Stromal cell–derived factor 1α (CXCL12) induces chemotaxis of lymphocytes through its receptor CXCR4. We examined the role of nonreceptor tyrosine kinases in CXCL12-induced chemotaxis of T cells and natural killer (NK) cells. Damnacanthal, a specific Lck inhibitor, but not the Syk inhibitor piceatannol, inhibited CXCL12-induced chemotaxis of both lymphocyte subsets. Similarly, damnacanthal was shown to inhibit CXCL12-induced chemotaxis of the Jurkat T-cell line. Stimulating T and NK cells with CXCL12 increased both the tyrosine phosphorylation and the kinase activity of Lck. A direct involvement of Lck in CXCL12-induced chemotaxis was demonstrated in the Lck-deficient Jurkat-derived cell line JCaM1.6. Although JCaM1.6 cells express CXCR4, no significant migration was detected after CXCL12 stimulation. Reconstitution with wild-type Lck restored both CXCL12-induced chemotaxis and Lck activation. Furthermore, cotransfection of wild-type Lck with C-terminal Src kinase (Csk) into JCaM1.6 failed to restore the chemotactic response induced by CXCL12. Finally, by targeting critical residues in the Src homology–2 (SH2) or SH3 domains of Lck, we observed that the SH3 domain is important for the function of Lck in CXCL12-mediated chemotaxis. Together, these results suggest a role for Lck in CXCL12-induced signaling pathways leading to lymphocyte chemotaxis.
Introduction
A superfamily of chemotactic cytokines, known as chemokines, induces the migration of leukocytes by promoting endothelial cell adhesion and transmigration toward a chemokine concentration gradient. Chemokines are small, secreted proteins of 8 to 10 kd and are subdivided into 4 families based upon a structurally related cystein motif in the amino terminal end. These are CXC (α), CC (β), C (γ), and CX3C (δ). Chemokines bind to receptors that are members of the 7 transmembrane–spanning domain receptor family that use heterotrimeric G proteins to transduce signals inside the cell. Receptor activation induces the release of Gβγ subunits from G proteins, which triggers a series of signaling events leading to cell movement.1 Stromal cell–derived factor 1α (CXCL12) (also known as SDF-1α) is a CXC chemokine and is the only known ligand for the chemokine receptor CXCR4.2CXCL12 induces the chemotaxis of CD34+ hematopoietic progenitor cells, T cells, B cells, natural killer (NK) cells, and monocytes, but not neutrophils.3-6
The Src family of tyrosine kinases is a closely related group of nonreceptor tyrosine kinases that modulate a variety of cellular functions.7 Most Src kinases consist of a unique N-terminal domain followed by an Src homology–3 (SH3), an SH2, and a catalytic domain.7 The Src kinase Lck is negatively regulated by phosphorylation of the conserved C-terminal tyrosine residue Tyr505 by the C-terminal Src kinase (Csk), while its kinase activity is promoted by autophosphorylation of Tyr394.8 The Src kinase Lck is confined to the lymphoid lineage,9 where it plays an important role in antigen receptor signaling. A physical coupling between Lck and CD4 has been found necessary for T-cell chemotaxis induced by the CD4 ligand interleukin 16 (IL-16).10,11 Moreover, IL-16 has been shown to induce an Lck-dependent inhibitory signal for CXCR4 chemotaxis, requiring the presence of the SH3 domain of Lck.11 Also, direct stimulation of the T-cell receptor inhibits CXCL12-induced chemotaxis,12 indicating a cross-talk between the T-cell receptor and CXCR4.
Lck is a promising candidate for orchestrating the signaling pathways necessary for chemotaxis. A recent report demonstrates that ZAP-70, which is a substrate for Lck, regulates chemotaxis induced by CXCL12,13 indicating a possible role for Lck in the signaling pathway leading to chemotaxis. ZAP-70 activation is suggested to be a necessary link to further downstream effectors such as the phosphatidylinositol-3 kinases (PI3 kinases),14 which are also implicated in the migrational response.15,16 It has been demonstrated that both the SH2 and the SH3 domains of Lck associate with a number of proteins implicated in the actin cytoskeletal reorganization events necessary for a migrational response, in particular the PI3 kinase, c-Cbl, and Vav.17-19 Also, Lck has been shown to be necessary for both CXCR4- and CD3-mediated activation of β1 and β2 integrins, suggesting an important role for Lck in mediating cell adhesion.20 21
Different Src kinases have recently been implicated in the signaling events leading to chemotaxis. In neutrophils, the Src kinases Hck and c-Fgr are essential for migration, while the Src kinase Lyn is required for chemotaxis of primary hematopoietic cells.22 23Although Lck is required for chemotaxis induced through CD4, it is not known whether Lck plays a similar role in chemotaxis mediated directly through chemokine receptors such as CXCR4. Here, we addressed the significance of Lck in CXCL12-induced chemotaxis of human T and NK cells. Through the use of a specific Lck inhibitor and the Lck-deficient cell line JCaM1.6, we demonstrate a novel role for Lck in CXCL12-induced chemotaxis, requiring the kinase activity of Lck as well as an intact SH3 domain.
Materials and methods
Reagents and materials
CXCL12 (SDF-1α) was purchased from PeproTech (Rocky Hill, NJ). Polyclonal and monoclonal antibodies against Lck and Protein A/G PLUS agarose beads were obtained from Santa Cruz Biotechnology (CA). Monoclonal antiphosphotyrosine antibody (4G10) and Src-family substrate peptide ([Lys19]cdc2(6-20)-NH2) were purchased from Upstate Biotechnology (Lake Placid, NY). Monoclonal anti-CXCR4 antibody was from R&D Systems Europe (Abingdon, Oxon, United Kingdom). Damnacanthal, piceatannol, and herbimycin A were from Biomol (Plymouth Meeting, PA).
Cell cultures and cell lines
Buffy coats from healthy human volunteers were obtained from the Red Cross Blood Bank at Ullevaal Hospital (Oslo, Norway). NK cells were generated from nylon-wool–nonadherent cells by means of adherence to plastic flasks in the presence of 1000 IU/mL IL-2 for 8 to 10 days as described.5 The culture medium consisted of RPMI 1640 supplemented with 10% human AB+serum (Ullevaal Hospital), 10 U/mL penicillin, 100 μg/mL streptomycin, 1 mM l-glutamine, 1% nonessential amino acids, and 5 × 10−5 M 2-ME (all from Life Technologies, Paisley, United Kingdom). Prior to use, NK cells were serum- and IL-2–starved for 18 hours by incubation in AIM-V medium (Life Technologies). This was followed by depletion of contaminating T cells by binding of the cells twice to Dynabeads coated with anti-CD3 (Dynal, Oslo, Norway). The result was more than 90% CD56+CD3− cells. Human naive T cells were enriched from nylon-wool–nonadherent cells and were more than 90% CD3+CD56−. The Jurkat cell line and JCaM1.6, a Jurkat cell line deficient in Lck, were treated as recently described.24
Chemotaxis assay
Blind-well chemotaxis chambers with a lower-well volume of 200 μL were used. We placed 200 μL RPMI medium containing 1% bovine serum albumin (BSA) in the lower wells in the presence or absence of CXCL12. Cells (4 × 105/100 μL NK cells, Jurkat cells, and JCaM1.6 cells and 1 × 106/100 μL T cells) were placed in the upper compartments of Boyden chambers above the filters. The chambers were incubated for 2 hours at 37°C in a 5% CO2 incubator. The filters were removed, dehydrated, fixed for 3 minutes in methanol, and stained with 15% Giemsa stain for 10 minutes. Cells in 10 high-power fields from 4 to 6 filters were counted and averaged for each sample. The migration index was calculated as the number of cells migrating toward the concentration gradient of chemokines divided by the number of cells migrating toward medium only.
Immunoprecipitation and immunoblotting
Cells were washed in RPMI containing 10 mM Hepes and resuspended in the same buffer. The cells were stimulated with CXCL12 at 37°C and lysed 1:1 vol on ice in 2 × NP-40 lysis buffer (2% Nonidet P-40; 50 mM Tris; 300 mM NaCl; 2 mM sodium orthovanadate; 20 mM NaF; and 2 mM phenylmethyl sulfonyl fluoride). The optimal concentration of CXCL12 was found to be 200 ng/mL for T cells and 80 ng/mL for NK cells. This could be due to higher-affinity CXCR4 receptors on activated NK cells, as compared with resting T cells. Cell lysates were clarified by centrifugation at 10 000 rpm for 10 minutes, then precleared with Protein G PLUS agarose beads for 30 minutes. The precleared lysates were immunoprecipitated for 2 hours with agarose conjugated with antiphosphotyrosine (PY99) (Santa Cruz Biotechnology). The immunoprecipitates were washed 3 times in 1 × NP-40 lysis buffer, separated on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels (BioRad, Uppsala, Sweden), and transferred to PVDF membranes (Millipore, Bedford, MA). The membranes were blocked for 30 minutes in 3% BSA or 5% skimmed milk, incubated with primary antibodies for 2 hours and with 1:5000 dilution of horseradish peroxidase–conjugated secondary antibodies (Jackson Immunotech, West Grove, PA) for 1 hour. Proteins were detected by means of Supersignal (Pierce, Rockford, IL).
Lck kinase assay
Cells (4 × 107/mL) were stimulated with CXCL12 at 37°C and lysed 1:1 vol on ice in 2 × NP-40 lysis buffer. Lck was immunoprecipitated from the lysates with monoclonal anti-Lck antibody (3A5) for 3 hours and Protein A/G PLUS agarose beads for 1 hour. One sample was immunoprecipitated with beads only as a background control. The immunoprecipitates were washed twice in lysis buffer and twice in kinase reaction buffer (100 mM Tris-HCl, pH 7.2, 125 mM MgCl2, 25 mM MnCl2, 2 mM ethyleneglycotetraacetic acid, 0.25 mM sodium orthovanadate, and 2 mM dithiothreitol). Two thirds of the purified Lck was added to 150 μM Src-family substrate peptide ([Lys19]cdc2(6-20)-NH2). Then, 100 μCi (3.7 MBq) [γ-32P]–adenosine 5′-triphosphate ([γ-32P]–ATP) (3000 Ci/mmol [110 TBq/mmol]) (Amersham Pharmacia Biotech, Uppsala, Sweden), 500 μM ATP, and kinase reaction buffer were added to a total of 40 μL. The mixture was incubated for 10 minutes at 30°C. The peptide was precipitated with 20 μL 40% trichloroacetic acid for 5 minutes at 30°C, transferred to P81 paper (Whatman, Maidstone, England), and washed twice in 0.75% phosphoric acid and once in acetone. The filters were counted in a scintillation counter. Kinase activity in stimulated samples was calculated as an increase in γ-32P incorporation compared with unstimulated samples. The remaining one third of the immunoprecipitated Lck was run on an SDS-PAGE gel, followed by anti-Lck immunoblotting to verify the immunoprecipitation of Lck.
Flow cytometric analysis
For the detection of CXCR4, JCaM1.6 cells (5 × 105 per sample) were stained with 1 μg anti-CXCR4 monoclonal antibody, or mouse immunoglobulin G as a control, for 45 minutes at 4°C. The cells were washed 3 times and then incubated 1:100 with a secondary rabbit anti–mouse phycoerythrin-conjugated antibody (Jackson Immunotech) for 30 minutes at 4°C. The samples were washed and examined in a FACS (Becton Dickinson, Mountain View, CA) flow cytometer.
Transient cell transfection
JCaM1.6 cells were washed once in Opti-MEM (Life Technologies) prior to transient transfection. Transfection was performed by electroporating 2 × 107 cells in 0.4 mL Opti-MEM mixed with 8 μg each DNA construct, in electroporation cuvettes with 0.4-cm electrode gap (BioRad). The cells were subjected to a field of 240 V/cm with 960 μF capacitance. The electroporated cells were expanded in complete medium with 10% fetal calf serum and harvested after 20 to 24 hours.
DNA constructs
The pEF-Neo vectors containing human Lck wild-type (wt) or Lck Tyr505Phe, and pEF-BOS/HA vectors containing human Csk were kind gifts from Dr K. Taskén (University of Oslo, Norway) and are as recently described.24 The Lck SH2 Arg154Lys or the Lck SH3 Trp98Ala were prepared by using the Stratagene QuickChange site-directed mutagenesis kit (Cedar Creek, TX) according to the manufacturer's instructions. The primers used were as follows: forward SH3, 5′-G-AGC-GGC-GAG-TGG-GCG-AAG-GCG-CAG-TC-3′; reverse SH3, 5′-GA-CTG-CGC-CTT-CGC-CCA-CTC-GCC-GCT-C-3′; forward SH2, 5′-C-GGC-TCC-TTC-CTC-ATC-AAA-GAG-AGC-GAG-AGC-ACC-G-3′; reverse SH2, 5′-C-GGT-GCT-CTC-GCT-CTC-TTT-GAT-GAG-GAA-GGA-GCC-G-3′. Constructs were verified by sequencing.
Statistical analysis
Data were analyzed with the unpaired Student ttest.
Results
The specific Lck inhibitor, damnacanthal, reduces CXCL12-induced lymphocyte chemotaxis
The signaling pathways leading from chemokine receptor activation to cell migration have not yet been fully elucidated. Nonreceptor tyrosine kinases, in particular the Src-family tyrosine kinases, have recently been implicated in the early signaling events after activation of G protein–coupled chemokine receptors.25 As the chemokine receptor CXCR4 is one of the most abundantly expressed chemokine receptors on both T and NK cells,5 26 we studied the role of Src kinases in chemotaxis induced by the CXCR4 ligand CXCL12 (SDF-1α).
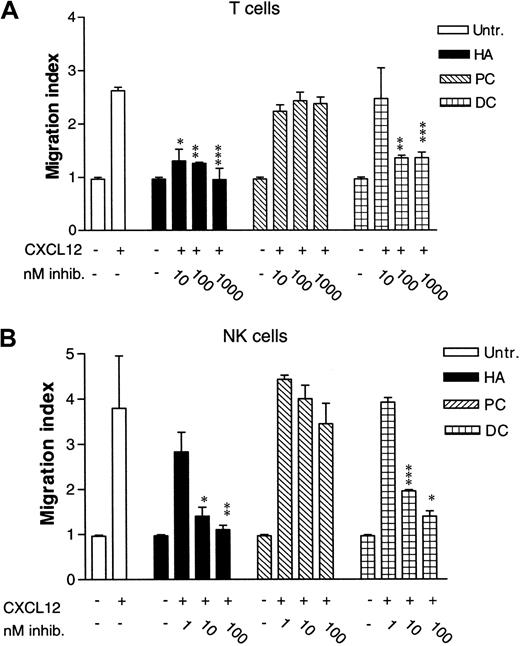
In Figure 1, the effect of 3 different inhibitors of nonreceptor tyrosine kinases on T- and NK-cell chemotaxis was studied. CXCL12 was used at 25 ng/mL, a concentration that is within the optimal response range for T lymphocytes.27Pretreating T cells with herbimycin A (HA), a general Src tyrosine kinase inhibitor, greatly reduced CXCL12-induced chemotaxis at 10 and 100 nM (P < .005 and P < .001, respectively), and the chemotactic response was almost totally abrogated at 1 μM concentration (P < .003; Figure 1A). This result indicated the importance of one or more Src-family kinases in CXCL12-induced chemotaxis of T cells. In contrast, chemotaxis induced by CXCL12 does not seem to depend on the Syk tyrosine kinase, another family of nonreceptor tyrosine kinases, as the Syk kinase inhibitor piceatannol (PC) did not reduce the chemotactic response even at 1 μM concentration. To dissect the type of Src kinase involved in the chemotactic response, we used a specific inhibitor of the Lck kinase, damnacanthal (DC).28 At 100 and 1000 nM DC, CXCL12-induced chemotaxis was greatly reduced (P < .001 and P < .003, respectively), indicating that the Src kinase Lck is important for chemotaxis. We did not use activated T cells since most known T-cell stimuli also activate Lck.10-12
Effect of tyrosine kinase inhibitors on CXCL12-induced chemotaxis of lymphocyte subsets.
(A) T cells either were left untreated or were pretreated with 10 to 1000 nM herbimycin A (HA), 10 to 1000 nM piceatannol (PC), or 10 to 1000 nM damnacanthal (DC) for 4 hours at 37°C. Chemotaxis assay was performed with 25 ng/mL CXCL12. Mean ± SD of 3 experiments (12 filters). *P < .005. **P < .001. ***P < .003. P values indicate comparison with cells not treated with inhibitors. (B) NK cells were either untreated or pretreated for 4 hours at 37°C with 1 to 100 nM HA, 1 to 100 nM PC, or 1 to 100 nM DC. Chemotaxis assay was performed with 10 ng/mL CXCL12. Mean ± SD of 3 experiments (18 filters). *P < .03. **P < .02. ***P < .07. P values indicate comparison with chemotaxis of cells not treated with inhibitors.
Effect of tyrosine kinase inhibitors on CXCL12-induced chemotaxis of lymphocyte subsets.
(A) T cells either were left untreated or were pretreated with 10 to 1000 nM herbimycin A (HA), 10 to 1000 nM piceatannol (PC), or 10 to 1000 nM damnacanthal (DC) for 4 hours at 37°C. Chemotaxis assay was performed with 25 ng/mL CXCL12. Mean ± SD of 3 experiments (12 filters). *P < .005. **P < .001. ***P < .003. P values indicate comparison with cells not treated with inhibitors. (B) NK cells were either untreated or pretreated for 4 hours at 37°C with 1 to 100 nM HA, 1 to 100 nM PC, or 1 to 100 nM DC. Chemotaxis assay was performed with 10 ng/mL CXCL12. Mean ± SD of 3 experiments (18 filters). *P < .03. **P < .02. ***P < .07. P values indicate comparison with chemotaxis of cells not treated with inhibitors.
Similar results were obtained with NK cells. While untreated NK cells showed a clear chemotactic response to CXCL12, this was inhibited by pretreating NK cells with 10 and 100 nM HA (P < .03 andP < .02, respectively; Figure 1B). PC, on the other hand, did not significantly reduce the chemotactic response to CXCL12 even at 100 nM. Using the Lck inhibitor DC at 1 nM concentration did not significantly reduce the chemotactic response toward CXCL12. However, increasing the concentration of DC to 10 and 100 nM reduced the chemotactic response (P < .07 and P < .03, respectively). Collectively, these results point toward a role for Lck in CXCL12-induced chemotaxis in both T and NK cells.
Stimulation of T and NK cells with CXCL12 induces tyrosine phosphorylation of Lck
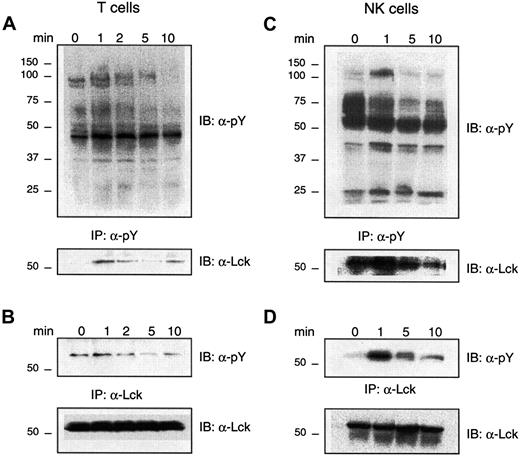
As Lck appears to be involved in CXCL12-induced chemotaxis of T cells and NK cells, we examined whether CXCL12 induces tyrosine phosphorylation of Lck in these cells. T cells and NK cells were stimulated with CXCL12 for 0 to 10 minutes. Figure2 demonstrates a rapid enhancement of tyrosine phosphorylation of several proteins in both T cells (panel A) and NK cells (panel C) after 1-minute stimulation with CXCL12. Phosphorylation was especially pronounced for proteins of molecular masses of approximately 120, 75, 50 to 60, 40, and 30 kd. These phosphoproteins probably represent members of the focal adhesion complex, tyrosine kinases and MAP kinases that have been previously described in CXCL12-induced signaling pathways.3,15 29 The general tyrosine phosphorylation response was transient in both lymphocyte subsets, as it decreased after 5 to 10 minutes of stimulation.
Effect of CXCL12 on tyrosine phosphorylation of Lck in lymphocytes.
CXCL12 induces tyrosine phosphorylation of Lck in lymphocytes. (A) (B) Lysates from T cells (4 × 107/mL) unstimulated (0) or stimulated with CXCL12 (200 ng/mL) for the indicated times, were immunoprecipitated (IP) with antiphosphotyrosine antibody (panel A) or anti-Lck (panel B) and immunoblotted with an antibody against phosphotyrosine (upper panels). The filters were stripped and reimmunoblotted with an antibody against Lck (lower panels). Representative for 4 independent experiments. (C) (D) Lysates from NK cells (4 × 107/mL) either unstimulated (0) or stimulated with CXCL12 (80 ng/mL) for the indicated times, were immunoprecipitated with either an antiphosphotyrosine antibody (panel C) or an anti-Lck antibody (panel D) and immunoblotted with an antibody against phosphotyrosine (upper panels). The filters were stripped and reimmunoblotted with an antibody against Lck (lower panels). Representative of 4 independent experiments.
Effect of CXCL12 on tyrosine phosphorylation of Lck in lymphocytes.
CXCL12 induces tyrosine phosphorylation of Lck in lymphocytes. (A) (B) Lysates from T cells (4 × 107/mL) unstimulated (0) or stimulated with CXCL12 (200 ng/mL) for the indicated times, were immunoprecipitated (IP) with antiphosphotyrosine antibody (panel A) or anti-Lck (panel B) and immunoblotted with an antibody against phosphotyrosine (upper panels). The filters were stripped and reimmunoblotted with an antibody against Lck (lower panels). Representative for 4 independent experiments. (C) (D) Lysates from NK cells (4 × 107/mL) either unstimulated (0) or stimulated with CXCL12 (80 ng/mL) for the indicated times, were immunoprecipitated with either an antiphosphotyrosine antibody (panel C) or an anti-Lck antibody (panel D) and immunoblotted with an antibody against phosphotyrosine (upper panels). The filters were stripped and reimmunoblotted with an antibody against Lck (lower panels). Representative of 4 independent experiments.
To examine whether one of the CXCL12-induced phosphorylated bands was the p56lck kinase, the filters were stripped and reblotted with an antibody toward Lck. The lower panel in Figure 2A shows a sharp increase in tyrosine phosphorylation of Lck from 0 to 1 minute in T cells, which then markedly decreased after 5 minutes of CXCL12-stimulation. A more direct demonstration of Lck tyrosine phosphorylation is shown in Figure 2B where T cells stimulated with CXCL12 are immunoprecipitated with an antibody against Lck and then immunoblotted with antiphosphotyrosine. Lck showed the same tyrosine phosphorylation pattern as seen with the phosphotyrosine immunoprecipitation in Figure 2A. The Lck phosphorylation appears to be biphasic, as the tyrosine phosphorylation content of Lck increased again after 10 minutes. An equal amount of Lck in the different lanes is demonstrated in Figure 2B, lower panel. Also in NK cells, CXCL12 induced a general increase in tyrosine-phosphorylated proteins after 1-minute stimulation with CXCL12 (Figure 2C, upper panel). A phosphorylated protein at approximately 56 kd was observed when the phosphotyrosine immunoblot was stripped and reblotted with anti-Lck (Figure 2C, lower panel). Reciprocally, when immunoprecipitating Lck after stimulation of NK cells with CXCL12 and immunoblotting it with antiphosphotyrosine, we were able to demonstrate phosphorylation of a band corresponding to Lck (Figure 2D, upper panel). Maximum tyrosine phosphorylation of Lck in NK cells appears to be after 1 minute of stimulation with CXCL12. Equal amount of Lck in each lane is demonstrated (Figure 2D, lower panel).
CXCL12 induces kinase activity of Lck in T and NK cells
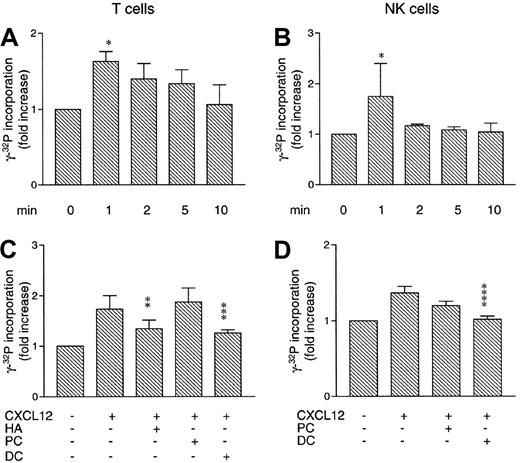
As phosphorylation of Lck can either activate or inhibit its enzymatic activity, we performed Lck kinase activity assays with either unstimulated or CXCL12-stimulated T cells or NK cells. As shown in Figure 3A, CXCL12 increased the kinase activity of Lck in T cells after 1 minute of stimulation (P < .05 compared with unstimulated cells), which slowly declined back to basal levels after 10 minutes. In NK cells, the Lck kinase activity increased markedly after 1 minute of CXCL12 stimulation (P < .05 compared with unstimulated cells), then returned to basal levels (Figure 3B).
Effect of CXCL12 on kinase activity of Lck in lymphocytes.
CXCL12 increases kinase activity of Lck in lymphocytes. (A) T cells (4 × 107/mL) were stimulated with CXCL12 (200 ng/mL) for the indicated times. Lysates were subjected to Lck kinase activity assay. Mean ± SD of 3 experiments. (B) NK cells (4 × 107/mL) were stimulated with CXCL12 (80 ng/mL) for the indicated times. Lysates were subjected to Lck kinase activity assay. Mean ± SD of 3 experiments. (C) T cells (4 × 107/mL) were either untreated or pretreated with 1 μM HA, 1 μM PC, or 1 μM DC for 4 hours at 37°C. Cells were stimulated with 200 ng/mL CXCL12 for 1 minute, then subjected to the Lck kinase activity assay. Mean ± SD of 3 experiments. (D) NK cells (4 × 107/mL) were either untreated or pretreated with 100 nM PC or 100 nM DC for 4 hours at 37°C. Cells were stimulated with 80 ng/mL CXCL12 for 1 minute, then subjected to the Lck kinase activity assay. Mean ± SD of 3 experiments. *P < .05. **P < .03. ***P < .02. ****P < .01.
Effect of CXCL12 on kinase activity of Lck in lymphocytes.
CXCL12 increases kinase activity of Lck in lymphocytes. (A) T cells (4 × 107/mL) were stimulated with CXCL12 (200 ng/mL) for the indicated times. Lysates were subjected to Lck kinase activity assay. Mean ± SD of 3 experiments. (B) NK cells (4 × 107/mL) were stimulated with CXCL12 (80 ng/mL) for the indicated times. Lysates were subjected to Lck kinase activity assay. Mean ± SD of 3 experiments. (C) T cells (4 × 107/mL) were either untreated or pretreated with 1 μM HA, 1 μM PC, or 1 μM DC for 4 hours at 37°C. Cells were stimulated with 200 ng/mL CXCL12 for 1 minute, then subjected to the Lck kinase activity assay. Mean ± SD of 3 experiments. (D) NK cells (4 × 107/mL) were either untreated or pretreated with 100 nM PC or 100 nM DC for 4 hours at 37°C. Cells were stimulated with 80 ng/mL CXCL12 for 1 minute, then subjected to the Lck kinase activity assay. Mean ± SD of 3 experiments. *P < .05. **P < .03. ***P < .02. ****P < .01.
T and NK cells were next pretreated with kinase inhibitors prior to performing Lck kinase assays. In Figure 3C, we demonstrate that pretreatment with 1 μM of both HA and DC inhibits the Lck kinase activity in T cells (P < .03 and P < .02, respectively), as opposed to pretreatment with 1 μM of the Syk inhibitor PC. Pretreating NK cells with 100 nM DC inhibited CXCL12-induced kinase activity of Lck (P < .01; Figure3D), comparable to untreated cells. In contrast, the Syk kinase inhibitor PC (100 nM) did not significantly block Lck kinase activity.
The Lck-deficient T-cell line JCaM1.6 does not migrate in response to CXCL12
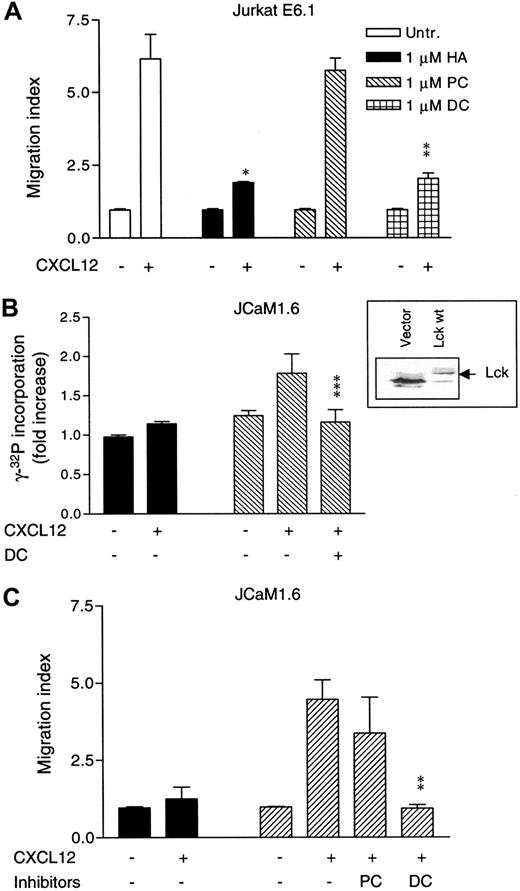
To further evaluate the role of Lck in CXCL12-induced chemotaxis, we extended the studies to the mutant Jurkat T-cell line JCaM1.6, which lacks functional Lck.30 The parental Jurkat cell line E6.1 behaved similarly to T and NK cells when treated with the different tyrosine kinase inhibitors prior to chemotaxis toward CXCL12. Previous work has shown optimal migration of Jurkat cells in response to CXCL12 to be in the range of 10 to 100 ng/mL.13 27 Figure4A shows that chemotaxis of Jurkat cells induced by 25 ng/mL CXCL12 was almost completely inhibited upon pretreatment with either 1 μM HA (P < .007) or 1 μM DC (P < .009). In contrast, PC did not affect Jurkat cell chemotaxis.
Role of Lck in CXCL12-induced chemotaxis of Lck-deficient JCaM1.6 cells.
Expression of Lck is necessary for CXCL12-induced chemotaxis of Lck-deficient JCaM1.6 cells. (A) Jurkat T cells were either left untreated or were pretreated with 1 μM HA, 1 μM PC, or 1 μM DC for 4 hours at 37°C and subjected to chemotaxis assay toward CXCL12 (25 ng/mL). Mean ± SD of 2 experiments (8 filters). (B) CXCL12-induced Lck kinase activity in JCaM1.6 cells transfected with empty vector (black bars) or with Lck wt (striped bars). A sample of Lck-transfected cells was pretreated with 100 nM DC for 4 hours. The different transfectants (4 × 107/mL) were either unstimulated or stimulated for 1 minute with CXCL12 (100 ng/mL). Mean ± SD of 3 experiments. Insert represents an immunoblot analysis of Lck in JCaM1.6 cells transfected with an empty vector or with wt Lck. (C) Chemotaxis of JCaM1.6 cells transfected with empty vector (black bars) or Lck wt (striped bars) toward CXCL12 (25 ng/mL). The Lck-transfected cells were either left untreated or pretreated with 100 nM of either PC or DC for 4 hours at 37°C. Mean ± SD of 3 experiments (18 filters). *P < .007. **P < .009. ***P < .05. P values indicate comparison with cells not treated with inhibitors.
Role of Lck in CXCL12-induced chemotaxis of Lck-deficient JCaM1.6 cells.
Expression of Lck is necessary for CXCL12-induced chemotaxis of Lck-deficient JCaM1.6 cells. (A) Jurkat T cells were either left untreated or were pretreated with 1 μM HA, 1 μM PC, or 1 μM DC for 4 hours at 37°C and subjected to chemotaxis assay toward CXCL12 (25 ng/mL). Mean ± SD of 2 experiments (8 filters). (B) CXCL12-induced Lck kinase activity in JCaM1.6 cells transfected with empty vector (black bars) or with Lck wt (striped bars). A sample of Lck-transfected cells was pretreated with 100 nM DC for 4 hours. The different transfectants (4 × 107/mL) were either unstimulated or stimulated for 1 minute with CXCL12 (100 ng/mL). Mean ± SD of 3 experiments. Insert represents an immunoblot analysis of Lck in JCaM1.6 cells transfected with an empty vector or with wt Lck. (C) Chemotaxis of JCaM1.6 cells transfected with empty vector (black bars) or Lck wt (striped bars) toward CXCL12 (25 ng/mL). The Lck-transfected cells were either left untreated or pretreated with 100 nM of either PC or DC for 4 hours at 37°C. Mean ± SD of 3 experiments (18 filters). *P < .007. **P < .009. ***P < .05. P values indicate comparison with cells not treated with inhibitors.
To compare CXCL12-induced chemotaxis in the Lck-deficient JCaM1.6 cells and Lck-reconstituted JCaM1.6 cells, we transiently transfected the JCaM1.6 cell line with either a wt Lck construct or an empty vector. Almost comparable levels of CXCR4 expression in both Lck-deficient and Lck-reconstituted JCaM1.6 cells were detected (Figure 6C), ruling out the possibility that any difference in chemotaxis might be due to a variation in CXCR4 expression. A successful transfection of Lck was demonstrated by a Western blot analysis (Figure 4B, insert). Also, we wanted to ascertain that the reintroduced wt Lck was enzymatically intact. Hence, an Lck kinase activity assay was performed with the wt Lck–transfected JCaM1.6 cells. As demonstrated in Figure 4B, cells transfected with Lck wt, and stimulated with CXCL12 for 1 minute, showed a marked increase in Lck kinase activity as compared with unstimulated Lck-transfected cells. No Lck kinase activity was observed above the background activity in CXCL12-stimulated JCaM1.6 cells transfected with an empty vector. Lck-transfected cells pretreated with 100 nM DC prior to stimulation with CXCL12 showed a decrease in the kinase activity as compared with DC-untreated cells (P < .05; Figure 4B). These results demonstrate a fully active Lck kinase after transfection of its construct into JCaM1.6 cells and show that CXCL12 is able to activate Lck in these cells.
The migratory behavior of Lck-deficient JCaM1.6 cells and Lck-transfected JCaM1.6 cells was compared in a chemotaxis assay toward CXCL12. We used CXCL12 at 25 ng/mL, the same concentration we used for the chemotactic assays with the parental E6.1 cell line. Figure 4C demonstrates that JCaM1.6 cells transfected with the empty vector migrated poorly in response to CXCL12. On the other hand, when these cells were being transiently transfected with wt Lck, a clear chemotactic response was observed (P < .005, compared with cells transfected with empty vector). When cells transfected with the wt Lck were pretreated with 100 nM DC or PC, the cells pretreated with PC still migrated potently, whereas cells pretreated with DC showed a low chemotactic response as compared with cells not treated with DC (P < .009).
Csk inhibits CXCL12-induced chemotaxis
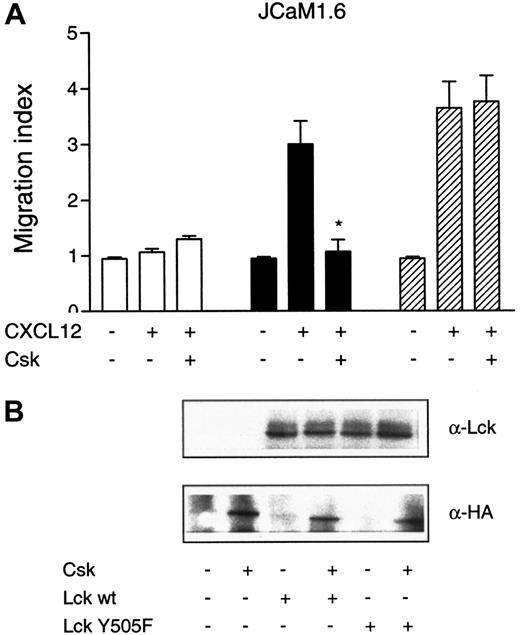
Csk negatively regulates Lck by phosphorylation of the C-terminal tyrosine residue Tyr505.31 To study the effect of Csk on CXCL12-induced chemotaxis, JCaM1.6 cells were transfected with an empty vector or the wt Lck construct in combination with an HA-tagged version of Csk. Figure 5demonstrates that cotransfection of Csk with wt Lck abrogates the chemotactic response as compared with cells transfected with wt Lck alone (P < .01). We next studied the effect of a mutated version of Lck, where the tyrosine at position 505 had been point mutated to phenylalanine (Tyr505Phe). As Csk cannot phosphorylate this mutated version of Lck, Lck Tyr505Phe is constitutively active. To further demonstrate the negative regulation of Lck by Csk, we transfected JCaM1.6 cells with Lck Tyr505Phe in combination with Csk. While cotransfection of Csk with wt Lck abrogated the chemotactic response to CXCL12, cotransfection of Csk with Lck Tyr505Phe had no inhibitory effect on the CXCL12-induced chemotactic response, reflecting that Lck Tyr505Phe is under no negative regulation (Figure5A). Also, a robust chemotactic response toward CXCL12 with the use of the Lck Tyr505Phe construct alone was observed (Figure 5A). JCaM1.6 cells cotransfected with Csk and an empty vector had no effect on chemotaxis induced by CXCL12 (Figure 5A). Control immunoblots of Csk and Lck were performed to ascertain successful transfections (Figure5B). The upper panel in Figure 5B shows the presence of Lck in JCaM1.6 cells transfected with either Lck wt or Lck Tyr505Phe. The lower panel demonstrates the presence of transfected Csk, detected with an anti-HA antibody. The above results indicate a specific requirement of Lck, as Csk cannot inhibit the chemotactic response of cells expressing a constitutively active version of Lck, suggesting that Csk in this particular signaling pathway specifically regulates Lck and no other Src kinase.
Effect of Csk on CXCL12-induced chemotaxis.
Csk inhibits CXCL12-induced chemotaxis. JCaM1.6 cells were transiently transfected with empty vector (■), Lck wt (■), or the constitutively active Lck Tyr505Phe construct (▨). All 3 constructs were also cotransfected with a vector encoding Csk, as indicated in the Figure. (A) The different transfectants were subjected to chemotaxis assay toward CXCL12 (25 ng/mL). Mean ± SD of 2 experiments (12 filters). *P < .01 as compared with cells transfected with Lck wt alone. (B) Lysates from transfected JCaM1.6 cells were immunoblotted with either an anti-Lck antibody or an anti-HA antibody to detect Csk.
Effect of Csk on CXCL12-induced chemotaxis.
Csk inhibits CXCL12-induced chemotaxis. JCaM1.6 cells were transiently transfected with empty vector (■), Lck wt (■), or the constitutively active Lck Tyr505Phe construct (▨). All 3 constructs were also cotransfected with a vector encoding Csk, as indicated in the Figure. (A) The different transfectants were subjected to chemotaxis assay toward CXCL12 (25 ng/mL). Mean ± SD of 2 experiments (12 filters). *P < .01 as compared with cells transfected with Lck wt alone. (B) Lysates from transfected JCaM1.6 cells were immunoblotted with either an anti-Lck antibody or an anti-HA antibody to detect Csk.
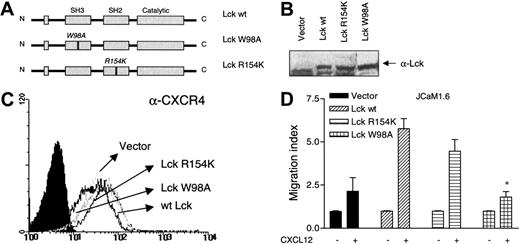
The SH3 domain of Lck is important for CXCL12-induced chemotaxis
Both SH2- and SH3-domain intermolecular and intramolecular interactions are proposed to regulate the kinase activity of the Src-family kinases.32 Therefore, we examined the effect of point mutations in either the SH2 or the SH3 domain of Lck on CXCL12-induced chemotaxis. The SH2 domain was point mutated in a single amino acid in position 154, from arginine to lysine (Arg154Lys), whereas the SH3 domain was point mutated in position 98, from tryptophane to alanine (Trp98Ala; Figure6A). Vectors expressing the 2 Lck mutants, wt Lck, or an empty vector were transiently transfected into the JCaM1.6 cells. The different Lck constructs were expressed at equal levels (Figure 6B). Also, CXCR4 was expressed similarly in all transfectants at levels comparable to untransfected cells (Figure 6C). The ability of Lck-deficient cells transiently transfected with the 2 Lck mutants to migrate toward CXCL12 was assessed in a chemotaxis assay. As shown in Figure 6D, the chemotactic response to CXCL12 was almost completely abrogated in cells expressing the Lck SH3 mutant, as compared with transfectants expressing the Lck SH2 mutant (P < .02) or the Lck wt (P < .004).
Role of the Lck SH3 domain in mediating CXCL12-induced chemotaxis.
The Lck SH3 domain is important for mediating CXCL12-induced chemotaxis. (A) A schematic drawing of Lck demonstrating the 3 different Lck constructs used and the position of the point mutations. (B) An immunoblot with an antibody against Lck, demonstrating successful transfection of Lck in the 3 different transfectants. (C) Analysis of surface expression of CXCR4 in untransfected cells and in the 3 different transfectants. (D) The 3 different constructs or an empty vector were transiently transfected into the JCaM1.6 cell line and subjected to a chemotaxis assay toward CXCL12 (25 ng/mL). Mean ± SD of 3 experiments (18 filters). *P < .004 as compared with Lck wt–transfected cells.
Role of the Lck SH3 domain in mediating CXCL12-induced chemotaxis.
The Lck SH3 domain is important for mediating CXCL12-induced chemotaxis. (A) A schematic drawing of Lck demonstrating the 3 different Lck constructs used and the position of the point mutations. (B) An immunoblot with an antibody against Lck, demonstrating successful transfection of Lck in the 3 different transfectants. (C) Analysis of surface expression of CXCR4 in untransfected cells and in the 3 different transfectants. (D) The 3 different constructs or an empty vector were transiently transfected into the JCaM1.6 cell line and subjected to a chemotaxis assay toward CXCL12 (25 ng/mL). Mean ± SD of 3 experiments (18 filters). *P < .004 as compared with Lck wt–transfected cells.
Discussion
The main role of Lck in T lymphocytes has traditionally been seen as the part it plays in the T-cell receptor complex through its association with CD4.33 A recent report, however, links Lck directly to G protein–coupled receptors, demonstrating that Lck can associate with other types of signaling receptor complexes in T cells.34 There is now accumulating evidence for a role of the different members of the Src-family tyrosine kinases in signaling events mediated through the G protein–coupled chemokine receptors. Stimulation of the neutrophil respiratory burst by chemoattractants is blocked by tyrosine kinase inhibitors, suggesting that one or more tyrosine kinases regulate the activity of critical signaling pathways used by these chemoattractants.35
We report in this paper that HA, a general Src-family inhibitor, and DC, a specific inhibitor of the Lck kinase, greatly reduce the chemotactic response of T cells and NK cells to CXCL12. We excluded the possibility that the observed inhibitory effect of either HA or DC is due to cytotoxic effects of the inhibitors by routinely performing trypan blue tests after inhibitor treatment. The inhibitory effect of the general Src kinase inhibitor and the specific Lck inhibitor is in line with other recent reports describing Src tyrosine kinases as central signaling molecules mediating migration. A study on CCL11 (eotaxin)–induced chemotaxis through the CCR3 receptor demonstrated an inhibitory effect of HA on eosinophil chemotaxis.36 Another study of eosinophils showed that PP1, a selective inhibitor of the Src kinases, abrogated the chemotactic response to the chemoattractant factor leukotriene B4.37 Also, mice deficient in Hck and Fgr demonstrate a reduced neutrophil migration into liver tissues, indicating a role of these kinases in neutrophil migration.23
An increased tyrosine phosphorylation of Lck after 1 minute of CXCL12 treatment of T cells and NK cells was observed. Su and coworkers38 have suggested that Lck is not tyrosine phosphorylated by CXCL12 in human CD4+ T cells. This was after 5 minutes of stimulation with CXCL12, however, and our results suggest that Lck phosphorylation in T cells is low after 5 minutes of CXCL12 stimulation prior to a second phase of tyrosine phosphorylation occurring after 10 minutes. Other groups also observed a biphasic tyrosine phosphorylation of Src kinases. In monocytes, Lyn is tyrosine phosphorylated with an initial peak after 30 seconds to 1 minute and a second peak after 10 to 30 minutes in response to soluble E-selectin.39 Also, the chemokine CCL2 (MCP-1) activates Src with an early peak at 1 to 2 minutes and a second peak after 15 minutes in monocytes.40 In NK cells, however, Lck tyrosine phosphorylation decreases gradually from 1 minute to 10 minutes of stimulation with CXCL12, corresponding to the observed time curve for Lck kinase activity in these cells. The observed biphasic Lck tyrosine phosphorylation in T cells was not observed in NK cells. This discrepancy between Lck phosphorylation in T cells and NK cells may reflect different signaling molecules that are active in these 2 cell types.
CXCL12 induces Lck kinase activity in both T cells and NK cells, reaching a maximum as early as 1 minute after stimulation in both cell types. This corresponds well with the observed increase in Lck tyrosine phosphorylation after 1 minute. A study on T cells has demonstrated that a physical coupling of Lck with CD4 is necessary for IL-16–induced migration. The enzymatic activity of Lck was not found to be necessary, as pretreatment with HA did not block chemotaxis.10 In contrast, the enzymatic activity of Lck seems to be essential for chemotaxis mediated through CXCR4 since CXCL12 induces Lck kinase activity and since CXCL12-induced Lck kinase activity and chemotaxis are inhibited by HA or DC in both T and NK cells. Gu et al34 have demonstrated that Lck increased its kinase activity after 1-minute treatment of S49 mouse lymphoma T cells with isoproterenol toward the β-adrenergic receptor, which is a G protein–coupled receptor, similar to CXCR4. This indicates that an early activation of Lck could be a general mechanism used by G protein–coupled receptors.
Both Jurkat T cells and an Lck-deficient Jurkat mutant, JCaM1.6, express the CXCR4 chemokine receptor at similar levels.12Previous analyses of JCaM1.6 cells have illustrated that the ability of CD4 signaling to enhance β1 integrin functional activity was dependent on Lck.21 Here, we report that JCaM1.6 cells exhibit poor migrational properties in response to the CXCR4 ligand CXCL12, in contrast to the result of others.12 Strikingly, the chemotactic response of JCaM1.6 cells in response to CXCL12 was restored when we reintroduced wt Lck into these cells. We were also able to inhibit the chemotactic response of CXCL12 in wt Lck–restored JCaM1.6 cells with the Lck inhibitor DC, which correlates with the initial results on T and NK cells pretreated with DC. Interestingly, a recent report by Feigelson and coworkers20 demonstrates poor adhesiveness of the integrin VLA-4 in JCaM1.6 cells in response to CXCL12, as compared with the parental Jurkat cell line, indicating that Lck plays a role in the activation of integrins, which is an essential step in chemotaxis.
Csk is the natural inhibitor of the Src kinase family in lymphocytes. By phosphorylating a tyrosine residue in the C-terminal tail of the kinases, it shuts down the enzymatic activity. We found that cotransfection of Csk with wt Lck leads to an inhibition of the CXCL12-induced chemotactic response observed in JCaM1.6 cells transfected with wt Lck only. The ability of Csk to block the chemotactic response clearly illustrates the that Src kinases are required in the initiation of a migrational signal. Furthermore, this suggests that release from Csk-mediated inhibition is a prerequisite for activation of a chemotactic response through chemokine receptors like CXCR4. The negative regulation by Csk of Lck in T-cell migration is thus similar to the role that these 2 signaling molecules play in T-cell receptor signaling. Collectively, these results point to a role for Lck in the signaling pathways leading to chemotaxis.
We demonstrate that a point mutation in an essential amino acid in the SH3 domain, but not the SH2 domain, of Lck abrogates the chemotactic response to CXCL12. Lck associates with and activates a number of downstream-signaling effectors through both its SH3 and its SH2 domains. Thus, the dramatic effect of just the mutated SH3 domain was somewhat surprising. This result could indicate that Lck SH3 domain interactions are critical for performing its task in the signaling transduction events. Actin reorganization and integrin activation are important events that lead to cell motility. Lck has been reported to bind proteins important for actin reorganization, such as c-Cbl, Ras–GTPase-activating protein, protein kinase Cθ, Vav, Itk, and the p85 subunit of PI3 kinase, through its SH3 domain.17,41-44This indicates a role for the Lck SH3 domain in providing the molecular interactions necessary for chemotaxis. Thus, Lck might mediate CXCR4 signaling to these effectors and other downstream effectors necessary for the actin reorganization events. A direct coupling of Lck to CXCR4 is unlikely, as the receptor contains no putative SH2 or SH3 binding domains. Heterotrimeric G proteins could mediate the possible connection between CXCR4 and Lck. In fact, Lck is shown to bind directly to the Gαs, but not the Gαq, subunit of G proteins after stimulation of the β-adrenergic receptor in S49 mouse lymphoma T cells.34 Similarly, c-Src and Hck can bind directly to the Gαs and the Gαisubunits of G proteins and can thereby be activated.45Another possibility is a coupling of Lck to CXCR4 via β-arrestin1, which contains SH3 binding domains.46 In this respect, it has been demonstrated that β-arrestin1 recruits c-Src to the β-adrenergic receptor through c-Src SH3 domain interaction.47
In summary, our study provides new information regarding the signaling pathways initiated by chemokine receptors leading to chemotaxis. Treating T cells or NK cells with the specific Lck inhibitor DC almost completely abrogated the chemotactic response to CXCL12. Also, stimulation with CXCL12 leads to phosphorylation and activation of Lck in both T and NK cells. The Lck-deficient Jurkat T-cell line, JCaM1.6, does not migrate in response to CXCL12, while reintroduction of wt Lck into these cells restores the chemotactic response. Both DC and Csk inhibit the chemotactic response of JCaM1.6 cells reconstituted with wt Lck. Further, we demonstrate that the Lck SH3 domain appears to be essential for chemotaxis. The almost similar results obtained with T cells and NK cells, in regard to the role of Lck in CXCL12-induced chemotaxis, strongly suggest that this could be a general mechanism used by CXCR4 and perhaps other chemokine receptors in lymphocytes. It remains to be seen what sorts of associations take place between this pathway and other intracellular signaling cascades induced by CXCL12 in other cell types.3,15 29
We thank Yenan Bryceson and Erik Dissen for valuable help in creating the Lck constructs and John Torgils Vaage for critically reading the manuscript.
Supported by grants from the Norwegian Research Council, the Norwegian Cancer Society, and Anders Jahre's Foundation; A.A.M. is a senior scientist of the Norwegian Cancer Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marit Inngjerdingen, Department of Anatomy, Institute of Basic Medical Sciences, University of Oslo, PO Box 1105 Blindern, N-0317 Oslo, Norway; e-mail:marit.inngjerdingen@basalmed.uio.no.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal