-binding sequence Asn226-Cys248 contains the congenital and artificial gain-of-function, valine substitution mutations at Gly233, Asp235, Lys237, and Met239 and the partial loss-of-function mutation, Ala238 to valine.18 The other component of the VM16d-binding site, Asp249-Gly268, constitutes the second disulfide loop of the C-terminal flank, formed by the Cys211-Cys264 and Cys209-Cys248 disulfide bridges.1 In studies of vWF binding, this Asp249-Gly268 loop was also found to lead to a gain-of-function when the human sequence was replaced by canine sequence in GPIbα (C249-268). Definition of the VM16d-binding site is significant in that this antibody not only inhibits vWF binding in the presence of botrocetin, it also inhibits the interaction of GPIbα and Mac-1.6 Other antibodies that strongly inhibit vWF binding to GPIbα do not inhibit Mac-1 binding. VM16d also inhibits thrombin-dependent platelet aggregation.29
Extending previous studies of the vWF-GPIbα interaction,2,11,18,19 the current experiments provide additional insight into the potential role of the C-terminal flank region of GPIbα in regulating vWF binding. One of the chimeras (C249-268) containing canine sequence only in the second disulfide loop (Asp249-Gly268) of the C-terminal flank bound vWF to a greater extent than wild-type GPIbα in the presence of ristocetin, and rolling of the cells to vWF under flow conditions in the absence of modulators was slower than in cells expressing wild-type GPIbα. These interactions are unlikely to have been affected by differences in the levels of receptor expression on different cell lines because any variation in expression levels on these cells (as assessed by WM23 binding) are well above levels of receptor where the interaction of cells with vWF under flow is significantly affected.21 31 These findings represent the first example where alterations to the second loop of the C-terminal flank result in a gain-of-function. The difference between human and canine sequences within Asp249-Gly268 include an alteration to charged residues from human to canine sequence at Asp249Val, Asp252Lys, Lys253Asn, Lys258Thr, and Gly263Asp that could account for differences in function.
An interesting feature of these results, combined with those from our earlier studies,11 is how the interaction with vWF under flow is recovered as canine GPIbα was incrementally made more human from the N-terminus. There was recovery of function to the extent of saltatory translocation of more than 90% of cells as human sequence was restored to the end of the fourth leucine-rich repeat (H128). Saltatory translocation occurs when cells jump along the direction of flow, where there is an increased off-rate/on-rate for adhesion compared with rolling cells.11,31 Restoring human sequence to the end of the fifth repeat (H152) resulted in rolling at 126 ± 7 μm/s compared with 42 ± 1 μm/s for wild-type GPIbα. However, as even more human sequence is restored, to the end of the sixth repeat (H176) there is decreased interaction, and less than 10% of cells display saltatory translocation (Figure 3C).11 Rolling was again observed when the receptor was made human to the end of the first loop of the C-terminal flank (C249-268). These results parallel the levels of ristocetin-dependent vWF binding to the chimeras, and they support previous studies showing that ristocetin-dependent vWF binding and adhesion to vWF under flow are comparable and distinct from botrocetin-dependent vWF binding.21 The simplest explanation for these results is that optimal binding of chimeric GPIbα-expressing cells to vWF, comparable to wild-type, only occurs when the sixth leucine-rich repeat and the first loop of the C-terminal flank (Phe201-Cys248) is of the same species, either both human (wild-type or C249-268) or both canine (H152) (Figure 3C). Saltatory translocation (or partial ristocetin-dependent binding), however, is present when leucine-rich repeats 2 to 4 are human (H128, H176, C201-248, C201-268), consistent with previous results.11Together, these findings raise the possibility of an interaction between sequences within the sixth leucine-rich repeat and Phe201-Cys248, an interaction that could be disrupted when respective sequences are cross-species. This may be an important consideration underlying the gain-of-function or loss-of-function mutations within the sequence spanning Gly233-Met239 within this first disulfide loop.18 In support of this supposition, alanine substitution point mutations of residues within the sixth leucine-rich repeat affected the capacity of recombinant GPIbα to support vWF binding.32 Definitive evidence awaits structural determination of the leucine-rich repeat and the C-terminal flank domains of GPIbα.
In conclusion, the current study has further defined the epitopes for 2 functionally important anti-GPIbα monoclonal antibodies, AP1 and VM16d, that inhibit interactions involving the platelet membrane GPIb-IX-V complex and vWF, Mac-1, and thrombin. In addition, analysis of canine–human chimeras of GPIbα expressed on CHO cells provides further evidence for a role of the C-terminal flank domain and of leucine-rich repeats in regulating vWF binding under static or flow conditions.
Supported in part by the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia.
Y.S. and J.D. are co-first authors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert K. Andrews, Baker Medical Research Institute, PO Box 6492, St Kilda Rd Central, Melbourne 8008, Australia; e-mail: rkandrews@hotmail.com.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal