Recipients of a partially T-cell–depleted (TCD) allogeneic stem cell transplantation (allo-SCT) developing reactivation of Epstein-Barr virus (EBV) with quantified viral DNA levels exceeding 1000 genome equivalents/milliliter (geq/mL) are at high risk for EBV–lymphoproliferative disease (EBV-LPD). We studied whether preemptive therapy with rituximab prevents EBV-LPD, LPD-mortality, and abrogates viral reactivation in high-risk patients. We monitored 49 recipients of a TCD allo-SCT weekly for EBV reactivation by quantitative real-time polymerase chain reaction (PCR). Preemptive therapy by a single infusion of rituximab was given to patients with viral reactivation more than or equal to 1000 geq/mL. Results were compared with an historical control group of patients retrospectively monitored for EBV reactivation at similar intervals. There were 17 prospectively monitored patients who showed EBV reactivation more than or equal to 1000 geq/mL and 15 received preemptive therapy. Median time to preemptive therapy was 113 days (range, 41-202 days) after SCT. There were 14 patients who showed complete response (CR) as characterized by prevention of EBV-LPD and complete clearance of EBV-DNA from plasma, which was achieved after a median number of 8 days (range, 1-46 days). One patient progressed to EBV-LPD despite pre-emptive therapy, but obtained CR after 2 infusions of rituximab and donor lymphocyte infusion. There were 2 patients who had already developed EBV-LPD prior to preemptive rituximab, but obtained CR following 2 rituximab infusions. Comparison of this prospectively followed series to our historical cohort with the same high-risk profile showed a reduction of EBV-LPD incidence (18% ± 9% versus 49% ± 11%, respectively) and a complete abrogation of LPD-mortality (0% versus 26% ± 10%, respectively) (P = .04) at 6 months from EBV-DNA more than or equal to 1000 geq/mL. Frequent quantitative monitoring of EBV reactivation and preemptive therapy by rituximab improves outcome in patients at high risk of EBV-LPD.
Introduction
Herpes viruses including cytomegalovirus (CMV) and Epstein-Barr virus (EBV) continue to affect outcome of allogeneic hematopoietic stem cell transplantation (allo-SCT) and solid organ transplantation. Considerable progress has been made in the last decade in the ability to prevent CMV infection and CMV disease.1 Key elements to that effect are the accurate identification of high-risk patients and the introduction of new effective antiviral agents.2 Currently, a risk-adapted strategy with preemptive or prophylactic ganciclovir in patients with a high-risk profile for CMV disease has become the preferred approach. 3
In contrast to CMV, the precise identification of patients at high risk for EBV–lymphoproliferative disease (EBV-LPD) has been hampered by lack of early and sensitive markers of EBV reactivation, which accurately predict impending EBV-LPD. The use of polymerase chain reaction (PCR)–based assays, however, has enabled the early diagnosis of EBV-LPD and also the monitoring of EBV reactivation.4-10 We recently showed a high incidence of EBV reactivation after allo-SCT.10 However, only recipients of a T-cell–depleted (TCD) allo-SCT appeared to be at risk for EBV-LPD. Furthermore, impending EBV-LPD could quantitatively be predicted by the frequent monitoring of viral load in plasma by quantitative real-time PCR. A viral load of 1000 genome equivalents per milliliter (geq/mL) proved to be a level of EBV reactivation associated with a high predictive value. Clearly, the prevention of EBV-LPD in high-risk patients would be preferable, as outcome of established EBV-LPD is still not optimal.9-14
The recent introduction of rituximab has provided a relatively simple and safe treatment modality,15,16 which has already been applied in LPD-treatment,10,14,17 18 but might be preferred for prevention in a selected group of high-risk patients. We set out to study whether the preemptive use of rituximab in TCD allo-SCT patients with viral reactivation of at least 1000 geq/mL would prevent the development of EBV-LPD. By comparing results with an historical control group of patients with a similar risk profile, it is shown that viral reactivation, progression to EBV-LPD, and mortality from EBV-LPD can effectively be abrogated by preemptive therapy selectively given to patients at high risk of developing EBV-LPD.
Patients, materials, and methods
Prospective cohort
We prospectively monitored 49 consecutively treated patients receiving a partial TCD allo-SCT either from an HLA antigen genotypically matched sibling donor (Sib; n = 35) or a matched unrelated donor (MUD; n = 14) following myeloablation at weekly intervals for EBV-DNA between January 1999 and March 2001 (Table1). There were 21 patients who had standard-risk underlying disease and 28 patients who suffered from poor-risk disease. Standard risk was defined by acute lymphoblastic leukemia (ALL) in first complete remission (CR1), acute myeloid leukemia (AML) in CR1, chronic myeloid leukemia (CML) in first chronic phase (CP1), and untreated (very) severe aplastic anemia (SAA). All other diagnoses were considered poor risk. One donor/recipient pair had negative EBV serology, 4 had discordant EBV serology, and the remaining donor/recipient pairs had positive EBV serology (antiviral capsid antigen IgG) prior to transplantation. Patients experiencing an EBV reactivation more than or equal to 1000 geq/mL were admitted to receive preemptive B-cell immunotherapy by use of a single infusion of rituximab (MabThera; Roche, Basel, Switzerland). Prior to infusion, patients were carefully examined for signs and symptoms of EBV-LPD. Patients with an established diagnosis of EBV-LPD were eligible for a therapeutic protocol including 2 infusions of rituximab followed by donor lymphocyte infusion (DLI) if the viral load had not been reduced to less than 50% by 72 hours after first rituximab infusion as previously described.9 Transplant and rituximab protocols were approved by local institutional review boards and all patients provided informed consent.
Patient characteristics
| Clinical parameter . | Study population (n = 49) . |
|---|---|
| Sex: male/female | 27/22 |
| Age in years: median (range) | 38 (16-56) |
| Diagnosis | |
| AML CR1 | 11 |
| AML > CR1 | 2 |
| ALL CR1 | 4 |
| ALL > CR1 | 2 |
| CML CP1 | 7 |
| CLL | 2 |
| Hodgkin | 1 |
| MDS | 1 |
| Multiple myeloma | 9 |
| NHL | 9 |
| SAA | 1 |
| Risk status: SR/PR | 21/28 |
| Donor type | |
| Sib | 35 |
| MUD | 14 |
| Conditioning regimen | |
| Cy/TBI | 33 |
| Cy/TBI/ATG | 15 |
| Bu/Cy | 1 |
| Graft characteristics | |
| MNC × 108/kg | 0.09 (0.01-0.74) |
| CD34 × 106/kg | 1.68 (0.53-11.1) |
| CD3 × 105/kg | 2.0 (1.0-4.0) |
| CFU-GM × 104/kg | 19.0 (3.0-128.0) |
| Stem cell source | |
| BM | 37 |
| PB | 12 |
| EBV serology (D/R) | |
| D+/R+ | 44 |
| D−/R− | 1 |
| D−/R+ | 1 |
| D+/R− | 3 |
| Clinical parameter . | Study population (n = 49) . |
|---|---|
| Sex: male/female | 27/22 |
| Age in years: median (range) | 38 (16-56) |
| Diagnosis | |
| AML CR1 | 11 |
| AML > CR1 | 2 |
| ALL CR1 | 4 |
| ALL > CR1 | 2 |
| CML CP1 | 7 |
| CLL | 2 |
| Hodgkin | 1 |
| MDS | 1 |
| Multiple myeloma | 9 |
| NHL | 9 |
| SAA | 1 |
| Risk status: SR/PR | 21/28 |
| Donor type | |
| Sib | 35 |
| MUD | 14 |
| Conditioning regimen | |
| Cy/TBI | 33 |
| Cy/TBI/ATG | 15 |
| Bu/Cy | 1 |
| Graft characteristics | |
| MNC × 108/kg | 0.09 (0.01-0.74) |
| CD34 × 106/kg | 1.68 (0.53-11.1) |
| CD3 × 105/kg | 2.0 (1.0-4.0) |
| CFU-GM × 104/kg | 19.0 (3.0-128.0) |
| Stem cell source | |
| BM | 37 |
| PB | 12 |
| EBV serology (D/R) | |
| D+/R+ | 44 |
| D−/R− | 1 |
| D−/R+ | 1 |
| D+/R− | 3 |
AML CR1/> CR1 indicates acute myeloid leukemia first/subsequent complete remission; ALL CR1/> CR1, acute lymphoblastic leukemia first/subsequent CR; CML CP1, chronic myeloid leukemia in first chronic phase; CLL, chronic lymphocytic leukemia; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma; SAA, severe aplastic anemia; SR/PR, standard-risk/poor-risk disease; Sib, genotypically matched sibling donor; MUD, matched unrelated donor; Cy, cyclophosphamide; TBI, total body irradiation; ATG, antithymocyte globulin; Bu, busulphan; MNC, mononuclear cells; CFU-GM, granulocyte macrophage-colony-forming units; BM, bone marrow; PB, peripheral blood; EBV, Epstein-Barr virus; D+/−, donor EBV-seropositive/-negative; R+/−, recipient EBV-seropositive/-negative.
Historical cohort
We included 85 consecutively treated patients receiving a TCD allo-SCT as controls. The retrospective monitoring of EBV load was performed at weekly intervals in these patients; the results were reported recently.10 Patients were treated at Utrecht Medical Center and at Erasmus Medical Center/Daniel den Hoed Cancer Center, Rotterdam, The Netherlands, using the same protocols for transplantation and graft manipulation. Records of these 85 patients were updated for the present study.
EBV reactivation and EBV-LPD
The real-time PCR assay for detection of EBV-DNA in plasma has been described before.8 In short, primers were selected from the EBV-DNA genome encoding the BNRF1-p143 protein and results were related to a reference standard quantified by electron microscopy. The assay accurately detects viral DNA in plasma over a linear span between 50 and 107 geq/mL. Reactivation was defined by a plasma EBV-DNA exceeding 50 geq/mL in donor/recipient pairs with positive EBV serology prior to transplantation. Viral load was prospectively monitored at weekly intervals starting at the time of SCT until day 180 after SCT and beyond day 180 in patients with chronic graft-versus-host disease (GVHD). A diagnosis of EBV-LPD was made on lymph node histology and/or cytology as described recently,9,10 and was classified according to the criteria of Knowles et al.19
Transplantation
Most patients received cyclophosphamide (120 mg/kg) and total body irradiation (TBI) (6 Gy on each of 2 successive days with partial shielding of the lungs for a total lung dose of 2 × 4.5 Gy) as a conditioning regimen (Table 1). Rabbit antithymocyte globulin (ATG) (Imtix Sangstat, Amstelveen, The Netherlands) was added (2 mg/kg from day −7 through day −3) for patients who received an unrelated donor graft for prevention of rejection (Table 1). Hematopoietic stem cells were obtained by bone marrow aspiration under general anesthesia or by peripheral blood stem cell collection. Grafts were partially depleted of T cells using sheep erythrocyte rosetting or CD34 selection (Miltenyi Biotech GmbH, Bergisch-Gladbach, Germany). The T-cell number in the graft was adjusted to a fixed low number of 105CD3+ T cells/kg, if the depletion procedure had resulted in less than 105 CD3+ T cells/kg (recipient body weight).20 Supportive care protocols were as previously described.9 10
Preemptive therapy
Patients with an EBV reactivation more than or equal to 1000 geq/mL within 180 days following allo-SCT were admitted to receive preemptive rituximab. In order to verify for EBV-LPD at initiation of preemptive therapy, physical examination, computerized tomography (CT), bone marrow morphology, and flow cytometry were performed. Rituximab was given as a single infusion (375 mg/m2 dissolved in 0.9% NaCl in a final concentration of 1 mg/mL) and immunosuppressive medication was continued. EBV load was monitored daily during the first 72 hours, then twice weekly until 2 negative test results were obtained and thereafter at each outpatient visit. Absolute B-cell numbers were evaluated prior to and within 1 week and at 1, 3, 6, 9, and 12 months following rituximab infusion. Complete response to preemptive rituximab was defined as clearance of EBV-DNA (< 50 geq/mL) from plasma and absence of signs and symptoms of EBV-LPD.
Endpoints and statistical analysis
Data were analyzed as of May 2001. Endpoints of the study included (1) incidence of viral reactivation more than or equal to 1000 geq/mL, (2) the incidence of EBV-LPD, (3) LPD mortality, (4) EBV load, and (5) B-cell recovery following preemptive rituximab infusion. Patient characteristics were compared with a group of previously described controls10 using the Fisher exact test or Pearson chi-square test, whichever appropriate in case of discrete variables, or the Wilcoxon rank-sum test in case of continuous variables. Time to EBV reactivation more than or equal to 1000 geq/mL was determined from the date of SCT until the date of last plasma sample. Time to EBV-LPD was measured from the date of viral reactivation more than or equal to 1000 geq/mL. Patients without EBV-LPD were censored at the date of death or last follow-up. LPD mortality was calculated from SCT until death due to progressive LPD. Time to viral reactivation, time to EBV-LPD, and LPD mortality were estimated by the Kaplan-Meier method,21 and the Kaplan-Meier curves of the 2 cohorts were compared using the logrank test.22 All reported P values are 2-sided and a significance level of α = .05 was used.
Results
EBV reactivation and preemptive therapy
There were 27 (55%) of 49 prospectively studied patients who showed EBV reactivation and 17 (35%) who progressed to a viral load of more than or equal to 1000 geq/mL. Median time to EBV reactivation more than or equal to 1000 geq/mL was 112 days (range, 39-189 days) after SCT. Median EBV-DNA level measured 2100 geq/mL (range, 500-14 000) prior to admission and a median of 3 days (range, 1-14) elapsed between that day and initiation of preemptive therapy. Of these 17 patients, 2 appeared to present with active EBV-LPD upon examination (see below). The other 15 patients were eligible for preemptive therapy and received rituximab at a median time of 113 days after SCT (range, 41-202 days). Of 15 treated patients, 14 had a complete and sustained response. EBV-DNA in plasma became undetectable after a median of 8 days (range, 1-46 days) (Table2). Recurrent reactivations were not observed in any of these patients with a median follow up of 12 months (range, 1-24 months). One patient (case no. 5) did not respond as was evident by a continuing increase of viral load and progression to EBV-LPD. This patient was treated with a second infusion of rituximab (375 mg/m2) and DLI (1 × 106CD3+ T cells/kg) (Table 2), and had a sustained response without subsequent EBV reactivation.
Preemptive rituximab after T-cell-depleted allogeneic stem cell transplantation for prevention of Epstein-Barr virus-lymphoproliferative disease
| Patient no. . | Donor type . | EBV ≥ 1 000 geq/mL . | Initiation of preemptive rituximab (d*) . | Max EBV-DNA level (geq/mL) . | EBV < 50 (geq/mL, d after start preemptive rituximab) . | EBV-LPD . | |
|---|---|---|---|---|---|---|---|
| Day of onset* . | EBV load . | ||||||
| 1 | Sib | 73 | 2 700 | 84 | 5 800 | 12 | − |
| 2 | Sib | 122 | 2 300 | 129 | 2 300 | 6 | − |
| 3 | Sib | 150 | 1 400 | 157 | 2 800 | 14 | − |
| 4 | Sib | 188 | 2 100 | 202 | 3 800 | 5 | − |
| 5 | MUD | 47 | 3 300 | 50 | 1 100 000 | 46 | + |
| 6 | MUD | 39 | 8 800 | 43 | 675 000 | 45 | − |
| 7 | Sib | 129 | 14 000 | 133 | 85 000 | 6 | − |
| 8 | Sib | 108 | 1 400 | 110 | 1 400 | 7 | − |
| 9 | Sib | 171 | 3 000 | 174 | 110 000 | 26 | − |
| 10 | MUD | 118 | 1 800 | 125 | 1 800 | 27 | − |
| 11 | MUD | 46 | 2 100 | 47 | 2 100 | 1 | − |
| 12 | Sib | 61 | 3 600 | 63 | 3 600 | 4 | − |
| 13 | Sib | 112 | 1 800 | 113 | 15 000 | 8 | − |
| 14 | MUD | 40 | 500 | 41 | 1 350 | 21 | − |
| 15 | Sib | 189 | 1 200 | 192 | 1 150 | 4 | − |
| Patient no. . | Donor type . | EBV ≥ 1 000 geq/mL . | Initiation of preemptive rituximab (d*) . | Max EBV-DNA level (geq/mL) . | EBV < 50 (geq/mL, d after start preemptive rituximab) . | EBV-LPD . | |
|---|---|---|---|---|---|---|---|
| Day of onset* . | EBV load . | ||||||
| 1 | Sib | 73 | 2 700 | 84 | 5 800 | 12 | − |
| 2 | Sib | 122 | 2 300 | 129 | 2 300 | 6 | − |
| 3 | Sib | 150 | 1 400 | 157 | 2 800 | 14 | − |
| 4 | Sib | 188 | 2 100 | 202 | 3 800 | 5 | − |
| 5 | MUD | 47 | 3 300 | 50 | 1 100 000 | 46 | + |
| 6 | MUD | 39 | 8 800 | 43 | 675 000 | 45 | − |
| 7 | Sib | 129 | 14 000 | 133 | 85 000 | 6 | − |
| 8 | Sib | 108 | 1 400 | 110 | 1 400 | 7 | − |
| 9 | Sib | 171 | 3 000 | 174 | 110 000 | 26 | − |
| 10 | MUD | 118 | 1 800 | 125 | 1 800 | 27 | − |
| 11 | MUD | 46 | 2 100 | 47 | 2 100 | 1 | − |
| 12 | Sib | 61 | 3 600 | 63 | 3 600 | 4 | − |
| 13 | Sib | 112 | 1 800 | 113 | 15 000 | 8 | − |
| 14 | MUD | 40 | 500 | 41 | 1 350 | 21 | − |
| 15 | Sib | 189 | 1 200 | 192 | 1 150 | 4 | − |
Days after stem cell transplantation.
Sib indicates HLA genotypically matched sibling donor; MUD, matched unrelated donor; EBV, Epstein-Barr virus; geq/mL, genome equivalents/milliliter; EBV-LPD, EBV-lymphoproliferative disease.
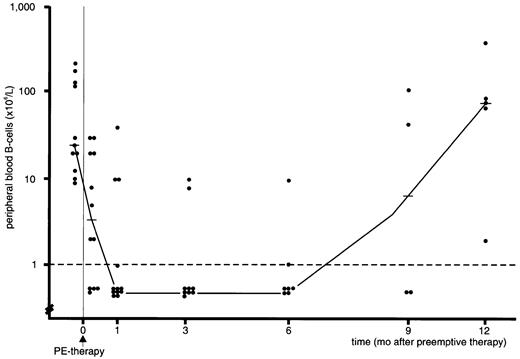
B-cell numbers rapidly declined following rituximab infusion and became undetectable in 12 out of 15 patients. B-cell lymphopenia in these patients persisted for several months (Figure1) and recovery started at approximately 6 months from preemptive therapy. Opportunistic infections (common toxicity criteria [CTC] grade 3 or 4) were observed in all 8 patients having extensive chronic GVHD. In contrast, only 3 out of 7 patients without extensive chronic GVHD experienced CTC grade 3 or 4 infections following rituximab treatment (P = .03). There were 4 B-cell lymphopenic patients who developed pneumonia, including 2 polymicrobial pneumonias (bacterial, 2; fungal, 4; viral, 1); all these 4 patients suffered at the time from extensive chronic GVHD necessitating intensive immunosuppressive therapy. Neutropenia (absolute neutrophil count < 0.5 × 109/L) within 4 weeks of infusion occurred in only 2 of the 15 patients; both had chronic extensive GVHD. The single patient (case no. 5, Table 2), who failed preemptive therapy and was treated using DLI, developed bronchiolitis obliterans organizing pneumonia, complicated by bacterial pneumonia.
B-cell lymphopenia following rituximab treatment.
Median and individual peripheral blood B-cell numbers in recipients of a TCD allo-SCT with EBV-DNA more than or equal to 1000 geq/mL before and after preemptive rituximab (PE-therapy) given at day 0 (dashed line denotes detection limit of assay; horizontal solid lines indicate median value).
B-cell lymphopenia following rituximab treatment.
Median and individual peripheral blood B-cell numbers in recipients of a TCD allo-SCT with EBV-DNA more than or equal to 1000 geq/mL before and after preemptive rituximab (PE-therapy) given at day 0 (dashed line denotes detection limit of assay; horizontal solid lines indicate median value).
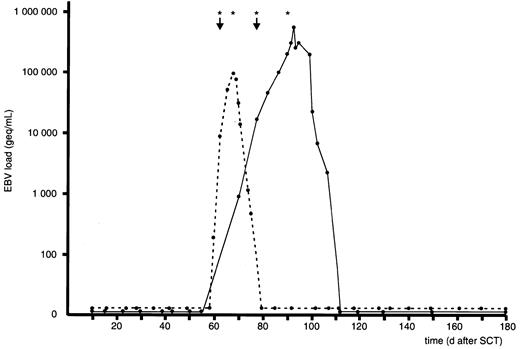
There were 2 patients who presented with lymphadenopathy at the time of admission and who were not eligible for preemptive therapy, because pathologic and immunologic examinations of lymph node biopsies were consistent with a diagnosis of EBV-LPD. Both patients had received a TCD unrelated donor graft and had also received ATG as part of the conditioning regimen. They showed rapid progression of viral reactivation with 2 and 7 days, respectively, between the first signs of reactivation and the onset of lymphadenopathy (Figure2). These 2 patients were enrolled in a therapeutical protocol, including 2 infusions of rituximab guided by viral load. Both patients obtained a complete response and they are alive at the date of last follow-up, day +338 and day +415, respectively. EBV-DNA levels were 8750 geq/mL and 17 500 geq/mL at the time of clinical admission and complete and persistent clearance of EBV-DNA was achieved after 16 days and 34 days, respectively (Figure2).
Viral load following therapy for established EBV-LPD.
EBV load in 2 recipients of a TCD allo-SCT with established EBV-LPD prior to planned preemptive therapy. EBV-LPD was diagnosed on lymph node biopsies in both patients at the day of first rituximab infusion. Both patients developed a sustained complete response after 2 successive infusions of rituximab combined with dose reduction of cyclosporin (↓ denotes diagnosis of EBV-LPD; asterisk indicates single infusion of 375 mg/m2 rituximab).
Viral load following therapy for established EBV-LPD.
EBV load in 2 recipients of a TCD allo-SCT with established EBV-LPD prior to planned preemptive therapy. EBV-LPD was diagnosed on lymph node biopsies in both patients at the day of first rituximab infusion. Both patients developed a sustained complete response after 2 successive infusions of rituximab combined with dose reduction of cyclosporin (↓ denotes diagnosis of EBV-LPD; asterisk indicates single infusion of 375 mg/m2 rituximab).
Comparison with historical cohort
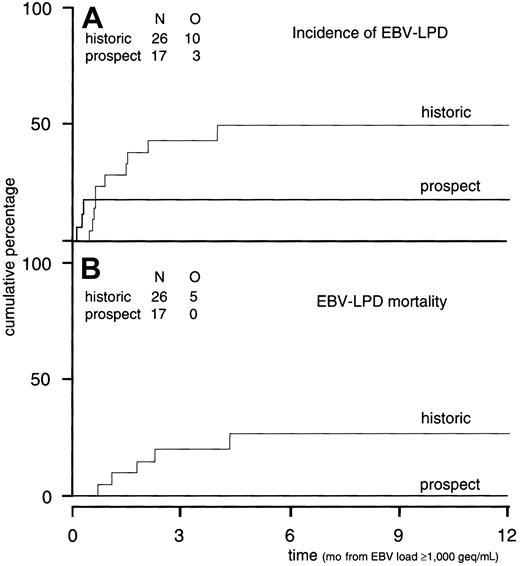
The positive and negative predictive values of a viral load of 1000 geq/mL have been established in a group of 85 recipients of a TCD allo-SCT.10 Plasma samples were retrospectively examined for EBV reactivation at weekly intervals in these 85 patients. Considering a threshold level of 1000 geq/mL, the negative predictive value was 100%. The cumulative probability of developing EBV-LPD was 38% ± 11% at 2 months and 49% ± 11% at 4 months from the date of EBV-DNA more than or equal to 1000 geq/mL (Figure3A). Results of the current prospective study were compared with those historical controls with respect to EBV reactivation, incidence of EBV-LPD, and LPD mortality. Patient characteristics of patients at high risk of progression to EBV-LPD, as defined by reactivation of more than or equal to 1000 geq/mL after a TCD allo-SCT, did not differ between prospectively followed patients (n = 17) and controls (n = 26, Table3). In both cohorts, the majority of patients suffered from poor-risk underlying disease. In the historical cohort, 42% of patients had received ATG and an unrelated donor graft, as compared with 41% in the prospective cohort. In addition, graft characteristics were similar (Table 3). GVHD prophylaxis was similar in both cohorts and consisted of TCD and cyclosporin A when a sibling donor was used; ATG was added in case of a MUD SCT. Cumulative incidences (CI) of acute and chronic GVHD did not differ between both cohorts. The CI of acute GVHD grade II to IV was 51% at 100 days post-SCT versus 54% at 100 days for prospectively followed patients and historical controls, respectively. The CI for chronic GVHD (limited and extensive) was 37% for prospectively followed patients versus 36% in the historical cohort.
Incidence of EBV-LPD and EBV-LPD mortality as compared to historical controls.
(A) Incidence of EBV-LPD. Cumulative incidence of EBV-LPD in historical control patients (n = 26) with EBV-DNA more than or equal to 1000 geq/mL versus the incidence of EBV-LPD in the prospectively followed group after EBV-DNA more than or equal to 1000 geq/mL (n = 17) (P = .13). (B) EBV-LPD mortality. Cumulative incidence of EBV-LPD mortality in historical control patients (n = 26) with EBV-DNA more than or equal to 1000 geq/mL versus the incidence of EBV-LPD mortality in patients (n = 17) prospectively studied (P = .04). N indicates numbers of patients studied; O, observations (endpoints) done in the study group.
Incidence of EBV-LPD and EBV-LPD mortality as compared to historical controls.
(A) Incidence of EBV-LPD. Cumulative incidence of EBV-LPD in historical control patients (n = 26) with EBV-DNA more than or equal to 1000 geq/mL versus the incidence of EBV-LPD in the prospectively followed group after EBV-DNA more than or equal to 1000 geq/mL (n = 17) (P = .13). (B) EBV-LPD mortality. Cumulative incidence of EBV-LPD mortality in historical control patients (n = 26) with EBV-DNA more than or equal to 1000 geq/mL versus the incidence of EBV-LPD mortality in patients (n = 17) prospectively studied (P = .04). N indicates numbers of patients studied; O, observations (endpoints) done in the study group.
Characteristics of high-risk patients
| Parameter . | Historical cohort (n = 26) . | Prospective study (n = 17) . |
|---|---|---|
| Age | 40 (18-55) | 40 (19-51) |
| Underlying disease | ||
| Risk status SR/PR | 2/24 | 4/13 |
| Donor type | ||
| Sib | 15 | 10 |
| MUD | 11 | 7 |
| ATG | 11 | 7 |
| Stem cell source | ||
| Bone marrow | 24 | 14 |
| Peripheral blood | 2 | 3 |
| Graft characteristics | ||
| CD3 (105/kg) | 2.0 (1.0-6.8) | 2.0 (1.0-4.0) |
| CD34 (106/kg) | 1.6 (0.26-4.57) | 1.7 (0.53-8.10) |
| Parameter . | Historical cohort (n = 26) . | Prospective study (n = 17) . |
|---|---|---|
| Age | 40 (18-55) | 40 (19-51) |
| Underlying disease | ||
| Risk status SR/PR | 2/24 | 4/13 |
| Donor type | ||
| Sib | 15 | 10 |
| MUD | 11 | 7 |
| ATG | 11 | 7 |
| Stem cell source | ||
| Bone marrow | 24 | 14 |
| Peripheral blood | 2 | 3 |
| Graft characteristics | ||
| CD3 (105/kg) | 2.0 (1.0-6.8) | 2.0 (1.0-4.0) |
| CD34 (106/kg) | 1.6 (0.26-4.57) | 1.7 (0.53-8.10) |
High-risk, as defined by (1) allogeneic stem cell transplantation by partial T-cell depletion, and (2) Epstein-Barr virus load ≥ 1000 geq/mL.
SR/PR indicates standard-risk/poor-risk disease; Sib, HLA-genotypically matched sibling donor; MUD, matched unrelated donor; ATG, antithymocyte globulin.
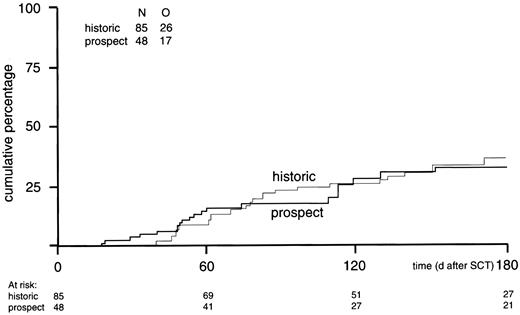
The probabilities of viral reactivation more than 50 geq/mL and more than or equal to 1000 geq/mL were similar in both cohorts. Probabilities of EBV reactivation (≥ 1000 geq/mL) at 4 months from SCT were 26% ± 5% versus 28% ± 7% for historical controls and prospectively monitored patients, respectively (Figure4).
Cumulative incidence of EBV reactivation more than or equal to 1000 geq/mL.
Cumulative incidence of EBV reactivation more than or equal to 1000 geq/mL in prospectively studied EBV-seropositive donor/recipient pairs (n = 48) versus the incidence in the historical control group (n = 85) (P = .86).
Cumulative incidence of EBV reactivation more than or equal to 1000 geq/mL.
Cumulative incidence of EBV reactivation more than or equal to 1000 geq/mL in prospectively studied EBV-seropositive donor/recipient pairs (n = 48) versus the incidence in the historical control group (n = 85) (P = .86).
Among the historical group, 10 of 26 patients developed EBV-LPD, of whom 5 died due to progressive EBV-LPD despite the therapeutic use of rituximab and DLI, and 3 other patients died due to extensive chronic GVHD secondary to DLI. Among the prospectively monitored patients, 1 of 15 patients treated preemptively developed EBV-LPD and 2 other patients presented with EBV-LPD before initiation of preemptive therapy (Figure 3A, P = .13). None of these 17 patients died from progressive EBV-LPD (Figure 3B, P = .04). Viral reactivation was abrogated in all of these 17 patients without any recurrences.
Discussion
The present study shows that preemptive rituximab selectively administered to high-risk patients abrogates EBV reactivation and reduces the incidence of EBV-LPD. Furthermore, mortality due to EBV-LPD no longer contributed to treatment-related mortality in these prospectively monitored patients with EBV reactivation after TCD allo-SCT.
Outcome of clinically established EBV-LPD is still not optimal, although new promising treatment modalities have been introduced, such as anti-CD20 immunotherapy12,14,17,18 and adoptive T-cell immunotherapy.13,23-25 Failure of treatment may be due to rapidly progressive EBV-LPD,9-11,14 development of resistance,26 viral immune evasion,27 and loss of CD20 antigen expression.28 Studies focusing on the therapeutic value of rituximab have shown a mortality of 17% to 25% due to progressive LPD.14,17 In addition, GVHD following donor lymphocyte infusion may also adversely affect outcome.9,10,13,23 Therefore, effective preventive approaches may be preferred to reduce mortality associated with EBV-LPD. Such approaches, however, should specifically be developed for high-risk patients, because EBV-LPD is a rare complication after allo-SCT and unnecessary treatment of patients with a low probability should be avoided. Retrospectively, we established a viral reactivation of 1000 geq/mL as a threshold value with high positive and negative predictive values in recipients of a TCD allo-SCT.10 Using that threshold value, we were now able to selectively administer preemptive therapy to 15 out of 49 patients. EBV-LPD was effectively prevented by rituximab in those recipients. Only 1 out of 15 patients receiving preemptive therapy progressed to EBV-LPD, but the patient was rescued by a second infusion of rituximab and DLI as well. This patient had received ATG prior to TCD allo-SCT, which may explain the more aggressive evolution of viral reactivation toward EBV-LPD. Retrospectively, ATG was strongly associated with a higher incidence of EBV reactivation and an earlier and more rapid evolution of reactivation and a higher incidence of EBV-LPD.10,11 29There were 2 other patients who had also received ATG and who showed early viral reactivation, followed by rapid progression to EBV-LPD before preemptive therapy could be instituted. However, both patients developed a sustained complete response after a second infusion of rituximab given at a relatively early time point in the course of their disease, and EBV-LPD mortality was effectively prevented. Although these patients escaped the preemptive approach, the frequent monitoring allowed an early diagnosis and thereby an early therapeutic intervention.
Peripheral B-cell numbers rapidly declined following a single infusion of rituximab and became undetectable in 12 patients. In addition, B-cell lymphopenia persisted for several months (Figure 1). Only a few relatively mild infections were observed in B-cell lymphopenic patients without chronic GVHD, which may be explained by unaffected plasma cell counts and thereby unaffected immunoglobulin production.15In contrast, patients with B-cell lymphopenia and chronic extensive GVHD appeared to be at higher risk for opportunistic pneumonias, which may reflect the immunodeficiency associated with chronic GVHD rather than with B-cell lymphopenia as such. So far, patients treated with more intensive rituximab immunotherapy in other studies have not shown an increased risk of opportunistic infections despite effective and prolonged B-cell lymphopenia.15 16
The development of EBV-LPD is the result of uncontrolled B-cell proliferation due to failure of immunologic control. Therefore, other investigators have focused on a preemptive approach of improving EBV-specific immunity in patients at high risk for EBV-LPD and reported on preemptive infusion of EBV-specific cytotoxic T cells (CTLs) in patients with elevated EBV-DNA levels.24,25 Effective prevention of EBV-LPD was strongly suggested by a decrease of viral DNA levels and a low incidence of EBV-LPD in the patient populations studied. The approach is, although attractive, hampered by the rather elaborate procedures needed to prepare EBV-specific CTLs. These and other studies have focused on detection of cellular EBV-DNA levels to identify patients at highest risk of EBV-LPD.4-7,24,25 We have used a quantitative PCR of EBV-DNA in plasma, which appeared to accurately predict impending EBV-LPD as well as response to therapy in recipients of a TCD allo-SCT.8-10 Especially in recipients of stem cell transplants, quantification of viral load in plasma may be advantageous, as the technique is relatively fast and simple and patients with lymphopenia may have insufficient cell numbers for reliable quantification of peripheral blood mononuclear cell viral load. Furthermore, response to therapy of EBV-LPD may be followed more accurately by plasma PCR, as suggested by our findings and those of Van Esser et al9 and Yang et al.30 Our assay may monitor lytic EBV infection and/or release of viral DNA from latently infected B cells. If viral DNA is mainly derived from lytic replication, our results may suggest that active lytic infection contributes to the development of EBV-LPD, which would be in line with a number of previous studies showing that active lytic infection participates in the development of EBV-LPD.31-35 The latter studies have raised the question whether the prophylactic or preemptive administration of antiviral agents such as aciclovir or ganciclovir would prevent EBV-LPD following transplantation of allogeneic stem cells or allogeneic solid organs. However, preventive approaches with these antiviral drugs have generally been disappointing (reviewed by Davis31), which may be explained by their inability to inhibit proliferating B cells, once these have acquired an autonomous growth pattern.
In conclusion, we developed a risk-adapted strategy to abrogate viral reactivation and prevent EBV-LPD. It is shown that a single infusion of rituximab is effective as preemptive therapy for EBV-LPD and prevention of EBV-LPD mortality in a selected group of allo-SCT recipients at high-risk for EBV-LPD. Considering the possible rapid and aggressive evolution of viral reactivation toward EBV-LPD in recipients having received ATG, the frequent monitoring of reactivation and early institution of preemptive therapy should be advocated in recipients treated with ATG.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
J. J. Cornelissen, Department of Hematology, Erasmus MC/Daniel den Hoed Cancer Center, Groene Hilledijk 301, 3075 EA Rotterdam, The Netherlands; e-mail: cornelissen@hemh.azr.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal