B-cell chronic lymphocytic leukemia (B-CLL) is considered an accumulative disease of antigen-naive CD5+ B lymphocytes that circulate in the resting state. However, to evaluate the possibility that B-CLL cells resemble antigen-experienced and activated B cells, we analyzed the expression of markers of cellular activation and differentiation on CD5+CD19+ cells from B-CLL patients and from age-matched healthy donors. The leukemic cells from all B-CLL patients, including those that lack significant numbers of V gene mutations, bear the phenotype of activated B cells based on the overexpression of the activation markers CD23, CD25, CD69, and CD71 and the underexpression of CD22, Fcγ receptor IIb, CD79b, and immunoglobulin D that are down-regulated by cell triggering and activation. Furthermore, these leukemic cells resemble antigen-experienced lymphocytes in the underexpression of molecules that are down-regulated by cell triggering and in the uniform expression of CD27, an identifier of memory B cells. A comparison of the phenotypes of B-CLL patients with and without immunoglobulin V gene mutations suggests that the 2 subgroups differ both in specific marker expression (CD69, CD71, CD62 L, CD40, CD39, and HLA-DR) and in the time since antigenic stimulation, based on the reciprocal relationship of CD69 and CD71 expression. These findings imply that the leukemic cells from all B-CLL cases (irrespective of V gene mutations) exhibit features of activated and of antigen-experienced B lymphocytes and that the B-CLL cells that differ in immunoglobulin V genotype may have different antigen-encounter histories.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) has been viewed as a homogeneous disease arising from the accumulation of naive, antigen-inexperienced B lymphocytes that circulate in the resting state (reviewed in Caligaris-Cappio et al1). Indeed, the view that B-CLL cells are antigen-naive and resting is consistent with their appearance as small lymphocytes with high nuclear-to-cytoplasmic ratios2 and their surface membrane coexpression of immunoglobulin M (IgM) and IgD, which usually marks virgin B cells.3
Nonetheless, the concepts that B-CLL cells are homogeneous and similar among patients and that they resemble antigen-naive and resting B cells are currently being called into question. For example, the documentation of somatic mutations in the leukemic lymphocytes of greater than 50% of these patients4-7 indicates that B-CLL cells are not necessarily similar among patients and that the disease is not homogeneous in this respect. In fact, B-CLL cases can be divided into at least 2 subgroups on the basis of the presence or absence of significant numbers of V gene mutations; these subgroups identify patients that follow strikingly dissimilar clinical courses.8,9 Since the induction of immunoglobulin V gene mutations requires cellular activation and maturation induced by antigen receptor engagement,10 11 B-CLL cells with immunoglobulin V gene mutations resemble antigen-experienced B cells that at one point in their evolution were activated and therefore are not antigen-naive. On the basis of this reasoning, the question arises as to whether B-CLL cells that exhibit immunoglobulin V genes with germ line–like sequences are indeed resting and antigen-naive or whether they also represent B cells that were stimulated out of the resting state by antigen but that did not accumulate mutations.
In addition, other data suggest that in vivo activation, albeit abortive, can occur in B-CLL. For example, B-CLL cells can express elevations of cyclin D2 without increases of the other cyclins that follow in cell cycle progression.12 Furthermore, B-CLL cells can exhibit constitutive translocation of nuclear factor of activated T cells (NF-ATp)13 and phosphorylation of signal transducer and activator of transcription 1 (STAT-1) and STAT-3.14 Finally, most B-CLL cells express CD23, a cell surface marker acquired after B-cell activation.15-18 These findings suggest that certain B-CLL cells or their precursors may have been triggered and may have attempted to traverse the cell cycle, and may therefore not be resting or antigen-naive.
To examine further the possibility that the leukemic cells from all B-CLL cases are antigen-experienced and exist in an activated state, we analyzed the expression of a panel of surface markers that define cellular activation and differentiation in a large cohort of B-CLL patients. The data indicate that all B-CLL cells, including those from patients with unmutated immunoglobulin V genes, bear the surface membrane phenotype of activated and antigen-experienced B lymphocytes. Furthermore, comparative analyses of the unmutated and mutated subgroups indicate different activation phenotypes, suggesting that they resemble B cells that differ in the time since their antigenic stimulation and, possibly, B cells activated by different activation stimuli.
Patients, materials, and methods
Patients and healthy donors
The Institutional Review Boards of North Shore University Hospital (Manhasset, NY) and Long Island Jewish Medical Center (New Hyde Park, NY) approved these studies. Informed consent was provided by all participants in accordance with the Declaration of Helsinki. A total of 61 cases diagnosed as B-CLL were studied. Laboratory data on a subset of these cases have been reported earlier.3,7,8 Patients were selected for the present study on the basis of the availability of DNA sequences for both the immunoglobulin VH and VL genes in each case.5,8,19 Patients lacking allelic exclusion of immunoglobulin V genes20 were not included. As a source of normal lymphocytes, leukocyte-enriched fractions from the blood of age-matched healthy volunteers were purchased from Long Island Blood Services (Melville, NY). These samples did not contain detectable titers of human immunodeficiency virus or hepatitis B virus antibodies.
Isolation of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMCs) were separated from heparinized venous blood of the B-CLL patients and from leukocyte fractions of the healthy donors by density gradient centrifugation using Ficoll-Paque (Pharmacia LKB Biotechnology, Piscataway, NJ). PBMCs were cryopreserved with a programmable cell-freezing machine (Cryomed, Mt Clemens, MI) and thawed at the time of analysis.
Cellular immunophenotypic analyses
The panel of antibodies described in Table1 was used to analyze subsets of CD5+ B cells expressing various surface markers that identify the activation/differentiation state of cells. Initially, each antibody preparation was titrated to determine the amount that identified positive cells with the optimal mean fluorescence intensity; this amount was then used in subsequent studies. For direct immunofluorescent analyses, PBMCs were suspended at a concentration of 107 cells per milliliter in fluorescent-activated cell sorter (FACS) buffer (1% bovine serum albumin and 0.1% sodium azide in phosphate-buffered saline [PBS], pH 7.2) and 50 μL cells were added to the standardized volumes of fluorochrome-conjugated antibodies. The cells were incubated for 30 minutes at 4°C, and excess, unbound antibodies were removed by 2 washes with FACS buffer. Following these washes, cells were fixed with PBS containing 2% formaldehyde for at least 2 hours before analysis on a flow cytometer (FACScan, Becton Dickinson, San Jose, CA). The CellQuest program was used for statistical analysis of the acquired data.
Fluorochrome-conjugated and unconjugated antibodies used in immunophenotyping
| Molecule with which antibody was reactive . | Fluorochrome . | Manufacturer/source . |
|---|---|---|
| CD5 | FITC, PE | Becton Dickinson (San Jose, CA) |
| CD19 | APC | Becton Dickinson |
| CD22 | FITC | Immunotech (Westbrook, ME) |
| CD23 | FITC | Immunotech |
| CD24 | FITC | Pharmingen (La Jolla, CA) |
| CD25 | FITC | Immunotech |
| CD27 | FITC | Pharmingen |
| CD30 | FITC | Pharmingen |
| CD38 | PE | Becton Dickinson |
| CD39 | PE | Pharmingen |
| CD40 | FITC | Pharmingen |
| CD40L | FITC | Ancell (Bayport, MN) |
| CD44 | FITC | Pharmingen |
| CD45RA | FITC | Immunotech |
| CD62L | FITC | Pharmingen |
| CD69 | FITC | Becton Dickinson |
| CD71 | FITC | Becton Dickinson |
| CD72 | FITC | Pharmingen |
| CD77 | Unconjugated | Immunotech |
| CD79b | PE | Immunotech |
| CD80 | PE | Becton Dickinson |
| CD86 | FITC | Pharmingen |
| CD95 | FITC | MBL (Nagoya, Japan) |
| HLA-DR | FITC | Pharmingen |
| Syndecan-1 | FITC | Serotec (Oxford, United Kingdom) |
| Murine IgG | FITC | Biosource International (Camarillo, CA) |
| Rat IgM | FITC | Biosource International |
| Human IgM, IgG, IgA, and IgD | FITC | Southern Biotechnology Associates (Birmingham, AL) |
| Human kappa light chain | FITC | Becton Dickinson |
| Human lambda light chain | FITC | Becton Dickinson |
| FcγRII (II1A5) | Pure | Dr Jurgen Frey (Weinrich et al64) |
| FcγRIIb (II8D2) | Pure | Dr Jurgen Frey (Weinrich et al64) |
| Molecule with which antibody was reactive . | Fluorochrome . | Manufacturer/source . |
|---|---|---|
| CD5 | FITC, PE | Becton Dickinson (San Jose, CA) |
| CD19 | APC | Becton Dickinson |
| CD22 | FITC | Immunotech (Westbrook, ME) |
| CD23 | FITC | Immunotech |
| CD24 | FITC | Pharmingen (La Jolla, CA) |
| CD25 | FITC | Immunotech |
| CD27 | FITC | Pharmingen |
| CD30 | FITC | Pharmingen |
| CD38 | PE | Becton Dickinson |
| CD39 | PE | Pharmingen |
| CD40 | FITC | Pharmingen |
| CD40L | FITC | Ancell (Bayport, MN) |
| CD44 | FITC | Pharmingen |
| CD45RA | FITC | Immunotech |
| CD62L | FITC | Pharmingen |
| CD69 | FITC | Becton Dickinson |
| CD71 | FITC | Becton Dickinson |
| CD72 | FITC | Pharmingen |
| CD77 | Unconjugated | Immunotech |
| CD79b | PE | Immunotech |
| CD80 | PE | Becton Dickinson |
| CD86 | FITC | Pharmingen |
| CD95 | FITC | MBL (Nagoya, Japan) |
| HLA-DR | FITC | Pharmingen |
| Syndecan-1 | FITC | Serotec (Oxford, United Kingdom) |
| Murine IgG | FITC | Biosource International (Camarillo, CA) |
| Rat IgM | FITC | Biosource International |
| Human IgM, IgG, IgA, and IgD | FITC | Southern Biotechnology Associates (Birmingham, AL) |
| Human kappa light chain | FITC | Becton Dickinson |
| Human lambda light chain | FITC | Becton Dickinson |
| FcγRII (II1A5) | Pure | Dr Jurgen Frey (Weinrich et al64) |
| FcγRIIb (II8D2) | Pure | Dr Jurgen Frey (Weinrich et al64) |
Ig indicates immunoglobulin; FITC, fluorescein isothiocyanate; PE, phycoerythrin; APC, allophycocyanin.
The expression of CD77, Fcγ receptor II (FcγRII), and FcγRIIb was determined by indirect immunofluorescence. PBMCs were incubated with primary antibody for 30 minutes. Following 2 washes with FACS buffer, cells were incubated with fluorochrome-conjugated secondary antibodies (fluorescein isothiocyanate [FITC]–conjugated goat anti–rat IgM for CD77, and FITC-conjugated goat anti–mouse IgG1 for FcγRII and FcγRIIb), and anti-CD5–phycoerythrin and anti-CD19–allophycocyanin. Following incubation and washing, the cells were fixed and analyzed as above.
Statistical analyses
The percentages of normal CD5+ B cells expressing various surface markers were compared with those of the leukemic cells from the B-CLL cases studied. The statistical significance of the observed differences was deduced by means of the Mann-Whitney test. Differences in the density of expression of surface markers were similarly analyzed to determine statistical significance.
Results
Comparison of surface membrane phenotypes of B-CLL cells and of normal peripheral blood CD5+ B cells
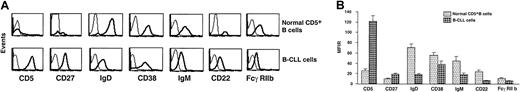
CD5+ B cells from B-CLL patients and age-matched healthy individuals were studied by 3-color immunofluorescence for expression of surface molecules that indicate different stages of cellular activation or differentiation. The percentages of B-CLL cells and normal CD5+ B cells expressing CD24, CD30, CD44, CD45RA, CD77, CD86, HLA-DR, and syndecan-1 were comparable. However, significantly higher numbers of B-CLL cells expressed the markers CD23, CD25, CD27, CD39, CD69, and CD71 (Figure1). In contrast, significantly fewer B-CLL cells expressed CD22, CD32/FcγRIIb, CD38, CD40, CD154/CD40 ligand (CD154/CD40L), CD62L, CD72, CD79b, CD80, and CD95 (Figure2).
Expression of surface membrane markers on B-CLL cells and normal CD5+ B cells.
Surface membrane markers overexpressed on B-CLL cells as compared with normal CD5+ B cells. PBMCs from B-CLL cases and age-matched healthy donors were analyzed for marker expression on CD5+CD19+ cells by means of 3-color immunofluorescence. (A) Representative flow cytometric profiles of expression of these markers on B cells from a 67-year-old healthy donor (N39) and a B-CLL patient (CLL 308). (B) Paired bars depict the mean ± SE of the percentage of CD5+CD19+cells expressing these markers in 61 (51 IgM+ and 10 IgG+) B-CLL patients and 20 age-matched healthy controls studied. B-CLL cells express significantly higher percentages (P < .0001) of CD27, CD39, CD23, CD25, CD69, and CD71 than normal CD5+CD19+ cells. The expression of these markers was variable both in the patients and in the markers, with the greatest variability seen for CD25. The SE and the SD for these markers ranged from 1.7 to 3.6 and from 13.1 to 28.0, respectively.
Expression of surface membrane markers on B-CLL cells and normal CD5+ B cells.
Surface membrane markers overexpressed on B-CLL cells as compared with normal CD5+ B cells. PBMCs from B-CLL cases and age-matched healthy donors were analyzed for marker expression on CD5+CD19+ cells by means of 3-color immunofluorescence. (A) Representative flow cytometric profiles of expression of these markers on B cells from a 67-year-old healthy donor (N39) and a B-CLL patient (CLL 308). (B) Paired bars depict the mean ± SE of the percentage of CD5+CD19+cells expressing these markers in 61 (51 IgM+ and 10 IgG+) B-CLL patients and 20 age-matched healthy controls studied. B-CLL cells express significantly higher percentages (P < .0001) of CD27, CD39, CD23, CD25, CD69, and CD71 than normal CD5+CD19+ cells. The expression of these markers was variable both in the patients and in the markers, with the greatest variability seen for CD25. The SE and the SD for these markers ranged from 1.7 to 3.6 and from 13.1 to 28.0, respectively.
Expression of surface membrane markers on B-CLL cells and normal CD5+ B cells.
Surface membrane markers underexpressed on B-CLL cells as compared with normal CD5+ B cells. PBMCs from B-CLL patients and age-matched healthy donors were analyzed for marker expression on CD5+CD19+ cells. (A) Representative flow cytometric profiles of expression of these markers on B cells from a 67-year-old healthy donor (N39) and CLL 308. (B) Paired bars depict the mean ± SE of the percentage of CD5+CD19+ cells expressing these markers in 51 IgM+ and 10 IgG+ B-CLL patients and 20 age-matched healthy controls. B-CLL cells expressed significantly lower percentages (P < .0001) of CD40, CD72, CD22, CD79b, CD62L, CD38, FcγRIIb, CD40L, CD95, and CD80 than normal CD5+CD19+ cells. The expression of these markers was variable both in the patients and in the markers, with the greatest variability seen for CD38. The SE and the SD for these markers ranged from 1.2 to 4.7 and from 8.9 to 35.5, respectively.
Expression of surface membrane markers on B-CLL cells and normal CD5+ B cells.
Surface membrane markers underexpressed on B-CLL cells as compared with normal CD5+ B cells. PBMCs from B-CLL patients and age-matched healthy donors were analyzed for marker expression on CD5+CD19+ cells. (A) Representative flow cytometric profiles of expression of these markers on B cells from a 67-year-old healthy donor (N39) and CLL 308. (B) Paired bars depict the mean ± SE of the percentage of CD5+CD19+ cells expressing these markers in 51 IgM+ and 10 IgG+ B-CLL patients and 20 age-matched healthy controls. B-CLL cells expressed significantly lower percentages (P < .0001) of CD40, CD72, CD22, CD79b, CD62L, CD38, FcγRIIb, CD40L, CD95, and CD80 than normal CD5+CD19+ cells. The expression of these markers was variable both in the patients and in the markers, with the greatest variability seen for CD38. The SE and the SD for these markers ranged from 1.2 to 4.7 and from 8.9 to 35.5, respectively.
Although the percentage of leukemic cells expressing CD38 among all the B-CLL patients studied was lower than the percentage of normal CD5+ B cells, when the 2 subgroups of B-CLL cases, defined on the basis of immunoglobulin V gene mutation status (see below), were independently compared with the CD5+ B cells from normal donors, the data were different. Specifically, in regard to the percentages of cells expressing CD38, the unmutated cases were similar to the normal CD5+ B cells (P = .083), whereas the mutated cases were different from normal CD5+ B cells (P < .001).
To measure the density of expression of an individual marker, we calculated the ratio of the mean fluorescence intensity (MFIR) of antibody binding to the surface molecule relative to the mean fluorescence intensity of nonspecific binding of a corresponding isotype “control” antibody. B-CLL cells and normal CD5+B cells expressed CD23, CD25, CD69, and CD71 at comparable densities (data not shown). However, CD5 and CD27 were expressed on B-CLL cells at significantly higher densities compared with normal CD5+cells, whereas CD22, FcγRIIb, and CD38 were expressed at significantly lower densities (Figure 3). The density of CD38 expression was less than that of normal CD5+ B cells, not only when the B-CLL cases were considered as a whole (P < .001) but also when they were segregated into unmutated (P < .001) and mutated (P < .01) subgroups.
Comparison of density of expression of surface membrane markers by B-CLL cells and normal CD5+ B cells.
The density of expression of CD5, CD27, CD38, CD22, FcγRIIb, IgD, and IgM on B-CLL cells was compared with that on CD5+CD19+ cells from age-matched controls. The density of expression of a membrane molecule was estimated as a ratio of specific-to-nonspecific binding of isotype “control” antibody and is expressed as the MFIR on the y-axis. Dark lines represent staining of cells using antibodies to the molecules listed whereas the thin lines represent the staining with isotype-matched control antibodies. (A) Representative flow cytometric profiles of expression of these markers on CD5+CD19+ B cells from a 67-year-old healthy donor (N39) and CLL 308. (B) Paired bars depict the mean ± SE of the MFIR of expression of these markers on CD5+CD19+ cells in 51 IgM+ B-CLL patients and 20 age-matched healthy controls. Although the IgG+ B-CLL patients expressed CD5, CD27, CD38, CD22, and FcγRIIb at densities identical to those of IgM+B-CLL patients, the data in this Figure do not include the IgG+ B-CLL patients since they do not express either smIgM (smIgM) or smIgD. All differences were statistically significant (P < .001).
Comparison of density of expression of surface membrane markers by B-CLL cells and normal CD5+ B cells.
The density of expression of CD5, CD27, CD38, CD22, FcγRIIb, IgD, and IgM on B-CLL cells was compared with that on CD5+CD19+ cells from age-matched controls. The density of expression of a membrane molecule was estimated as a ratio of specific-to-nonspecific binding of isotype “control” antibody and is expressed as the MFIR on the y-axis. Dark lines represent staining of cells using antibodies to the molecules listed whereas the thin lines represent the staining with isotype-matched control antibodies. (A) Representative flow cytometric profiles of expression of these markers on CD5+CD19+ B cells from a 67-year-old healthy donor (N39) and CLL 308. (B) Paired bars depict the mean ± SE of the MFIR of expression of these markers on CD5+CD19+ cells in 51 IgM+ B-CLL patients and 20 age-matched healthy controls. Although the IgG+ B-CLL patients expressed CD5, CD27, CD38, CD22, and FcγRIIb at densities identical to those of IgM+B-CLL patients, the data in this Figure do not include the IgG+ B-CLL patients since they do not express either smIgM (smIgM) or smIgD. All differences were statistically significant (P < .001).
Although the density of expression of surface membrane IgM (smIgM) and smIgD on B-CLL cells was significantly lower than on normal CD5+ B cells (P < .001; Figure 3), the ratio of surface IgM-to-IgD on B-CLL cells was significantly higher (mean, 1.58) compared with normal CD5+ B cells (mean, 0.66;P < .05).
Comparison of surface phenotypes of IgM+ and IgG+ B-CLL patients
Although most B-CLL patients bear smIgM, some express non-IgM isotypes.21 22 When we analyzed the same markers on the 10 IgG+ B-CLL patients in our cohort, we found significantly higher percentages of cells expressing CD27 (mean, 97.5% versus 85.6%), CD62L (mean, 59.7% versus 34.5%), and CD72 (mean, 89.8% versus 75.3%), and significantly lower percentages of cells expressing syndecan-1 (mean, 2.7% versus 14.9%), as compared with the 51 IgM+ cases (P < .001). These markers were expressed at similar densities in the IgG+ and IgM+ cases.
Phenotypic differences in B-CLL cases grouped by immunoglobulin V gene mutation status
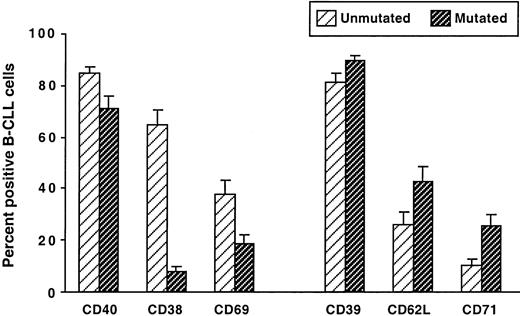
Because immunoglobulin V gene mutation status segregates B-CLL cases into 2 subgroups7 with significantly different clinical courses,8 9 we compared the phenotypes of our B-CLL cases stratified in this manner. The cases were classified as unmutated if both their immunoglobulin VH and their VL genes differed by less than 2% from the most similar germ line counterpart; they were classified as mutated if either their immunoglobulin VH or their VL genes differed by 2% or more from the corresponding germ line gene. The unmutated group contained significantly higher percentages of leukemic cells expressing CD38, CD69, and CD40, whereas the mutated group contained significantly higher percentages of B-CLL cells expressing CD62L, CD71, and CD39 (Figure 4).
Comparison of surface membrane phenotypes of IgM+ B-CLL cases grouped on the basis of immunoglobulin V gene mutation.
PBMCs from 51 IgM+ B-CLL cases were analyzed for the expression of CD38, CD39, CD40, CD62L, CD69, and CD71 on CD5+CD19+ cells. The B-CLL cases were segregated into 2 groups: unmutated, in which the expressed immunoglobulin V genes showed less than a 2.0% difference from the most similar germ line gene, and mutated, in which the expressed immunoglobulin V genes showed a 2.0% or greater difference from the most similar germ line. The mean ± SE of the percentages of CD5+CD19+ cells expressing these markers in the unmutated and mutated cases are represented. All differences were statistically significant (P < .001).
Comparison of surface membrane phenotypes of IgM+ B-CLL cases grouped on the basis of immunoglobulin V gene mutation.
PBMCs from 51 IgM+ B-CLL cases were analyzed for the expression of CD38, CD39, CD40, CD62L, CD69, and CD71 on CD5+CD19+ cells. The B-CLL cases were segregated into 2 groups: unmutated, in which the expressed immunoglobulin V genes showed less than a 2.0% difference from the most similar germ line gene, and mutated, in which the expressed immunoglobulin V genes showed a 2.0% or greater difference from the most similar germ line. The mean ± SE of the percentages of CD5+CD19+ cells expressing these markers in the unmutated and mutated cases are represented. All differences were statistically significant (P < .001).
The density of expression of these molecules was comparable in the 2 groups. This was also true for CD38, despite the marked difference between the unmutated and mutated groups (P = .39) in the percentage of cells expressing this molecule. In contrast, HLA-DR was expressed at a significantly higher density on the leukemic cells of the unmutated B-CLL group (P < .01; Figure5).
Comparison of density of HLA-DR expression on B-CLL cells and normal CD5+ B cells.
PBMCs from 61 B-CLL cases and 20 age-matched normal donors were analyzed for the expression of HLA-DR on CD5+CD19+ cells. Bars represent the mean ± SE of the MFIR of HLA-DR expression on CD5+CD19+ cells. The differences between the unmutated and mutated subgroups and between the CD38+and the CD38− subgroups are significant (P < .001). In addition, the differences between the unmutated subgroup and the normal CD5+ B cells, and between the CD38+ subgroup and the normal CD5+ B cells, are also significant (P < .001).
Comparison of density of HLA-DR expression on B-CLL cells and normal CD5+ B cells.
PBMCs from 61 B-CLL cases and 20 age-matched normal donors were analyzed for the expression of HLA-DR on CD5+CD19+ cells. Bars represent the mean ± SE of the MFIR of HLA-DR expression on CD5+CD19+ cells. The differences between the unmutated and mutated subgroups and between the CD38+and the CD38− subgroups are significant (P < .001). In addition, the differences between the unmutated subgroup and the normal CD5+ B cells, and between the CD38+ subgroup and the normal CD5+ B cells, are also significant (P < .001).
CD69 and CD71 are indicators of cellular activation that follow different kinetics of expression. CD69 is expressed very quickly (within 4 hours) after cell triggering, whereas CD71 is expressed much later.23-25 Since the unmutated B-CLL cases contain significantly higher percentages of CD69+ cells and lower percentages of CD71+ cells than the mutated group (Figure4), we determined the ratio of CD69 to CD71 expression in the 2 groups. When the IgM+ and IgG+ cases were analyzed together, a significant difference in the CD69-to-CD71 ratios was found (P < .0001). When the IgM+ and IgG+ cases were analyzed separately, the IgM+B-CLL cases exhibited this difference (P < .0001), whereas the IgG+ cases did not. This discordance may reflect the limited number of isotype-switched samples available for study (n = 10).
When we plotted the percentages of cells expressing these 2 markers in each individual B-CLL case, we observed strikingly different patterns. In general, a reciprocal relationship was found between CD69 and CD71 expression (Figure 6). In approximately 80% of unmutated cases, the percentage of CD69+ B-CLL cells was higher than that of CD71+cells; in contrast, only approximately 20% of mutated cases showed a higher percentage of CD69 expression.
Expression of CD69 and CD71 by B-CLL cells from unmutated and mutated cases.
PBMCs from 61 B-CLL cases were analyzed specifically for the expression of CD69 and CD71 on CD5+CD19+ cells. The percentages of CD5+CD19+ cells expressing CD69 or CD71 were plotted independently in each of the 2 adjacent scattergrams. The symbol representing the percentage of cells expressing CD69 is connected with a line to the symbol representing the percentage of cells expressing CD71 in each individual unmutated (left panel) and mutated case (right panel). Overall, the percentages of cells expressing CD69 inversely correlated with those expressing CD71 (P < .001).
Expression of CD69 and CD71 by B-CLL cells from unmutated and mutated cases.
PBMCs from 61 B-CLL cases were analyzed specifically for the expression of CD69 and CD71 on CD5+CD19+ cells. The percentages of CD5+CD19+ cells expressing CD69 or CD71 were plotted independently in each of the 2 adjacent scattergrams. The symbol representing the percentage of cells expressing CD69 is connected with a line to the symbol representing the percentage of cells expressing CD71 in each individual unmutated (left panel) and mutated case (right panel). Overall, the percentages of cells expressing CD69 inversely correlated with those expressing CD71 (P < .001).
Phenotypic differences in B-CLL cases grouped by CD38 expression
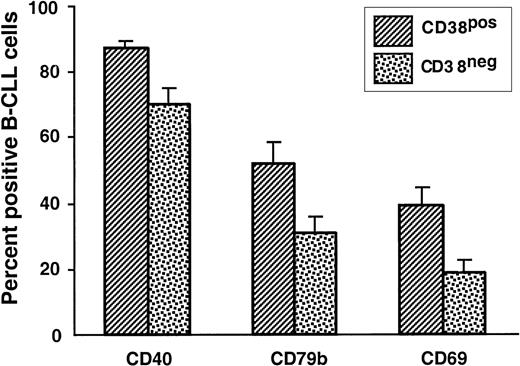
We previously reported that B-CLL cases can be subdivided into 2 groups on the basis of the percentage of leukemic cells that express CD38.8 26 Therefore, we subdivided our patients on the basis of CD38 expression (CD38+ for 30% or more CD38-expressing cells; CD38− for 30% or fewer CD38-expressing cells) and reanalyzed the surface marker expression data.
A significantly higher percentage of the CD38+ cases expressed CD40, CD69, and CD79b (Figure7). In addition, the density of expression of HLA-DR by the CD38+ group was significantly higher compared with the CD38− group (P < .01; Figure 5); these results were similar to those obtained when the cases were segregated with the use of immunoglobulin V gene mutation status as the discriminating criterion.
Comparison of surface membrane phenotypes of IgM+ B-CLL cases grouped on the basis of CD38 expression.
PBMCs from 51 IgM+ B-CLL cases were analyzed for the expression of CD38, CD40, CD69, CD79b, and HLA-DR on CD5+CD19+ cells. The B-CLL cases were segregated into 2 groups: CD38+, in which 30% or more CD5+CD19+ B cells expressed CD38, and CD38−, in which 30% or fewer CD5+CD19+ B cells expressed CD38. The mean ± SE of the percentages of CD5+CD19+ cells expressing CD40, CD69, or CD79b in the CD38+ and CD38− cases are provided.
Comparison of surface membrane phenotypes of IgM+ B-CLL cases grouped on the basis of CD38 expression.
PBMCs from 51 IgM+ B-CLL cases were analyzed for the expression of CD38, CD40, CD69, CD79b, and HLA-DR on CD5+CD19+ cells. The B-CLL cases were segregated into 2 groups: CD38+, in which 30% or more CD5+CD19+ B cells expressed CD38, and CD38−, in which 30% or fewer CD5+CD19+ B cells expressed CD38. The mean ± SE of the percentages of CD5+CD19+ cells expressing CD40, CD69, or CD79b in the CD38+ and CD38− cases are provided.
However, the expression of CD39, CD62L, and CD71 was not different in the CD38+ and CD38− groups; this contrasts with the results obtained when the cases were segregated according to immunoglobulin V gene mutation status. Nevertheless, the ratios of CD69-to-CD71 expression were significantly different in the CD38 subgroups (P < .001 for all B-CLL cases; P < .01 for IgM+ B-CLL cases), as was seen when the cases were compared on the basis of V gene mutation.
Discussion
The preceding data demonstrate that the leukemic cells from all B-CLL cases studied differ significantly in their surface membrane phenotypes from normal antigen-naive and resting CD5+ B cells. Indeed, the phenotypic picture that emerges is that B-CLL resembles the clonal expansion of mature antigen-experienced (not naive) B lymphocytes and that these leukemic cells exhibit an activated (not resting) state that is more advanced than that of normal circulating CD5+ B cells from age-matched controls.
B-CLL cells from all cases display an activated surface membrane phenotype
B-cell activation is generally accompanied by changes in the cell surface expression and/or density of certain critical functional molecules. Since our studies indicate that B-CLL cells from all cases express the classical activation markers CD23, CD25, CD69, and CD71 (albeit in varying degrees; see below), these cells clearly display an activated surface membrane phenotype. Furthermore, the increased density of expression of CD5 and CD27 on B-CLL cells (Figure 3) is in line with the activation hypothesis, since the level of both of these markers can be up-regulated upon cellular activation.27-30 Additional support for this hypothesis comes from data indicating that B lymphocytes from B-CLL patients express CD23 and other activation or costimulatory molecules and receptor/counter-receptor pairs16-18,31-33 and can express intracellular signaling intermediates.12-14
The reduction in the percentage of positivity and density of expression of CD22, CD32, CD79b, and IgD (Figures 2-3) is also consistent with cellular activation. B cells stimulated via the B-cell antigen receptor (BCR) down-regulate CD79b, either by modulating transcription or by splicing of messenger RNA.34,35 Furthermore, the reduced smIgD-to-smIgM ratio reported here may also reflect activation-induced modulation since mature B cells that coexpress IgM and IgD down-regulate IgD upon BCR signaling.36,37 Indeed, B-CLL cells from most patients exhibit diminished levels of surface immunoglobulin.38-41
Of interest is the reduced expression of CD22 and CD32 (Figures 2-3; Bourgois et al36; Merson and Brochier39), molecules that participate in the negative regulation of BCR-mediated42-45 and CD38-mediated46responses. Since B cells down-regulate CD22 and probably FcγRIIb after BCR-mediated B-cell activation47,48 and/or cytokine exposure,49-51 the finding of decreased expression of CD22 and FcγRIIb strengthens the concept that B-CLL cells are triggered and consequently lose these regulatory molecules. Our observations are in line with previous studies indicating decreased levels of surface membrane CD22.40,52 53
B-CLL cases that differ in immunoglobulin V gene mutation status also differ in activation phenotype
In addition to documenting the activated state of the leukemic cells of B-CLL cases as a whole, we have identified differences between the B-CLL subgroups defined by immunoglobulin V gene mutations. The V gene unmutated subgroup contains more cells expressing CD38, CD69, and CD40 and fewer cells expressing CD71, CD62L, and CD39 (Figure 4). In addition, the cells in this group express considerably more HLA-DR than the mutated group (Figure 5).
The time interval between cellular activation and the modulation of these markers differs considerably among normal B cells, in that the up-regulation of CD69 and HLA-DR and the down-regulation of CD62L occur more proximally to the inductive stimulus than the up-regulation of CD71.23-25 54 The strong inverse correlation between CD69 and CD71 expression (P < .001) by the unmutated versus the mutated subgroups (Figure 6) supports the notion that these subgroups approximate cells that differ in the interval since cellular triggering, with the unmutated group resembling B cells at an earlier state of activation than the mutated group.
Finally, our criterion for assigning a B-CLL case to the mutated category (ie, a 2% or greater difference from the most similar germ line gene counterpart) is based on a convention decided upon several years ago when the degree of polymorphism of the human VHlocus was less rigorously understood. Since this locus has now been sequenced, it may be reasonable to set the cutoff for this category at 1% or greater difference from the most similar germ line gene. Among the cohort of B-CLL patients studied here, 4 (all IgM+) exhibited mutations in the 1% to 2% range; these would be considered mutated if the cutoff were moved to a 1% or greater difference. Nevertheless, when we did a statistical analysis of our cases using a 1% mutation as the cutoff point, essentially the same significant differences in marker expression were observed as when we considered 2% mutation as the cutoff. Specifically, the percentage of B-CLL cells expressing CD38, CD39, CD40, CD69, and CD71 in patients with less than a 1% difference from the most similar germ line gene was significantly different from the patients with a 1% or greater difference (P < .05 for all). The difference in the percentage of B-CLL cells expressing CD62L among these 2 groups, however, did not achieve statistical significance with the 1% cutoff (P = .14). Furthermore, the difference in the density of expression of HLA-DR by the CLL cells of these 2 groups also remained statistically significant when the 1% cutoff was used (P < .05).
B-CLL cases that differ in CD38 expression also differ in activation phenotype
Since CD38 expression defines 2 groups of B-CLL cases8 that follow different clinical courses,8 55 we also analyzed our data to determine if a correlation exists between CD38 expression and a composite surface membrane phenotype.
The CD38+ cases show significantly more B-CLL cells expressing CD40, CD69, and CD79b (Figure 7) and a higher density of expression of HLA-DR as compared with the CD38−group (Figure 5). Although the CD38− group did not show an increase in CD71 expression, the ratios of CD69 to CD71 expression were significantly different in the 2 CD38 subgroups (P < .01; data not shown).
When these results were compared with those based on V gene mutation status, the B-CLL cells in the CD38+ group resembled the unmutated group with respect to CD40 and CD69 expression and the ratios of CD69 to CD71 expression. However, they differed in regard to CD39, CD62L, and CD79b expression. These results are consistent with the idea that these 2 sets of subgroups overlap but are not identical.8,9 26 The major phenotypic feature that discriminates the CD38+ from the CD38−subgroup and the unmutated from the mutated subgroup is the expression of markers indicative of temporal differences in cellular activation and possibly of the mechanisms leading to activation.
B-CLL cases do not appear to be antigen-naive
The assignment of the mutated cases to the memory compartment seems incontrovertible since these cells express significant numbers of immunoglobulin V gene mutations that are markers of BCR triggering and antigen encounter. However, since CD27 is a memory cell marker,56-58 the presence of very large numbers of CD27+ cells (approximately 90%) in all B-CLL cases demonstrated in this study (Figure 1) and others59-61implies that all B-CLL cases resemble antigen-experienced and “memory” B lymphocytes. Indeed, it is intriguing to consider the possibility that the expression of CD27 as well as the down-regulation of CD22, FcγRIIb, CD79b, and IgD (Figure 2) on the unmutated cases is also a reflection of previous triggering and memory. If so, then it is necessary to consider why these cases lack, or have very few, immunoglobulin V gene mutations. There are several possibilities. For example, these B cells may not have developed mutations because the appropriate mutational machinery was not activated. This could result because (1) the B cells were stimulated by antigens that could not turn on the mutation machinery and/or the germinal center reaction (eg, by T-cell–independent antigens, including autoantigens or other autologous structures); (2) the B cells were activated in a T-cell–dependent manner but were transformed before entering a germinal center; or (3) the B cells, after transformation, were prevented from participating in the germinal center reaction. Alternatively, these cases could have arisen from B cells that were activated and subsequently selected by antigens that are most reactive with unmutated immunoglobulin V genes. The CD38 data support the first possibility since CD38 can be expressed with cellular activation59,60 and induced by T cell–independent stimuli,30 whereas the restricted VH CDR3 features that are characteristic of the unmutated B-CLL cases7,62 63 support the last possibility.
Concluding considerations
All of the above conclusions are based on the hypothesis that the surface membrane phenotypes of B-CLL cells reflect their antigenic and activation experiences. Nevertheless, we cannot rule out the possibility that the expression of these marker proteins is a consequence of genetic abnormalities that occurred during leukemic transformation. However, if this is the case, then either different transformation mechanisms exist for the 2 subgroups or a common transforming event exists that is accompanied by other variables (such as in vivo activation) as an explanation for the differences in marker expression. Further studies will be necessary to clarify this issue.
We thank Ms Cathy Rapelje and Ms Grace Lee for performing flow cytometric analyses.
Supported in part by US Public Health Service grants CA81554, CA87956, and AI 10811 from the National Institutes of Health; the Jean Walton Fund for Lymphoma and Myeloma Research; the Joseph Eletto Leukemia Research Fund; the Sass Foundation for Medical Research; and the Richard and Nancy Leeds Fund of North Shore University Hospital.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nicholas Chiorazzi, North Shore–Long Island Jewish Research Institute, 350 Community Dr, Manhasset, NY 11030; e-mail: nchizzi@nshs.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal