Molecular abnormalities caused by the hybrid Bcr-Abl gene are causally associated with the development and progression of Philadelphia chromosome–positive (Ph+) chronic myelogenous leukemia (CML). Imatinib mesylate (STI571), a specific Bcr-Abl tyrosine-kinase signal-transduction inhibitor, has shown encouraging activity in phase I and II studies of CML. Here, we describe the use of imatinib mesylate to treat 75 patients in blast-phase CML (median age, 53 years; 65 with nonlymphoid and 10 with lymphoid blasts), and compare the results with those of a historical control group treated with standard cytarabine-based therapy. Imatinib mesylate was given as oral doses at 300 to 1000 mg per day and was the first salvage therapy for 47 patients. The objective response rate was 52% (39 of 75 patients: 16 had complete and 3 had partial hematologic response; 12 had hematologic improvement; 7 returned to second chronic phase; and 1 had a complete response in extramedullary blastic disease). Response rates were not different between nonlymphoid and lymphoid groups. The cytogenetic response rate was 16% (12 patients: 5 complete, 3 partial [Ph+ below 35%], and 4 minor [Ph+, 34% to 90%]). The estimated median overall survival was 6.5 months; the estimated 1-year survival was 22%. Response to therapy (landmark analysis at 8 weeks) was associated with survival prolongation. Compared with standard cytarabine combinations, imatinib mesylate therapy was less toxic and produced a higher response rate (55% versus 29%, P = .001), longer median survival (7 versus 4 months, P = .04), and lower 4-week induction mortality (4% versus 15%, P = .07). Imatinib mesylate is currently being tested in combination with other drugs to improve the prognosis for blast-phase CML.
Introduction
Chronic myelogenous leukemia (CML) is expressed in several phases—an indolent or chronic phase, an accelerated phase, and a blastic phase.1-3 The hallmark of CML is the Philadelphia (Ph) chromosome, a translocation abnormality that leads to production of the Bcr-Abl fusion protein p210. The activation of p210 is causally related to the development and progression of CML.4,5 The development of a blast phase in CML carries an extremely poor prognosis.6 In most cases, the blasts are of myeloid or undifferentiated origin, but in about 25% of cases, blasts originate from lymphoid cell types. Treatment of lymphoid blast-phase CML with regimens used to treat acute lymphocytic leukemia (such as hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) produces a response rate of 50% to 60% and a median survival of 9 to 12 months.7 With cytarabine-containing combination regimens, the corresponding values for myeloid or undifferentiated blast-phase CML are 20% to 30% and 3 to 5 months.6,8 Attempts to improve prognosis for blast-phase CML have included the use of allogeneic stem cell transplantation (SCT), decitabine, troxacitabine, and tiazofurin.8-10
Phase I and II studies of imatinib mesylate (STI571, Gleevec [Novartis Pharmaceuticals, East Hanover, NJ]), a selective Bcr-Abl tyrosine kinase inhibitor,11,12 for CML in different phases have yielded extremely encouraging results.13-18 In patients with chronic-phase CML whose disease had failed to respond to interferon-alpha, imatinib mesylate produced a complete hematologic response (CHR) rate of 98% and a cytogenetic response rate of 65% (a major response [suppression of Ph+ cells to below 35%] in 50% and a complete response [CR] [Ph, 0%] in 39%).15Imatinib mesylate also produced impressive—but less durable—results in accelerated- and blast-phase disease.16-18 In accelerated-phase CML, the CHR rate was about 40% and the complete cytogenetic response rate was 20%; the estimated 12-month survival rate was 75%.16 In blast-phase CML, the complete bone-marrow response rate (5% of fewer blasts in the marrow) was 28% in myeloid blast-phase disease and 60% in lymphoid blast-phase disease or Ph+ acute lymphocytic leukemia.13,17,18However, studies of blast-phase CML to date have involved relatively small numbers of patients, and the follow-up periods have been short. Moreover, because of the multinational, multi-institutional design of the studies17 18 and central data collection, incomplete information makes comparing results from different laboratories difficult. Here we describe our use of imatinib mesylate to treat Ph+ CML in blast phase. The largest single-institution study of its kind to date, it includes a comprehensive analysis of the association pretreatment factors and disease features with response and prognosis. A unique feature of this analysis is the comparison of the results of imatinib mesylate therapy with those of cytarabine-containing regimens (standard of care), in our historical data for frontline therapy of nonlymphoid blast-phase CML.
Patients and methods
Patients
Adults with confirmed Ph+ blast-phase CML who were referred to the Leukemia Department at The University of Texas MD Anderson Cancer Center participated in this study. Informed consent was obtained according to institutional guidelines. Entry criteria were (1) morphologic and cytogenetic evidence of Ph+ blast-phase CML; (2) age 18 years or older; (3) adequate performance status (level 0 to 2 on the Eastern Cooperative Oncology Group scale); (4) normal renal function (creatinine level lower than 2 mg/100 mL); and (5) normal hepatic function (bilirubin, aspartate aminotransferase, and alanine aminotransferase levels less than twice the upper limits of normal).
The diagnosis of blast-phase CML was confirmed on the basis of morphologic and staining characteristics, cytogenetic analysis, and immunophenotyping.19 Blast-phase CML was defined by the presence of 30% or more blasts in the blood or bone marrow. Cytogenetic studies were used to confirm the presence of Ph+ metaphases [t(9;22) (q34;q11) or Ph variants] and to identify other chromosomal abnormalities. Blasts were considered to be of myeloid origin if 3% or more blasts stained positively for myeloperoxidase; of lymphoid origin if myeloperoxidase staining was negative and terminal deoxynucleotide transferase staining was positive in 40% or more blasts; and of undifferentiated origin if all stains were negative and immunophenotyping demonstrated the presence of myeloid markers (CD13 or CD33) in 20% or more cells or the c-kit in 5% or more cells.
Therapy
Imatinib mesylate was given in oral doses ranging from 300 to 1000 mg daily according to 1 of 3 Novartis-sponsored protocols for blast-phase CML: the phase I study (Novartis 001; 29 patients, 20 reported in the previous study)13; a phase II multi-institutional, multinational study (Novartis 102; 13 patients)17; and a phase II “expanded access” study (Novartis 115; 33 patients). Treatment was continued until disease progression, death, failure from other causes, or eligibility for and initiation of SCT.
Response criteria
Response criteria have been described previously.7 8 Briefly, CHR required normalization of peripheral counts and differentials (granulocyte count of 109/L or higher, white blood cell count of 2 to 10 × 109/L, and platelet count of 100 × 109/L or higher) with 5% or fewer blasts in the bone marrow for at least 4 weeks. Hematologic improvement (HI) was based on the same criteria as CHR except for allowing persistent thrombocytopenia (lower than 100 × 109/L) and the presence of few immature cells (no blasts or promyelocytes, 5% or fewer myelocytes plus metamyelocytes) in the peripheral blood. Patients who achieved CHR or HI were further categorized by their cytogenetic response as follows: complete (no Ph+metaphases), partial (1% to 34% Ph+ metaphases), and minor (35% to 90% Ph+ metaphases). A partial hematologic response (PHR) was based on the same criteria used for CHR but allowed persistent palpable splenomegaly (although spleen size had to have been reduced by 50% or more), thrombocytosis (platelet count exceeding 450 × 109/L), and/or the presence of few immature cells (no blasts or promyelocytes, 5% or fewer myelocytes plus metamyelocytes) in the peripheral blood. A return to second chronic phase was defined by the disappearance of blast-phase features and a return to chronic-phase features, ie, fewer than 15% peripheral blasts, fewer than 30% peripheral blasts plus promyelocytes, fewer than 20% peripheral basophils, and platelet count exceeding 100 × 109/L.
In this study, we defined 2 marrow responses to allow comparison with previously reported imatinib mesylate studies in CML blastic phase.13,17 18 Marrow CR required 5% or fewer marrow blasts without normalizing peripheral counts. Marrow PR allowed 15% or fewer marrow blasts without normalizing peripheral counts. These responses were coded if the patients did not meet the above response criteria.
Patients were also coded and analyzed overall by the response criteria used in the imatinib mesylate studies,13,17 18 ie, by whether they achieved marrow CR (5% or fewer marrow blasts) with recovery of peripheral counts (identical to CHR) or without recovery of peripheral counts (marrow CR, no recovery), or marrow PR (15% or fewer marrow blasts).
All other responses were considered treatment failures and were categorized as follows: (1) early death if death occurred within 2 weeks from the start of therapy; (2) aplastic death if the patient died during remission induction with hypocellular marrow and a marrow leukemic infiltrate (MLI) (percentage of marrow blasts multiplied by marrow cellularity) of lower than 20%; (3) resistant disease if the bone marrow MLI was persistently greater than 20% (primary) or if it decreased to lower than 20% with subsequent regrowth of leukemia (secondary). Response criteria for patients with extramedullary disease were the complete disappearance of disease for a CR, and a disease reduction of 50% or more for a PR.
Statistical analyses
Survival analysis was based on the Kaplan-Meier method.20 Differences among groups were compared by the log-rank test.21 Survival was calculated from the initiation of treatment for blast-phase CML until death or last follow-up. Response duration was calculated from the date of the response until the recurrence of blast-phase CML. Two approaches were used to evaluate the association of response criteria with survival: comparison of survival among response groups by landmark analysis,22 and use of a proportional-hazards regression model for survival that included significant pretreatment factors.23
For the landmark analysis, a landmark time of 8 weeks (from the initiation of the treatment) was chosen since almost all responses had occurred by that time. Patients who died before 8 weeks were excluded from this analysis. In the second approach, the association of pretreatment factors with survival was first assessed individually by using the log-rank test, and factors found to be relatively significant (P < .1) in that analysis were included in the final multivariate model. For those tests, the Cox proportional-hazards regression model was used, with a stepwise procedure to evaluate the independent significance of different variables on survival.23 In multivariate analyses,P ≤ .05 was considered statistically significant. Treatment response rates among subgroups were compared with a 2-sided Fisher exact test.
Historical control group
Because of the results with imatinib mesylate studies, current and future studies in blast-phase CML are not likely to compare, in a randomized design, therapy with imatinib mesylate versus combination chemotherapy with cytarabine-containing regimens (the standard of care), but will be more likely to combine imatinib mesylate with chemotherapy. Therefore, we felt it useful to compare the results of imatinib mesylate as frontline therapy in nonlymphoid blast-phase CML with the results of cytarabine-containing regimens. We therefore reviewed our experience with cytarabine regimens for frontline therapy of nonlymphoid blast-phase CML in 133 patients referred to our service between 1972 and 2000.
Results
Patients
The pretreatment characteristics of the 75 patients (median age, 53 years) are listed in Table 1. The distribution of imatinib mesylate doses, all given orally, was as follows: 9 patients were given 300 to 500 mg daily; 58 were given 600 mg daily; 3 were given 750 mg daily; and 5 were given 400 to 500 mg twice daily (800 to 1000 mg per day). Five patients received 20 mg/m2 cytarabine daily × 10 every month, in addition to imatinib mesylate.
Characteristics of the study group before treatment
| Feature . | No. (% of 75 patients) . |
|---|---|
| Age, y | |
| Median | 53 |
| 60 or older | 27 (36) |
| Splenomegaly > 5 cm bcm | 28/68 (41) |
| Hemoglobin < 10 g/dL | 38 (51) |
| White blood cell count > 50 × 109/L | 21 (28) |
| Platelet count < 100 × 109/L | 41 (55) |
| Other chromosomal abnormalities | 43/71 (61) |
| Peripheral blasts > 30% | 52 (69) |
| Marrow blasts > 50% | 40 (53) |
| Blast morphology | |
| Lymphoid | 10 (13) |
| Myeloid, undifferentiated | 65 (87) |
| Time from diagnosis to blastic phase, mo | |
| < 12 | 17 (23) |
| 12-35 | 20 (27) |
| ≥ 36 | 38 (51) |
| Blastic phase salvage | |
| First | 50 (67) |
| ≥ Second | 25 (33) |
| Feature . | No. (% of 75 patients) . |
|---|---|
| Age, y | |
| Median | 53 |
| 60 or older | 27 (36) |
| Splenomegaly > 5 cm bcm | 28/68 (41) |
| Hemoglobin < 10 g/dL | 38 (51) |
| White blood cell count > 50 × 109/L | 21 (28) |
| Platelet count < 100 × 109/L | 41 (55) |
| Other chromosomal abnormalities | 43/71 (61) |
| Peripheral blasts > 30% | 52 (69) |
| Marrow blasts > 50% | 40 (53) |
| Blast morphology | |
| Lymphoid | 10 (13) |
| Myeloid, undifferentiated | 65 (87) |
| Time from diagnosis to blastic phase, mo | |
| < 12 | 17 (23) |
| 12-35 | 20 (27) |
| ≥ 36 | 38 (51) |
| Blastic phase salvage | |
| First | 50 (67) |
| ≥ Second | 25 (33) |
bcm indicates below costal margin.
Responses
Responses for the entire group and for those with nonlymphoid or lymphoid blast morphology are shown in Table2. Among the 65 patients with nonlymphoid blast-phase CML, 15 (23%) achieved CHR, 11 (17%) had HI, 3 (5%) had PHR, and 6 (8%) returned to a second chronic phase. One patient with extramedullary blastic phase achieved CR. Another 6 patients (9%) achieved marrow CR or marrow PR. The overall objective response rate was 55% (36 of 65) if established response criteria were used, and 65% (42 of 55) if the criteria were expanded to include bone-marrow response. Fifteen patients (23%) achieved marrow CR with recovery of peripheral blood counts, and 19 patients (29%) achieved marrow CR without recovery of peripheral counts.
Responses to imatinib mesylate therapy
| Type of response . | No. of patients (%) . | ||
|---|---|---|---|
| Total . | Blast type . | ||
| Nonlymphoid . | Lymphoid . | ||
| Total treated | 75 | 65 | 10 |
| CHR | |||
| Total | 16 (21) | 15 (23) | 1 (10) |
| Cytogenetic response | 8 (11) | 7 (11) | 1 (10) |
| Minor | 2 (3) | 2 (3) | |
| Partial | 2 (3) | 2 (3) | |
| Complete | 4 (6) | 3 (5) | 1 (10) |
| HI | |||
| Total | 12 (16) | 11 (17) | 1 (10) |
| Cytogenetic response | 4 (5) | 4 (6) | |
| Minor | 2 (3) | 4 (6) | |
| Partial | 1 (1) | 1 (2) | |
| Complete | 1 (1) | 1 (2) | |
| PHR | 3 (4) | 3 (5) | |
| Second chronic phase | 7 (9) | 6 (8) | 1 (10) |
| Early death | 2 (3) | 2 (3) | |
| Aplastic death | 0 | 0 | 0 |
| Resistance disease | 21 (28) | 18 (28) | 3 (30) |
| Inevaluable | 5 | 3 | 2 |
| Complete/partial marrow response | 2 + 5 (9) | 2 + 4 (9) | 0 + 1 (10) |
| Imatinib mesylate response criteria met by patient13 | |||
| Marrow CR + recovery (ie, CHR) | 16 (21) | 15 (23) | 1 (10) |
| Marrow CR, no recovery | 20 (27) | 19 (29) | 1 (10) |
| Marrow PR | 8 (11) | 6 (9) | 2 (20) |
| Type of response . | No. of patients (%) . | ||
|---|---|---|---|
| Total . | Blast type . | ||
| Nonlymphoid . | Lymphoid . | ||
| Total treated | 75 | 65 | 10 |
| CHR | |||
| Total | 16 (21) | 15 (23) | 1 (10) |
| Cytogenetic response | 8 (11) | 7 (11) | 1 (10) |
| Minor | 2 (3) | 2 (3) | |
| Partial | 2 (3) | 2 (3) | |
| Complete | 4 (6) | 3 (5) | 1 (10) |
| HI | |||
| Total | 12 (16) | 11 (17) | 1 (10) |
| Cytogenetic response | 4 (5) | 4 (6) | |
| Minor | 2 (3) | 4 (6) | |
| Partial | 1 (1) | 1 (2) | |
| Complete | 1 (1) | 1 (2) | |
| PHR | 3 (4) | 3 (5) | |
| Second chronic phase | 7 (9) | 6 (8) | 1 (10) |
| Early death | 2 (3) | 2 (3) | |
| Aplastic death | 0 | 0 | 0 |
| Resistance disease | 21 (28) | 18 (28) | 3 (30) |
| Inevaluable | 5 | 3 | 2 |
| Complete/partial marrow response | 2 + 5 (9) | 2 + 4 (9) | 0 + 1 (10) |
| Imatinib mesylate response criteria met by patient13 | |||
| Marrow CR + recovery (ie, CHR) | 16 (21) | 15 (23) | 1 (10) |
| Marrow CR, no recovery | 20 (27) | 19 (29) | 1 (10) |
| Marrow PR | 8 (11) | 6 (9) | 2 (20) |
Data are given as number (%) of patients. CHR indicates complete hematologic response; HI, hematologic improvement; PHR, partial hematologic response; CR, complete response; PR, partial response.
Among the 10 patients with lymphoid blast-phase disease, 1 achieved CHR (and had a complete cytogenetic response), 1 had HI, 1 had a second chronic phase, and 1 had marrow PR. Thus, the overall objective response rate was 30% (3 of 10) according to established response criteria.
Twelve patients (16%) had a cytogenetic response (5 complete, 3 partial, and 4 minor). All cytogenetic responses were among patients who achieved either CHR (8 of 16, 50%) or HI (4 of 12, 33%).
Response duration and survival
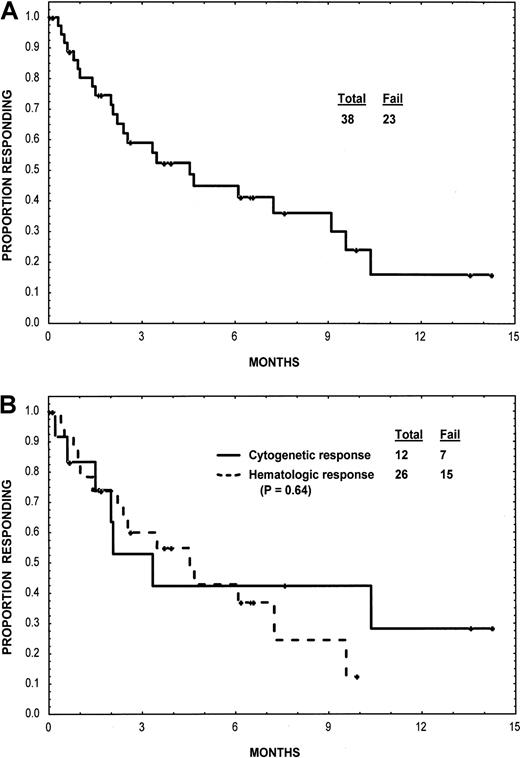
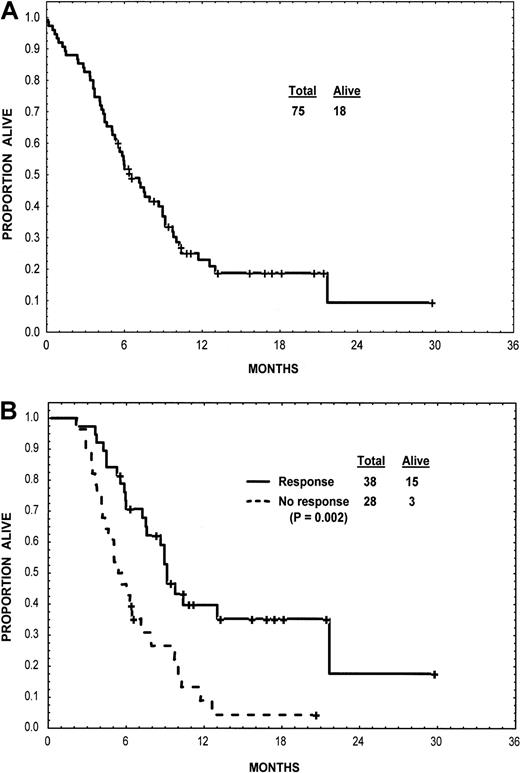
At a median follow-up of 11 months (cutoff for analysis, November 15, 2001), 57 patients had died and 8 others were still taking imatinib mesylate. The duration of response for the entire group and according to type response is shown in Figure 1. Cytogenetic responses were short lived and were not associated with a survival advantage. Survival for the entire group and survival according to type of response are shown in Figure2. Among patients with nonlymphoid blast-phase CML, the median survival was 6.5 months; the estimated 1-year survival rate was 28%. Among the 10 patients with lymphoid blast-phase CML, the median survival was 7 months.
Duration of response.
(A) Response duration for the group as a whole. (B) Response duration according to the type of response (cytogenetic or hematologic).
Duration of response.
(A) Response duration for the group as a whole. (B) Response duration according to the type of response (cytogenetic or hematologic).
Survival.
Survival is shown for the group as a whole (panel A) and according to type of response (panel B). Landmark analysis was dated at 8 weeks into therapy and excluded deaths that had taken place between the initiation of therapy and that 8-week point.
Survival.
Survival is shown for the group as a whole (panel A) and according to type of response (panel B). Landmark analysis was dated at 8 weeks into therapy and excluded deaths that had taken place between the initiation of therapy and that 8-week point.
Imatinib mesylate versus cytarabine
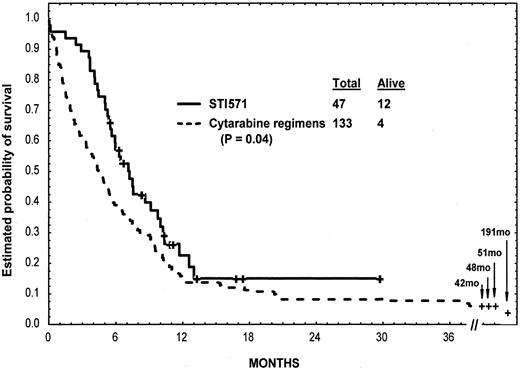
We compared the outcome of patients in this study given imatinib mesylate as the frontline therapy for nonlymphoid blast-phase CML (n = 47) with outcome in a historical control group, specifically patients given other cytarabine-based regimens as frontline treatment for nonlymphoid blast-phase CML at the MD Anderson Cancer Center between 1972 and 2000 (n = 133). The age, presence of splenomegaly or anemia, and proportion of blasts (previously established prognostic factors), as well as other characteristics,6 8 were similar in the 2 groups except for the younger age of the cytarabine study group (Table 3). Objective response rates (including CHR, PHR, HI, and return to second chronic phase) were 55% in the imatinib mesylate group and 29% in the cytarabine group (P = .001). The 4-week mortality rates were 4% in the imatinib mesylate group and 15% in the cytarabine group (P = .07). Survival was longer among patients treated with imatinib mesylate than those treated with cytarabine regimens (median survival, 7 months versus 4 months, P = .04; 1-year survival rates, 23% versus 15%) (Figure3).
Characteristics of the 47 patients treated with imatinib mesylate and the 133 historical control patients treated with cytarabine-containing regimens (1972-2000) for frontline therapy of myeloid blast-phase chronic myelogenous leukemia
| Characteristic . | Treatment, % . | |
|---|---|---|
| Imatinib mesylate . | Cytarabine regimens . | |
| Age > 60 years (median) | 47 (57) | 20 (45) |
| Splenomegaly > 5 cm bcm | 36 | 32 |
| Hemoglobin < 10 g/dL | 43 | 52 |
| WBC > 50 × 109/L | 28 | 31 |
| Peripheral blasts > 30% | 74 | 66 |
| Marrow blasts > 50% | 47 | 42 |
| Time from diagnosis to blastic phase ≥ 36 mo | 53 | 42 |
| Characteristic . | Treatment, % . | |
|---|---|---|
| Imatinib mesylate . | Cytarabine regimens . | |
| Age > 60 years (median) | 47 (57) | 20 (45) |
| Splenomegaly > 5 cm bcm | 36 | 32 |
| Hemoglobin < 10 g/dL | 43 | 52 |
| WBC > 50 × 109/L | 28 | 31 |
| Peripheral blasts > 30% | 74 | 66 |
| Marrow blasts > 50% | 47 | 42 |
| Time from diagnosis to blastic phase ≥ 36 mo | 53 | 42 |
bcm indicates below costal margin; WBC, white blood count.
Survival of patients given imatinib mesylate versus cytarabine.
Survival of 47 patients given imatinib mesylate and 133 patients given cytarabine-containing regimens as first salvage therapy for nonlymphoid blast-phase CML (2 of the 4 long-term survivors in the cytarabine group had allogeneic SCT and are alive without evidence of disease at longer than 191 and longer than 48 months).
Survival of patients given imatinib mesylate versus cytarabine.
Survival of 47 patients given imatinib mesylate and 133 patients given cytarabine-containing regimens as first salvage therapy for nonlymphoid blast-phase CML (2 of the 4 long-term survivors in the cytarabine group had allogeneic SCT and are alive without evidence of disease at longer than 191 and longer than 48 months).
Predictors of response and survival
The associations of pretreatment characteristics with achievement of objective response (CHR, PHR, HI, or second chronic phase) or survival are shown in Table 4. Only peripheral blasts predicted for response. Only platelet count was associated with survival, with higher counts predicting longer survival (Table 4). Multivariate analysis of pretreatment characteristics and response at the selected landmark time of 8 weeks identified treatment response (P < .05) as being an independent prognostic factor for survival.
Associations between pretreatment factors and response to imatinib mesylate
| Characteristic . | No. . | No. responding (%) . | P . | Median survival, mo . | P . |
|---|---|---|---|---|---|
| Age, y | |||||
| < 60 | 48 | 21 (44) | .09 | 6.0 | .86 |
| ≥ 60 | 27 | 18 (67) | |||
| Splenomegaly, cm bcm | |||||
| ≤ 5 | 40 | 21 (53) | .62 | 7.1 | .46 |
| > 5 | 28 | 17 (61) | 7.5 | ||
| Hemoglobin, g/dL | |||||
| < 10 | 38 | 21 (55) | .65 | 5.2 | .49 |
| ≥ 10 | 37 | 18 (49) | 5.2 | ||
| White blood cells, × 109/L | |||||
| < 30 | 38 | 22 (58) | .36 | 9.0 | .08 |
| ≥ 30 | 37 | 17 (46) | 5.4 | ||
| Platelets, × 109/L | |||||
| ≤ 50 | 25 | 10 (40) | .15 | 4.2 | .02 |
| > 50 | 50 | 29 (58) | 7.6 | ||
| % Peripheral blood blasts | |||||
| < 50 | 48 | 30 (63) | .02 | 7.6 | .18 |
| ≥ 50 | 27 | 9 (33) | 6.0 | ||
| % Bone marrow blasts | |||||
| < 50 | 35 | 20 (57) | .49 | 7.2 | .28 |
| ≥ 50 | 40 | 19 (48) | 6.2 | ||
| Blast morphology | |||||
| Lymphoid | 10 | 3 (30) | .18 | 6.8 | .20 |
| Nonlymphoid | 65 | 36 (55) | 6.5 | ||
| Time from diagnosis to blast phase, mo | |||||
| < 12 | 17 | 8 (47) | .89 | 6.0 | .96 |
| 12-36 | 21 | 11 (52) | 6.5 | ||
| > 36 | 37 | 20 (54) | 7.5 | ||
| Salvage status | |||||
| First | 50 | 28 (56) | .34 | 7.5 | .32 |
| ≥ Second | 25 | 11 (44) | 4.5 | ||
| Additional chromosomal abnormalities | |||||
| No | 28 | 18 (64) | .15 | 7.5 | .49 |
| Yes | 43 | 20 (47) | 4.5 | ||
| Objective response (landmark 8 weeks) | |||||
| No | 38 | — | 9.2 | .002 | |
| Yes | 28 | — | 5.6 |
| Characteristic . | No. . | No. responding (%) . | P . | Median survival, mo . | P . |
|---|---|---|---|---|---|
| Age, y | |||||
| < 60 | 48 | 21 (44) | .09 | 6.0 | .86 |
| ≥ 60 | 27 | 18 (67) | |||
| Splenomegaly, cm bcm | |||||
| ≤ 5 | 40 | 21 (53) | .62 | 7.1 | .46 |
| > 5 | 28 | 17 (61) | 7.5 | ||
| Hemoglobin, g/dL | |||||
| < 10 | 38 | 21 (55) | .65 | 5.2 | .49 |
| ≥ 10 | 37 | 18 (49) | 5.2 | ||
| White blood cells, × 109/L | |||||
| < 30 | 38 | 22 (58) | .36 | 9.0 | .08 |
| ≥ 30 | 37 | 17 (46) | 5.4 | ||
| Platelets, × 109/L | |||||
| ≤ 50 | 25 | 10 (40) | .15 | 4.2 | .02 |
| > 50 | 50 | 29 (58) | 7.6 | ||
| % Peripheral blood blasts | |||||
| < 50 | 48 | 30 (63) | .02 | 7.6 | .18 |
| ≥ 50 | 27 | 9 (33) | 6.0 | ||
| % Bone marrow blasts | |||||
| < 50 | 35 | 20 (57) | .49 | 7.2 | .28 |
| ≥ 50 | 40 | 19 (48) | 6.2 | ||
| Blast morphology | |||||
| Lymphoid | 10 | 3 (30) | .18 | 6.8 | .20 |
| Nonlymphoid | 65 | 36 (55) | 6.5 | ||
| Time from diagnosis to blast phase, mo | |||||
| < 12 | 17 | 8 (47) | .89 | 6.0 | .96 |
| 12-36 | 21 | 11 (52) | 6.5 | ||
| > 36 | 37 | 20 (54) | 7.5 | ||
| Salvage status | |||||
| First | 50 | 28 (56) | .34 | 7.5 | .32 |
| ≥ Second | 25 | 11 (44) | 4.5 | ||
| Additional chromosomal abnormalities | |||||
| No | 28 | 18 (64) | .15 | 7.5 | .49 |
| Yes | 43 | 20 (47) | 4.5 | ||
| Objective response (landmark 8 weeks) | |||||
| No | 38 | — | 9.2 | .002 | |
| Yes | 28 | — | 5.6 |
Side effects
Side effects were consistent with previous reports.13 14 Nonhematologic grade 3-4 side effects included nausea and vomiting (1%), liver dysfunction (8%), and fluid retention and edema (8%) (Table 5). Hematologic reactions were difficult to separate from the pretreatment degree of myelosuppression. However, among patients with pretreatment granulocyte counts exceeding 1 × 109/L and platelet counts exceeding 100 × 109/L, suppression of granulocyte counts to less than 0.5 × 109/L was noted in 24 of 48 patients (50%), and suppression of platelet count to less than 50 × 109/L was noted in 15 of 34 patients (44%). Febrile episodes were recorded in 13 patients (17%): fever of unknown origin in 6 (8%) and documented infections in 7 (10%). Some patients had multiple febrile episodes, resulting in a total of 13 febrile events, including 8 documented infections. Among the 57 patients who died, 10 died of disease progression during imatinib mesylate therapy, and 47 died after imatinib mesylate was stopped and the patient was undergoing other treatment. Cause of death was unknown for 9 patients (death outside the institution), infection for 2, bleeding for 2, a combination of infection and persistent disease for 12, disease progression in 31, and other causes in 1. No imatinib mesylate–associated deaths were observed in this study.
Side effects from imatinib mesylate
| Side effect . | No. patients (%) . | ||
|---|---|---|---|
| Grade 1-2 . | Grade 3-4 . | Any grade . | |
| Nausea, vomiting | 35 (47) | 1 (1) | |
| Diarrhea | 14 (19) | ||
| Skin rashes | 17 (23) | ||
| Muscle cramps, bone and joint aches | 23 (31) | 1 (1) | |
| Fluid retention | 27 (36) | 5 (7) | |
| Periorbital | 8 (11) | ||
| Lower extremities | 10 (13) | 1 (1) | |
| Liver dysfunction | 35 (47) | 6 (8) | |
| Other (congestive heart failure, renal) | 2 (3) | 1 (1) | |
| Granulocytopenia < 0.5 × 109/L | 24/48 (50)5-150 | ||
| Thrombocytopenia | |||
| < 50 × 109/L | 15/34 (44)5-150 | ||
| < 10 × 109/L | 5/34 (15)5-150 | ||
| Febrile episodes | 13 (17) | ||
| Unknown origin | 6 (8) | ||
| Bacterial infection | 5 (7) | ||
| Other infection | 2 (3) | ||
| Side effect . | No. patients (%) . | ||
|---|---|---|---|
| Grade 1-2 . | Grade 3-4 . | Any grade . | |
| Nausea, vomiting | 35 (47) | 1 (1) | |
| Diarrhea | 14 (19) | ||
| Skin rashes | 17 (23) | ||
| Muscle cramps, bone and joint aches | 23 (31) | 1 (1) | |
| Fluid retention | 27 (36) | 5 (7) | |
| Periorbital | 8 (11) | ||
| Lower extremities | 10 (13) | 1 (1) | |
| Liver dysfunction | 35 (47) | 6 (8) | |
| Other (congestive heart failure, renal) | 2 (3) | 1 (1) | |
| Granulocytopenia < 0.5 × 109/L | 24/48 (50)5-150 | ||
| Thrombocytopenia | |||
| < 50 × 109/L | 15/34 (44)5-150 | ||
| < 10 × 109/L | 5/34 (15)5-150 | ||
| Febrile episodes | 13 (17) | ||
| Unknown origin | 6 (8) | ||
| Bacterial infection | 5 (7) | ||
| Other infection | 2 (3) | ||
Denominators indicate the numbers of patients showing granulocytes > 109/L or platelets ≥ 100 × 109/L before treatment.
We also compared mortality at 4 weeks of patients given imatinib mesylate in this study with that of the historical control group given cytarabine-based therapy, as described earlier. Although attributing mortality to one event rather than another can be difficult, death within the first 4 weeks of therapy often reflects a combination of myelosuppression-associated complications, failure to control the disease, or both. We found that the rate of induction mortality during the first 4 weeks among patients undergoing first salvage therapy for nonlymphoid blast-phase CML in this study was 4% (2 of 47 patients); the corresponding rate among the historical controls treated with cytarabine regimens was 15% (20 of 133 patients) (P = .07).
Follow-up
At a median follow-up of 11 months, 57 patients (76%) had died (of causes described above) and 18 were alive, 8 of whom were continuing to take imatinib mesylate. Seven patients were able to undergo allogeneic SCT immediately after imatinib mesylate therapy. Of these patients, 3 were alive with no evidence of disease at 6+, 8+, and 17+ months; 1 is alive with CML relapse after SCT; 1 died of complications of the SCT; 1 died with recurrent blastic phase; and 1 was lost to follow-up.
Discussion
Although the prognosis for patients with CML in blast phase has always been considered extremely poor, the availability of imatinib mesylate has produced the first realistic hope of improving the outcome of blast-phase CML. In this, the largest single-institution experience with the use of imatinib mesylate to treat blast-phase CML, the objective response rate was 52%, and the cytogenetic response rate was 16%. At a median follow-up of 11 months, the estimated median survival of patients was 6.5 months. For patients given imatinib mesylate as the frontline therapy for nonlymphoid blast-phase disease, the objective response rate was higher with imatinib mesylate as compared with cytarabine-based chemotherapy (55% versus 29%;P = .001). The median survival with imatinib mesylate therapy was also longer than with standard intensive cytarabine-based chemotherapy (7 versus 4 months; P = .04). Side effects from imatinib mesylate treatment were manageable and less severe than those associated with standard chemotherapy. The number of deaths during the first 4 weeks of therapy was lower with imatinib mesylate than we found in our experience with other regimens (4% versus 15%;P = .07), reflecting that imatinib mesylate was both better tolerated and more efficacious. However, in this study of patients with blast-phase CML, the incidences of granulocytopenia (50%) and thrombocytopenia (44%) were higher than those observed among patients in chronic-phase CML, which may reflect either the poor normal bone-marrow reserve in patients with blast-phase disease or the higher imatinib mesylate doses that were used in this study.
In this study, response to imatinib mesylate was an independent predictor of survival. Lymphoid blast morphology, a known favorable prognostic factor for response and survival after treatment with anti–acute lymphocytic leukemia regimens, could not be evaluated because of the small number of patients treated.
The median survival of 7 months achieved by using imatinib mesylate as frontline therapy for nonlymphoid blast-phase disease is favorable and has not been matched by other regimens in large numbers of patients. Nevertheless, the overall prognosis remains unsatisfactory. One potential strategy for improving this prognosis is the use of allogeneic SCT at the stage of minimal residual disease. However, the evidence is that this approach is not broadly applicable, in that only 7 of the 75 patients (9%) in this study were able to proceed to allogeneic SCT. It can be argued that no patient with CML should ever reach the transformation phase if an appropriate matched donor can be identified, since allogeneic SCT ideally should be performed in the preblastic phase. Alternatives to allogeneic SCT include using imatinib mesylate in combination with established regimens, eg, idarubicin and cytarabine in standard or in modified lower-dose schedules, or with investigational agents such as low-dose decitabine, troxacitabine, or homoharringtonine.24-27 In our preliminary experience, troxacitabine produced objective responses in 5 of 16 evaluable patients (31%) and was associated with a median survival of 14 months.24 If these findings can be confirmed in a larger study group, a combination regimen of imatinib mesylate and troxacitabine may prove beneficial. In other studies, the DNA-methylation inhibitor decitabine, given at 500 to 1000 mg/m2 per course, produced an objective response rate of 28% and a median survival of 7 months.8,25 Lower-dose schedules of decitabine given over longer periods (eg, 5 to 20 mg/m2 over 1 hour daily for 10 to 15 days) may be superior and less toxic than the higher-dose decitabine schedules (J. P. Issa, unpublished data, January 2002). Another compound, homoharringtonine, has been shown to be superior to low-dose cytarabine against late chronic-phase CML26,27 and should also be considered for use in combination with imatinib mesylate for blast-phase CML. The advent of imatinib mesylate as the new cornerstone of therapy for blast-phase CML should encourage investigation of it in combination with one or more of the agents discussed above, or with some other promising investigational agents such as farnesyl transferase inhibitors28,29 or others.30
In summary, imatinib mesylate therapy seems to be the most active agent yet identified against blast-phase CML, raising hopes that future imatinib mesylate–based combination approaches could significantly improve the prognosis associated with this most resistant form of adult leukemia.
Supported by Novartis Oncology research grants.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hagop M. Kantarjian, Department of Leukemia, Box 428, MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: hkantarj@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal