Recombinant factor VIIa (rFVIIa) is a novel prohemostatic drug for patients with hemophilia who have developed inhibitory antibodies. The postulation has been made that hemophilia is not only a disorder of coagulation, but that hyperfibrinolysis due to a defective activation of thrombin activatable fibrinolysis inhibitor (TAFI) might also play a role. In this in vitro study, the potential of rFVIIa to down-regulate fibrinolysis via activation of TAFI was investigated. rFVIIa was able to prolong clot lysis time in plasmas from 17 patients with severe hemophilia A. The prolongation of clot lysis time by rFVIIa was completely abolished by addition of an inhibitor of activated TAFI. The concentration of rFVIIa required for half maximal prolongation of clot lysis time (Clys½-VIIa) varied widely between patients (median, 73.0 U/mL; range, 10.8-250 U/mL). The concentration of rFVIIa required for half maximal reduction of clotting time (Cclot½-VIIa) was approximately 10-fold lower than the Clys½-VIIa value (median, 8.4 U/mL; range, 1.7-22.5 U/mL). Inhibition of TFPI with a polyclonal antibody significantly decreased Clys½-VIIa values (median, 2.6 U/mL; range, 0-86.9 U/mL), whereas Cclot½-VIIa values did not change (median, 7.2 U/mL; range, 2.2-22.5 U/mL). On addition of 100 ng/mL recombinant full-length TFPI, a nonsignificant increase of Clys½-VIIa values was observed (median, 119.2 U/mL; range, 12.3-375.0 U/mL), whereas Cclot½-VIIa values did not change (median, 8.8 U/mL; range, 2.6-34.6 U/mL). In conclusion, this study shows that rFVIIa both accelerates clot formation and inhibits fibrinolysis by activation of TAFI in factor VIII-deficient plasma. However, a large variability in antifibrinolytic potential of rFVIIa exists between patients.
Introduction
Treatment of patients suffering from hemophilia is often complicated by the development of inhibitory antibodies. In approximately 25% to 30% of patients with hemophilia A1and in approximately 1% to 3% of the patients with hemophilia B,2 inhibitors develop during their lifetime. A novel way to treat hemophilia patients with inhibitors is the administration of recombinant factor VIIa (rFVIIa, NovoSeven, Novo Nordisk, Bagsvaerd, Denmark).3 rFVIIa has been shown to be a safe and effective prohemostatic drug during bleeding episodes or surgery.4,5 Advantages over traditional treatment (ie, factor concentrates) are the lack of antigenicity and viral safety of rFVIIa.6 7
rFVIIa exerts its prohemostatic effect via enhancement of the extrinsic coagulation pathway in a tissue factor–dependent manner.8On binding of factor VIIa to tissue factor, factor VIIa is able to activate both factors IX and X, thereby leading to a primary generation of thrombin. At high concentrations of tissue factor, factor X is the preferred substrate for the tissue factor-VIIa complex, whereas at low tissue factor concentrations factor IX is preferably activated (for a review, see Rapaport and Rao9). The ability of high-dose rFVIIa to overcome the inhibitory effect of plasma factor VII on coagulation might play a role in the enhancement of thrombin generation by rFVIIa.10 After formation of the fibrin clot, thrombin generation proceeds via the intrinsic pathway through activation of factor XI by thrombin.11 The secondary burst of thrombin, which is formed via this factor XI feedback loop, results in the activation of thrombin activatable fibrinolysis inhibitor (TAFI).12 Activated TAFI down-regulates fibrinolysis by cleaving C-terminal lysine and arginine residues from partially degraded fibrin, thereby attenuating tissue-type plasminogen activator (t-PA)–mediated fibrinolysis.13 Activation of TAFI is stimulated approximately 1200-fold on thrombin binding to the cell surface receptor thrombomodulin.14
The discovery of TAFI as an important link between coagulation and fibrinolysis stimulated the development of new concepts on the regulation of the hemostatic system. A clotting factor deficiency such as hemophilia A might not only result in defective clot formation, but also in accelerated clot degradation due to diminished TAFI activation. Indeed, it has been shown that factor VIII–deficient plasma shows premature lysis of tissue factor–induced clots due to a lack of TAFI activation, which could be restored by addition of factor VIII.15,16 Clinical evidence that supports the hypothesis of hemophilia A being a defect in both coagulation and fibrinolysis has been obtained from skin biopsy studies in patients with severe hemophilia A in which abnormalities in a skin wound could not be observed until 2 hours after the wound was made.17Although these abnormalities were initially explained by the decreased fibrin-forming capacity of these patients, an increased fibrinolytic potential could also explain these observations. Another indication of disturbed down-regulation of fibrinolysis in hemophilia is the efficacy of antifibrinolytic drugs such as tranexamic acid and ε-aminocaproic acid in controlling bleedings in regions of the body with high fibrinolytic activity (eg, the oral cavity).18 19
Alternative mechanisms for the therapeutic effects of rFVIIa have been proposed. A potential mechanism, hypothesized in the literature,20 involves enhancement of activation of TAFI. Another possible alternative mechanism involves thrombin formation on activated platelets or monocytes independently of tissue factor.21 22
In this study, the antifibrinolytic potential of rFVIIa in plasma from different patients with severe hemophilia A was explored. Also, the involvement of tissue factor pathway inhibitor (TFPI) on rFVIIa-mediated clot protection was investigated.
Patients, materials, and methods
Patients
Plasma samples from 17 patients with severe hemophilia A were used. Of these 17, 8 patients had an inhibitor titer above 1 Bethesda unit (BU)/mL. Patients had not received treatment with products containing factor VIII for at least 72 hours before blood sampling. Pooled normal plasma was obtained by combining plasma from 40 healthy volunteers. Blood samples were obtained by venipuncture from the antecubital vein into 3.2% sodium citrate (9:1, vol/vol). To obtain platelet-poor plasma, the samples were centrifuged twice at 2000g for 15 minutes. Plasma samples were stored at −70°C until use.
Materials
Recombinant human tissue factor (Innovin) was from Dade Behring (Marburg, Germany). Carboxypeptidase inhibitor from potato (CPI) was purchased from Calbiochem (La Jolla, CA). The t-PA was from Chromogenix (Mölndal, Sweden). rFVIIa, recombinant full-length TFPI (rFL-TFPI), a polyclonal inhibitory antibody against tissue factor, and a polyclonal inhibitory antibody against human factor VIII were generous gifts from Drs U. Hedner and M. Kjalke (tissue factor/factor VII research, Novo Nordisk, Måløv, Denmark). A polyclonal inhibitory antibody against TFPI (13 mg/mL) was a generous gift from Dr Walter Kisiel (University of New Mexico, Albuquerque, NM) and was used in a dilution of 1:100. Purified human factor VIII (Monoclate-P) was from Armour Pharmaceuticals (Collegeville, PA).
The TAFI antigen levels were determined by a sandwich-type enzyme-linked immunosorbent assay (ELISA), using a monoclonal capturing antibody and a polyclonal detection antibody as described.23 TAFI levels were expressed as percentage of pooled normal plasma. TFPI activity levels were determined according to Sandset et al.24 Factor II, VII, and X activity levels were determined by a one-stage clotting assay using factor II– and factor X–deficient plasma from Boeringer Mannheim (Mannheim, Germany), and factor VII–deficient plasma from Helena Laboratories (Beaumont, TX). Thromborel S from Dade Behring (Mannheim, Germany) was used as thromboplastin reagent. Fibrinogen levels were determined according to Clauss,25 using thrombin obtained from Sigma (St Louis, MO). Levels of plasminogen were determined using the Coamatic plasminogen kit from Chromogenix. Soluble thrombomodulin levels were determined using the Asserachrom Thrombomodulin ELISA kit from Diagnostica Stago (Asnières, France).
Phospholipid vesicles consisting of 40%l-α-dioleoylphosphatidylcholine, 20%l-α-dioleoylphosphatidylserine, and 40%l-α-dioleoylphosphatidylcholine (all from Sigma) were prepared according to Brunner et al26 with minor modifications as described by Van Wijnen et al.27 Total phospholipid content of the vesicles was determined by phosphate analysis according to Rouser et al.28
Clot lysis assay
Lysis of a tissue factor–induced clot by exogenous t-PA was studied by monitoring changes in turbidity during clot formation and subsequent lysis essentially as described previously.29 A mixture of tissue factor (diluted Innovin, final dilution 105 times), CaCl2 (final concentration 17 mM), t-PA (final concentration 30 U/mL; 56 ng/mL), and phospholipid vesicles (final concentration 10 μM) was added to 75 μL citrated plasma. The volume was adjusted to 150 μL with Hepes buffer (25 mM Hepes, 137 mM NaCl, 3.5 mM KCl, 3 mM CaCl2, 0.1% bovine serum albumin, pH 7.4), resulting in a final plasma concentration of 50%. After mixing thoroughly, 100 μL of this mixture was transferred to a microtiter plate and turbidity at 405 nm was measured in time at 37°C in a Spectramax 340 kinetic microplate reader (Molecular Devices, Menlo Park, CA). Clot lysis time was defined as the time from the midpoint of the clear-to-maximum turbid transition, which is defined as clotting time, to the midpoint of the maximum turbid-to-clear transition. To block possible residual factor VIII activity in hemophilic plasma, which did not contain an inhibitor, 10 BU/mL of a polyclonal antibody against human factor VIII was added and preincubated at room temperature for 45 minutes. To assess the contribution of TAFI activation to clot lysis time, experiments were performed in which CPI (25 μg/mL), a specific inhibitor of activated TAFI,13was added to the plasma. The effect of rFVIIa on fibrinolysis in hemophilic plasma was determined by performing clot lysis assays with factor VIII–deficient plasma to which different concentrations of rFVIIa were added.
Statistical analysis
Statistical analysis was performed using the GraphPad InStat (GraphPad, San Diego, CA) software package. Statistical significance of the increase in mean clot lysis time on addition of rFVIIa was determined by a repeated measures ANOVA followed by the Dunnett post test. Statistical differences between clotting times in the presence or absence or an inhibitory antibody against tissue factor was determined by the standard t test. Correlations were calculated using the Pearson correlation coefficient. Statistical significance between differences in clot lysis time (Clys½-VIIa) or clotting time (Cclot½-VIIa) on manipulation of TFPI was calculated by a repeated measures ANOVA followed by the Tukey post test. P < .05 was considered statistically significant.
Results
rFVIIa mediates down-regulation of fibrinolysis and acceleration of clot formation in factor VIII–deficient plasma
To investigate whether rFVIIa restores down-regulation of fibrinolysis in factor VIII–deficient plasma, clot lysis assays were performed using plasma from 17 patients with severe hemophilia A in the presence of different concentrations of rFVIIa. A polyclonal inhibitory antibody to human factor VIII was added to the plasma samples in which no inhibitor was present, because a previous study showed that as little as 0.01% of factor VIII present in pooled normal plasma is sufficient to completely restore down-regulation of fibrinolysis in factor VIII–deficient plasma.16 A typical example of the effect of rVIIa on clot lysis time in hemophilic plasma is shown in Figure 1A. rFVIIa was able to prolong clot lysis time in all patient samples. The rFVIIa-mediated prolongation of clot lysis time could be completely abolished by the addition of a specific inhibitor of activated TAFI (CPI). Figure 1B shows mean clot lysis times for the 17 patient samples at increasing concentrations of rFVIIa. At rFVIIa concentrations of 31.3 U/mL and higher the increase in clot lysis time was statistically significant (P < .01). From the rFVIIa titration curves the concentrations of rFVIIa required for half maximal prolongation of clot lysis time (Clys½-VIIa) were determined as shown in Figure 1C.
Effect of rFVIIa on clot formation and clot lysis time in plasma from patients with severe hemophilia A.
(A) Increase in clot lysis time on addition of increasing concentrations of rFVIIa (closed symbols). The increase in clot lysis time on addition of 500 U/mL rFVIIa could be completely abolished by addition of CPI (open symbol). (B) Mean clot lysis times of 17 patients with severe hemophilia A on addition of increasing concentrations of rFVIIa. At concentrations of rFVIIa of 31.3 U/mL and higher, the increase in clot lysis time was statistically significant. Error bars indicate SEM. *P < .01 versus clot lysis time in the absence of rFVIIa. (C) The data from panel A were fitted by an exponential function. From this curve the concentration of rFVIIa required for half maximal prolongation of clot lysis time (Clys½-VIIa) was calculated. (D) The decrease in clotting time on addition of rFVIIa. These data were fitted by an exponential function and from this curve the concentration of rFVIIa required for half maximal reduction of clotting time (Cclot½-VIIa) was calculated.
Effect of rFVIIa on clot formation and clot lysis time in plasma from patients with severe hemophilia A.
(A) Increase in clot lysis time on addition of increasing concentrations of rFVIIa (closed symbols). The increase in clot lysis time on addition of 500 U/mL rFVIIa could be completely abolished by addition of CPI (open symbol). (B) Mean clot lysis times of 17 patients with severe hemophilia A on addition of increasing concentrations of rFVIIa. At concentrations of rFVIIa of 31.3 U/mL and higher, the increase in clot lysis time was statistically significant. Error bars indicate SEM. *P < .01 versus clot lysis time in the absence of rFVIIa. (C) The data from panel A were fitted by an exponential function. From this curve the concentration of rFVIIa required for half maximal prolongation of clot lysis time (Clys½-VIIa) was calculated. (D) The decrease in clotting time on addition of rFVIIa. These data were fitted by an exponential function and from this curve the concentration of rFVIIa required for half maximal reduction of clotting time (Cclot½-VIIa) was calculated.
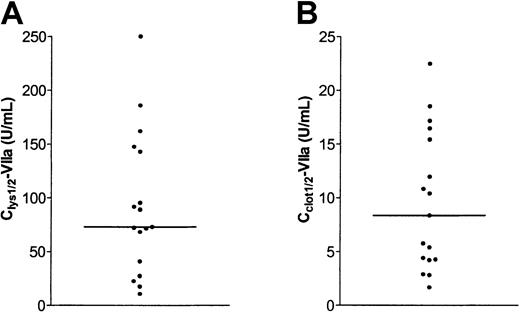
A wide variation of Clys½-VIIa values was observed as shown in Figure 2A (median, 73.0 U/mL; range, 10.7-250.0 U/mL). Clys½-VIIa values were similar in plasma samples from patients without (median, 73.0 U/mL; range, 17.4-185.9 U/mL) and with (median, 81.8 U/mL; range 10.7-250.0 U/mL) inhibitory antibodies. To examine whether the antifibrinolytic potential of rVIIa remained constant over time in a single patient, Clys½-VIIa values of 2 unrelated patients were determined in 4 plasma samples collected at intervals of approximately 3 months. These experiments showed that Clys½-VIIa values remained constant in a period of 1 year (patient 1, range 238-267 U/mL; patient 2, range 91-150 U/mL).
Variability in antifibrinolytic and procoagulant effect of rFVIIa in 17 plasma samples from patients with severe hemophilia A.
(A) Clys½-VIIa values were determined in plasma samples from 17 patients with severe hemophilia A following the example in Figure 1B. (B) Cclot½-VIIa values were determined in plasma samples from 17 patients with severe hemophilia A following the example in Figure 1C. The horizontal line indicates medians.
Variability in antifibrinolytic and procoagulant effect of rFVIIa in 17 plasma samples from patients with severe hemophilia A.
(A) Clys½-VIIa values were determined in plasma samples from 17 patients with severe hemophilia A following the example in Figure 1B. (B) Cclot½-VIIa values were determined in plasma samples from 17 patients with severe hemophilia A following the example in Figure 1C. The horizontal line indicates medians.
To investigate the effect of tissue factor concentration on Clys½-VIIa values, clot lysis assays were performed using plasma of a single patient in which coagulation was initiated by increasing amounts of tissue factor. Clys½-VIIa was 85 U/mL at the tissue factor dilution used throughout this study. When the tissue factor concentration was doubled or quintupled, Clys½-VIIa values decreased to 26 and 11 U/mL, respectively.
Correlation of Clys½-VIIa values with levels of several plasma proteins
To study possible parameters that influence Clys½-VIIa values, levels of several plasma proteins were measured and the correlation with Clys½-VIIa values was determined. Clys½-VIIa values were not correlated with TFPI activity (r = 0.190, P = .468), TAFI antigen (r = 0.089, P = .729), soluble thrombomodulin antigen (r = 0.032, P = .895), fibrinogen (r = 0.054,P = .861), factor II activity (r = 0.071,P = .785), factor VII activity (r = 0.286,P = .265), factor X activity (r = 0.302,P = .238), and plasminogen activity (r = 0.032,P = .902) levels.
To exclude the possibility that differences in trace amounts of factor VIII activity left in the plasma samples account for the large variation in Clys½-VIIa values, an experiment was performed using a plasma sample from a patient with severe hemophilia A due to an intron 22 gene inversion, who did not receive any treatment for more than a year. The plasma in this patient was considered to completely lack factor VIII activity. Clys½-VIIa values were determined in this plasma sample in the presence of the polyclonal inhibitory antibody in the absence or presence of 1 mU/mL factor VIII, which represents 0.1% of the factor VIII found in pooled normal plasma. Clys½-VIIa did not change on addition of factor VIII, indicating sufficient inhibition of 0.1% factor VIII by the antibody (data not shown).
Improvement of clot formation requires around 10-fold less rFVIIa than improvement of fibrinolysis
In all patients, a significant reduction in clotting time on addition of rFVIIa was observed. A typical example of the effect of rFVIIa on clotting time is shown in Figure 1D. Cclot½-VIIa values were defined as the concentration of rVIIa required to half-maximally reduce clotting time. Cclot½-VIIa values were approximately 10-fold lower than Clys½-VIIa values as shown in Figure 2B (median, 8.4 U/mL; range, 1.7-22.5 U/mL). Cclot½-VIIa values were not correlated with Clys½-VIIa values (r = 0.187,P = .470). Cclot½-VIIa for pooled normal plasma was 9.9 U/mL.
Cclot½-VIIa values correlate with endogenous factor VII levels
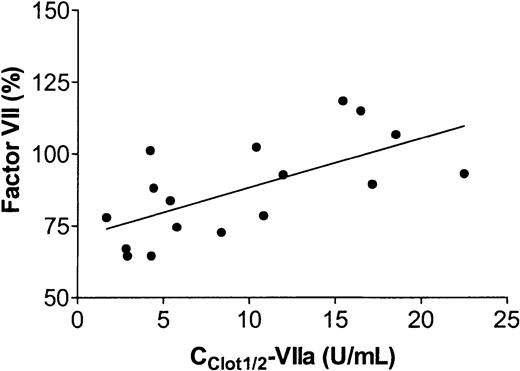
The Cclot½-VIIa values were positively correlated with endogenous factor VII levels as shown in Figure3 (r = 0.653, P < .005). Cclot½-VIIa values were not correlated with plasma levels of factor X (r = 0.311, P = .223), factor II (r = 0.164, P = .530), and fibrinogen (r = 0.206,P = .499).
Correlation between Cclot½-VIIa levels and endogenous factor VII levels.
Tissue factor dependency of clot formation in hemophilic plasma
To investigate whether clot formation in factor VIII–deficient plasma was completely dependent on tissue factor, clot lysis assays were performed using plasma samples from 4 patients (all with inhibitory antibodies against factor VIII) in the presence of increasing concentrations of rFVIIa and in the presence or absence of an inhibitory antibody against tissue factor (500 μg/mL). As shown in Figure 4, clot formation was significantly inhibited (clotting time > 30 minutes) on inhibition of tissue factor. When rFVIIa was added to the plasma, clotting did occur during the time span of our experiment when tissue factor was inhibited. Inhibition of tissue factor attenuated clot formation at all rFVIIa concentrations tested. However, at 125 and 250 U/mL rFVIIa the increase in clotting time on inhibition of tissue factor was no longer statistically significant.
Effect of the inhibition of tissue factor on the clotting time in plasma from 4 patients with severe hemophilia A at different rFVIIa concentrations.
Clotting times were determined using the same conditions as used for the clot lysis assay in the absence (open circles) or presence (closed circles) of a polyclonal inhibitory antibody against tissue factor. Error bars indicate SD. Asterisk indicates P < .05.
Effect of the inhibition of tissue factor on the clotting time in plasma from 4 patients with severe hemophilia A at different rFVIIa concentrations.
Clotting times were determined using the same conditions as used for the clot lysis assay in the absence (open circles) or presence (closed circles) of a polyclonal inhibitory antibody against tissue factor. Error bars indicate SD. Asterisk indicates P < .05.
Effects of modulation of TFPI on Clys½-VIIa and Cclot½-VIIa values
The effect of TFPI on Clys½-VIIa and Cclot½-VIIa values was investigated in all patient samples. As shown in Figure 5A, addition of rFL-TFPI resulted in an increase in Clys½-VIIa values (median, 119.2 U/mL; range, 12.3-375 U/mL), but this did not reach statistical significance. Inhibition of TFPI by an inhibitory antibody resulted in a significant decrease of Clys½-VIIa values (median, 2.6 U/mL; range, 0-86.9 U/mL; P < .05).
Effect of TFPI on antifibrinolytic and procoagulant potential of rFVIIa.
(A) Clys½-VIIa values of the 17 plasma samples as shown in Figure 2A (no additions), and Clys½-VIIa values of the same samples in the presence of 100 ng/mL rFL-TFPI (+ TFPI), or an inhibitory antibody against TFPI (+ α-TFPI). (B) Cclot½-VIIa values of the 17 plasma samples as shown in Figure 2B (no additions), and Cclot½-VIIa values of the same samples in the presence of 100 ng/mL rFL-TFPI (+ TFPI), or an inhibitory antibody against TFPI (+ α-TFPI).
Effect of TFPI on antifibrinolytic and procoagulant potential of rFVIIa.
(A) Clys½-VIIa values of the 17 plasma samples as shown in Figure 2A (no additions), and Clys½-VIIa values of the same samples in the presence of 100 ng/mL rFL-TFPI (+ TFPI), or an inhibitory antibody against TFPI (+ α-TFPI). (B) Cclot½-VIIa values of the 17 plasma samples as shown in Figure 2B (no additions), and Cclot½-VIIa values of the same samples in the presence of 100 ng/mL rFL-TFPI (+ TFPI), or an inhibitory antibody against TFPI (+ α-TFPI).
As shown in Figure 5B, addition of rFL-TFPI did not change Cclot½-VIIa values (median, 7.2 U/mL; range, 2.2-20.5 U/mL). Also addition of a blocking antibody against TFPI did not change Cclot½-VIIa values (median, 8.8 U/mL; range, 2.6-34.6 U/mL). However, clotting times in the absence of rFVIIa were decreased by addition of a blocking antibody against TFPI, whereas clotting times in the absence of rFVII increased by addition of rFL-TFPI (data not shown).
Discussion
This in vitro study shows that rFVIIa both accelerates clot formation and inhibits fibrinolysis by activation of TAFI in factor VIII–deficient plasma. In agreement with previous studies, we have shown a lack of TAFI activation in plasma from patients with severe hemophilia A at low tissue factor concentrations, implicating that hemophilia is a disorder of both clot formation and down-regulation of clot breakdown. In a previous study, we showed that as little as 0.01% of the amount of factor VIII present in pooled normal plasma completely restores TAFI-mediated down-regulation of fibrinolysis in factor VIII–deficient plasma.16 Here, we showed that high rFVIIa levels are required for inhibition of fibrinolysis. Values required for half maximal effect range from therapeutic (10.7 U/mL) to supratherapeutic (250 U/mL) levels.
We have not been able to determine specific factors that determine Clys½-VIIa values. We speculate that the antifibrinolytic potential of rVIIa is determined by a combination of both thrombin-generating capacity and plasma fibrinolytic potential.
We have shown that the amount of rFVIIa required to restore fibrinolysis is dependent on the concentration of tissue factor. The efficacy of a certain dose of rFVIIa in a patient is probably highly dependent on the site of injury and the extent of vascular damage. It is likely also that in vivo rFVIIa is more effective in down-regulation of the fibrinolytic system at wounds where large amounts of tissue factor are exposed. Another important factor in the efficacy of rFVIIa is the amount of thrombomodulin present. Thrombomodulin is capable of enhancing TAFI activation by approximately 3 orders of magnitude.14 However, a high concentration of thrombomodulin down-regulates TAFI activation by protein C activation.30 Thus, it is likely that both tissue factor and thrombomodulin present at the site of injury determine the antifibrinolytic potential of rFVIIa in a patient with hemophilia A.
The regulatory effect of TFPI in rVIIa-mediated inhibition of fibrinolysis becomes evident on evaluation of Clys½-VIIa values, which are determined in the presence of a blocking antibody against TFPI. In the absence of the inhibitory activity of TFPI, little rFVIIa is needed to restore fibrinolysis, and in some patients fibrinolysis was already maximally inhibited in the absence of rFVIIa. The addition of rFL-TFPI did not lead to a significant inhibition of the antifibrinolytic effect of rFVIIa, probably because TFPI concentrations present in plasma were nearly saturating at the low tissue factor concentrations used in our assay.
The effect of rFVIIa on clot formation was more pronounced than the effect on TAFI activation; the levels of rFVIIa required to half maximally reduce clot formation were approximately 10-fold lower than those required to half maximally inhibit fibrinolysis. Moreover Clys½-VIIa values were not correlated with Cclot½-VIIa values. This indicates that primary and secondary thrombin formation in factor VIII–deficient plasma are differently regulated processes. This phenomenon is supported by the observation that modulation of TFPI activity clearly affects Clys½-VIIa values, whereas Cclot½-VIIa values are unchanged. The inhibitory activity of TFPI on clot formation is overruled by rVIIa. This might be due to kinetic limitations combined with the rate-limiting concentration of factor Xa. The thrombin generation that occurs after formation of the fibrin clot is efficiently inhibited by TFPI even in the presence of rVIIa, as inhibition of TFPI markedly reduces Clys½-VIIa values.
The hypothesis that primary and secondary thrombin formation in our system are differently regulated processes is further supported by the observation that endogenous factor VII levels positively correlate with Cclot½-VIIa but not with Clys½-VIIa values. The correlation of endogenous factor VII levels with Cclot½-VIIa corresponds to the inhibitory activity of zymogen factor VII on tissue factor–induced coagulation as described by van 't Veer et al.10 Apparently this inhibitory activity is not important for secondary thrombin generation.
In conclusion, the efficacy of rFVIIa infusion in patients with hemophilia who developed inhibitory antibodies may be explained by a combination of the following factors: (1) enhancement of clot formation via the tissue factor pathway, possibly by overruling the inhibitory activity of zymogen factor VII, and (2) down-regulation of fibrinolysis by TAFI activation, which contributes to the stability of the clot. However, until now we have had no evidence that the antifibrinolytic effect of rFVIIa also occurs in the in vivo situation. More sophisticated techniques for TAFI activation in vivo will be required to address this question. Whether the in vitro variations in antifibrinolytic potential of rFVIIa may have clinical relevance (eg, in predicting clinical efficacy of rFVIIa in patients with severe hemophilia) will be investigated in future studies.
The authors would like to thank Drs U. Hedner and M. Kjalke for their generous gift of rFVIIa, rFL-TFPI, and the antibodies against factor VIII and tissue factor, and Dr W. Kisiel for his generous gift of the antibody against TFPI.
Supported in part by an unrestricted educational grant of Novo Nordisk, and a grant from the Netherlands Heart Foundation (grant 96.088). J.C.M.M. is an Established Investigator of the Netherlands Heart Foundation (grant D96.021).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ton Lisman, Thrombosis and Haemostasis Laboratory, Dept of Haematology, G03.647, University Medical Center, Heidelberglaan 100, 3584 CX, Utrecht, The Netherlands; e-mail: j.a.lisman@lab.azu.nl.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal