We report here the identification and characterization of a new platelet alloantigen, Duva+, implicated in a case of neonatal thrombocytopenia. Immunochemical studies demonstrated that the epitope was localized on glycoprotein (GP) IIIa. Sequencing of the exons 2 to 15 of GP IIIa gene polymerase chain reaction products from both parents revealed a single base substitution 517C>T (complementary DNA) present in a heterozygous state in DNA from the father leading to amino acid substitution Thr140Ile (ACC>ATC) within the Arg-Gly-Asp binding domain of GP IIIa. Flow cytometry and immunoprecipitation studies of IIb-C517 or T517 IIIa transfected Cos cells allowed us to demonstrate this mutation was responsible for expression of the Duva+epitope. By polymerase chain reaction–single-strand conformational-polymorphism analysis, the mutated allele could not be detected in a population of 100 healthy unrelated donors, indicating a low frequency of occurrence. The Thr140/Ile dimorphism, localized 3 amino acids upstream from the Arg143 involved in the expression of HPA-4a, did not interfere with the binding of an anti–HPA-4a antibody in flow cytometry. Results of functional analysis of wild-type or mutated transfected CHO cells—(1) aggregation in the presence of Ca++ and soluble fibrinogen after complex activation by dithiothreitol, (2) adhesion on coated fibrinogen, (3) binding of monoclonal antibody PAC-1 or LIBS antibody D3, and (4) outside-in signaling—all suggest that the Thr140Ile polymorphism localized in the Arg-Gly-Asp binding domain of GP IIIa does not affect significantly, if at all, the integrin function. We have shown that the anti-Duva+ antibody may inhibit platelet GP IIb-IIIa function.
Introduction
The platelet membrane glycoprotein (GP) IIb-IIIa complex or αIIbβ3 belongs to the integrin family of heterodimeric adhesion receptors involved in cell-matrix and cell-cell interactions.1 This complex can bind ligands containing the Arg-Gly-Asp (RGD) sequence, such as fibrinogen, von Willebrand factor, vitronectin, and fibronectin. It plays a major role in platelet aggregation by interacting with the C-terminal part of the γ chain of fibrinogen.2,3 The genes coding for GP IIb and GP IIIa are highly polymorphic. Most of the human platelet alloantigen (HPA) systems described are carried by the GP IIb-IIIa complex, and most of them are localized on the GP IIIa subunit: HPA-1, -4, -6, -7, -8, -10, -11, and one unclassified private antigen Oea.4-11 Only 2 of the platelet antigen systems, HPA-3 and -9, reside on GP IIb,12,13 and the genetic origin of the Vaa antigen remains to be determined.14 These antigenic systems result from a single base-pair substitution with the exception of Oea, which depends on an in-frame deletion of 3 bases.11 Platelet alloantigen systems are involved in neonatal alloimmune thrombocytopenia (NAIT), posttransfusion purpura, and refractoriness to platelet transfusions. NAIT is characterized by the destruction of fetal or newborn platelets by a maternal antibody elicited by the maternofetal platelet antigen incompatibility. During the course of the severe thrombocytopenia in the fetus or the newborn, there is a risk of intracerebral hemorrhage leading to death or neurologic impairment. The incidence of NAIT has been shown to be 1 per 800 to 1000 live births.15 Most NAIT in Caucasians involves HPA-116 or HPA-517 (GP Ia polymorphism18) maternofetal incompatibilities.
We describe here a case of severe NAIT due to a new alloantigen Duva+ localized on GP IIIa. The genetic determination revealed a single base-pair substitution, C>T, at the GP IIIa complementary DNA (cDNA) position 517. This leads to a threonine/isoleucine dimorphism at position 140 of the mature GP IIIa. The Duva+ form of the GP IIb-IIIa complex expressed in Cos cells shows this polymorphism is responsible for the alloimmunization and the consequent NAIT. The Duva+ polymorphism that was localized in the RGD binding site of GP IIIa could affect the αIIbβ3 function. Because no individuals homozygous for Duva+ were available, stable cell lines expressing the Duva+ form of GP IIb-IIIa were produced to study a possible effect of the Duva+ substitution on integrin function.
Materials and methods
Case report
We report a 30-year-old white woman (Duv), para 4, gravida 4, who gave birth to a full-term male child with a severe thrombocytopenia at birth (platelets 17 × 109/L) associated with minor bruises and petechiae. The child was otherwise healthy (Apgar score 9 of 10). He was treated with a total of 1.6 g/kg intravenous immunoglobulin G (IgG) over 24 hours. On day 3, the platelet count had increased to 139 × 109/L, and the outcome was favorable. Neonatal thrombocytopenia was not recorded in any of the siblings.
Blood samples
Blood samples from the mother, the father, the infant, and healthy adult human volunteers were collected by venipuncture using 5 mM ethylenediaminetetraacetic acid as an anticoagulant. Samples were not available from the siblings. Platelets were separated by differential centrifugation and stored until use at 4°C in isotonic solution containing 0.1% sodium azide.
Platelet serology
Characterization of the maternal serum alloantibody and platelet phenotyping were performed with the monoclonal antibody–specific immobilization of platelet antigen assay (MAIPA).19 The following monoclonal antibodies (Moabs) were used: Gi9 to GP Ia and B1G6 to the β2-microglobulin (Immunotech, Marseille, France), Pl1-64 and Pl2-46 to GP IIb-IIIa (from the authors' laboratory), and GR-P to GP Ib-IX (from Dr Garrido, Granada, Spain). A serum containing an anti–HPA-1a alloantibody from a case of NAIT was used as a positive control for the GP IIb-IIIa complex.
Isolation of genomic DNA
Genomic DNA was isolated from peripheral blood according to published procedures.20
Genotyping
Western blot analysis
The Western blot procedure was used as previously described.24
Sequencing and SSCP analysis
Site-directed mutagenesis
The mutation Duv was introduced into wild-type GP IIIa cDNA cloned in the eukaryotic expression vector by using the GeneEditor in vitro mutagenesis system (Promega, Lyon, France) according to the manufacturer's instructions. We used the mutagenic oligonucleotide 5′-GCTTTCGCATCTGGATGGCCAGCTTGGTAC-3′, where the underlined “A” replaced a “G” in the wild-type sequence.26The presence of the mutation was confirmed by sequencing the region of interest.
Cell culture/transfection
Cos-7 cells were cultivated in Iscoves Dulbecco modified Eagle medium supplemented with 10% fetal calf serum and 1% antimycotic/antibiotic reagent (Life Technologies, Cergy-Pontoise, France) in a 5% CO2 saturating-humidity atmosphere. Petri dishes (10 cm) were seeded with 1.5 × 106 cells and incubated for 12 hours. Cells rinsed with phosphate-buffered saline (PBS) were transfected by incubation at 37°C for 5 hours in 3 mL Opti-MEM containing 5 μg of the pcDNA3 plasmid containing IIb cDNA and 5 μg of the pcDNA3 plasmid containing IIIa cDNA (from Dr P. J. Newman, The Blood Research Institute, The Blood Center of Southeastern Wisconsin, Milwaukee, WI) and 40 μL Lipofectamine (Life Technologies). The incubation was continued overnight after adding 15 mL complete medium with 20% fetal calf serum. The medium was replaced by 15 mL medium supplemented with 10% fetal calf serum and the culture incubated for an additional 48 hours before transfected cells were harvested by using trypsin–ethylenediaminetetraacetic acid (Life Technologies). Cells were washed 3 times, resuspended in PBS (108/mL), and kept on ice until used.
For stable expression, CHO cells were transfected with 2 μg of each plasmid and 20 μL Lipofectamine according to the procedure described above. Forty-eight hours after transfection the cells were subjected to drug selection in media containing 800 μg/mL G 418 (Genecitin, Life Technologies) for 10 days. Resistant cells expressing the GP IIb-IIIa complex were isolated by using the anticomplex-specific Moab AP-2 (GTI, Brookfield, WI) and the Dynabeads M-450 GAM IgG system (Dynal, Bromborough, United Kingdom) according to the manufacturer's instructions. After cloning, stable cell lines were selected by flow cytometric analysis on their ability to bind the anti-GP IIb-IIIa Moab AP-2.
Flow cytometry
Surface expression and fibrinogen binding function of the transfected cells were analyzed by flow cytometry. For each analysis, 5 × 105 cells were incubated in 100 μL PBS containing 4 mg/mL bovine serum albumin (BSA) and 30 μL human sera or 1 μg/mL purified Moabs SZ 22 to GP IIb, SZ 21 to GP IIIa (Immunotech), D3 to a ligand-induced binding site (LIBS) GP IIIa epitope (from Dr L. Jennings, Memphis, TN), or the 1:1000 diluted AP-3 (anti-GP IIIa) and AP-2 ascites (GTI). Cells were incubated for 30 minutes at room temperature before being washed twice in 3 mL PBS-BSA. They were resuspended in 100 μL PBS-BSA containing a 1:50 dilution of R-phycoerythrin–conjugated goat antihuman or antimouse IgGs (Fc-specific) (Immunotech). Cells were incubated for 30 minutes, washed twice, and analyzed on a FACSCalibur flow cytometer (Becton Dickinson, Le Pont de Claix, France).
For binding analysis of the fibrinogen and the ligand mimetic Moab PAC-1 (Becton Dickinson), cells were treated by dithiothreitol (DTT) in Tyrode buffer as described in Lyman et al27 to “activate” the GP IIb-IIIa complex receptor function. A total of 106 treated or untreated cells were incubated in 100 μL Tyrode solution containing 200 μg/mL fibrinogen conjugated to the fluorochrome A-488 (Molecular Probes, Eugene, OR), 2 mM CaCl2, and 4 mg/mL BSA for 20 minutes at room temperature. Cells were washed once and resuspended in 500 μL Tyrode solution containing BSA and calcium prior to being analyzed by flow cytometry. PAC-1 binding was analyzed as previously described by using a R-phycoerythrin–conjugated polyclonal goat antimouse IgM (Immunotech). In some experiments the complex was “activated” by incubating transfected cells with 200 μg/mL of the anti-LIBS Moab D3.28
Inhibition of the anti-Duva+ alloantibody binding to transfected cells by the Arg-Gly-Asp-Ser (RGDS) or the Arg-Gly-Glu-Ser (RGES) peptides was studied by flow cytometry. For each analysis, 2.5 × 105 cells were incubated for 30 minutes at 4°C in 100 μL PBS containing 2% BSA, 100 mM MgCl2, 100 mM CaCl2, and 25 mM RGDS or RGES peptides prior the addition of 10 μL of the maternal or a control serum. Cells were incubated for 90 minutes at 4°C before being washed and analyzed as described above.
Cell aggregation and adhesion
Cell aggregation and adhesion experiments were performed as described by Lyman et al27 with a single modification for adhesion tests: 96-well culture plates were coated with 50 μL PBS containing different concentrations of fibrinogen or BSA or 20 μg/mL AP-2.
In inhibition experiments, prior to performing adhesion tests, cells were incubated for 30 minutes at room temperature with either 20 μg/mL of the anti-GP IIb-IIIa Moab AP-2 or a nonrelevant polyclonal mouse IgG, or with 5 mM of the RGDS or the RGES peptides. In these experiments, microtiter plates were coated by 20 μg/mL fibrinogen.
To assess the effect of the anti-Duva+ antibody on cell adhesion, cells were incubated with 30 μL human sera for 90 minutes at 4°C prior to plating onto wells coated with 250 ng fibrinogen or BSA. Human sera used in these experiments were incubated for 1 hour at room temperature with CHO cells expressing wild-type GP IIb-IIIa to minimize nonspecific binding in subsequent adhesion assays.
Tyrosine phosphorylation of the focal adhesion kinase
Tyrosine phosphorylation and focal adhesion kinase (pp125FAK) immunoprecipitation assays were performed according to Schaffner-Reckinger et al.29Western blotting was performed as previously described24except that saturation and Moab incubation steps were performed in buffer II (10 mM Tris-HCl pH 7.5, 100 mM NaCl, 0.1% Tween 20) containing 1% BSA. Washing steps were performed in buffer II alone. The pp125FAK and phosphotyrosine were detected, respectively, by using a Moab to the pp125FAK and the Moab PY-20 (Transduction Laboratory, Lexington, KY) diluted 1:1000. Blots probed with the Moab PY-20 were stripped for 30 minutes at 50°C in 62.5 mM Tris-HCL (pH 6.7), 2% sodium dodecyl sulfate (SDS), and 100 mM β-mercaptoethanol before being probed with the Moab directed against the pp125FAK. Blot densitometric analysis was performed to compare the phoshorylation percentage of the different transfected cell types with regard to their adherent or nonadherent status. For each cell type, the phosphorylation percentage is expressed as the difference between ratios obtained for adherent (A) and nonadherent (S) cells as follows:
Results
Serologic studies
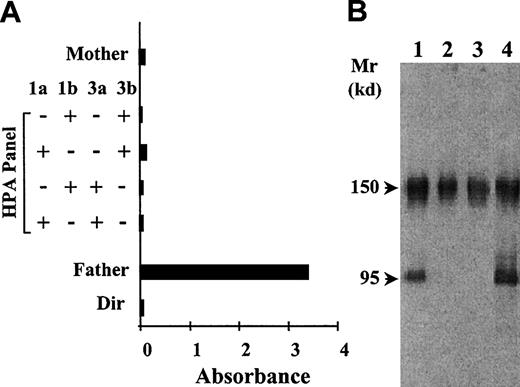
The maternal serum was tested against the paternal platelets and a panel by the MAIPA assay. The maternal serum gave a selective and strong positive reaction with the father's platelets in MAIPA using the Moab Pl1-64 directed against the GP IIb-IIIa complex (Figure1A). Results were negative with Moabs against other membrane GPs (Ia-IIa, Ib-IX, or HLA). No positive reaction was observed with the panel regardless of the Moab used in the reaction and the platelet alloantigenic status despite incompatibility in the HPA-3 system between the mother and the father or the child (Table 1). No autoantibodies were identified that reacted with the father's or mother's platelets. Moreover, genotypes of both parents did not show any incompatibility in the HPA-4 and the HPA-6w to -11w alloantigenic systems (excluding HPA-8w, which was not tested) (Table 1). These results were in favor of a new platelet alloantigen localized on the GP IIb-IIIa complex.
Identification and characterization of the anti-Duva+ antibody by the techniques of MAIPA and Western blot.
(A) The reactivity of the serum Duv was tested in MAIPA with the mother's platelets, 4 panel platelets, and the father's platelets by using the Moab Pl1-64 to capture the GP IIb-IIIa complex. Paternal autoantibodies were searched for by direct incubation of the platelets with the Moabs (Dir). (B) Immunoblot of SDS-soluble platelet proteins subjected to SDS–polyacrylamide gel electrophoresis under nonreduced conditions and transferred to nitrocellulose membrane. The maternal serum Duv was tested with father's platelets (lane 1), mother's platelets (lane 2), and a control donor (lane 3). An anti–HPA-1a serum (anti–GP IIIa) was used as positive control (lane 4). Human IgGs were revealed by using an antihuman IgG conjugated to the peroxidase associated to the chemiluminescence detection technique. The maternal serum Duv bound specifically to a protein migrating in the position of GP IIIa of the father's platelets.
Identification and characterization of the anti-Duva+ antibody by the techniques of MAIPA and Western blot.
(A) The reactivity of the serum Duv was tested in MAIPA with the mother's platelets, 4 panel platelets, and the father's platelets by using the Moab Pl1-64 to capture the GP IIb-IIIa complex. Paternal autoantibodies were searched for by direct incubation of the platelets with the Moabs (Dir). (B) Immunoblot of SDS-soluble platelet proteins subjected to SDS–polyacrylamide gel electrophoresis under nonreduced conditions and transferred to nitrocellulose membrane. The maternal serum Duv was tested with father's platelets (lane 1), mother's platelets (lane 2), and a control donor (lane 3). An anti–HPA-1a serum (anti–GP IIIa) was used as positive control (lane 4). Human IgGs were revealed by using an antihuman IgG conjugated to the peroxidase associated to the chemiluminescence detection technique. The maternal serum Duv bound specifically to a protein migrating in the position of GP IIIa of the father's platelets.
Western blotting experiments were performed to identify the subunit that carried the Duva+ epitope. Maternal antibody reacted specifically with a protein migrating under nonreduced conditions in the position of GP IIIa (95 kd) of the father's platelets (Figure 1B, lane 1) identified by the reactivity of a well-characterized anti–HPA-1a antibody (Figure 1B, lane 4). The band at 150 kd detected in all lysates corresponds to platelet-associated IgG. These results were in favor of the localization of the putative antigen on GP IIIa. Disruption of disulfide bonds by DTT treatment of the lysates abolished the maternal antibody binding to GP IIIa. When paternal platelets were treated with α-chymotrypsin, the epitope carried on GP IIIa was no longer recognized by the maternal antibody (data not shown).
Genetic analysis
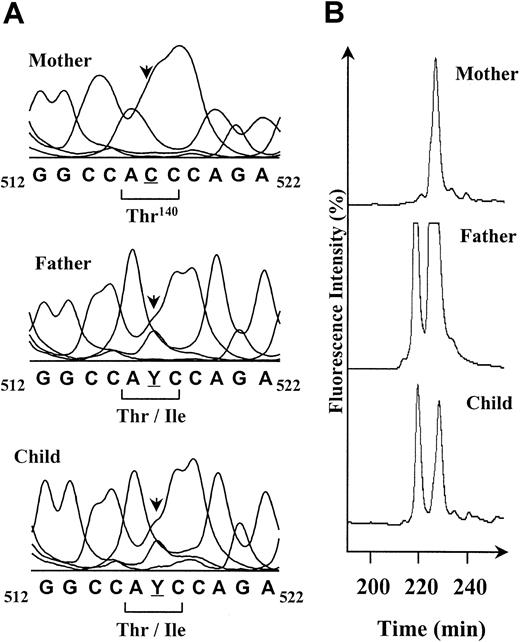
To identify the mutation responsible for the expression of this new antigen, genomic DNA from the father, the mother, and the child was amplified for all coding sequences of the GP IIIa gene. Each PCR product was directly sequenced by using an automatic DNA-analyzer system (AlF-Express, Amersham Pharmacia Biotech, Saclay, France). A single nucleotide change corresponding to a C>T mutation in a heterozygous state was identified in the exon 4 of the DNA from the father and his child (Figure2A). This C>T mutation localized at the position 517 of the cDNA leads to the Thr140Ile substitution in the peptide sequence of the mature GP IIIa. This mutation was confirmed by SSCP analysis showing an alteration of the migration profile of exon 4–PCR products from the father and child compared with the mother's (Figure 2B). The 3 profiles showed the presence of a common peak at 230 minutes that corresponds to the C allele, whereas an additional peak on the father and child profiles at 220 minutes revealed the C>T change in the nucleotide sequence. The presence of 2 peaks confirms the heterozygous state for the father and his child. This mutation was not identified in DNA from a population of 100 unrelated healthy donors using the SSCP technique (not shown).
Direct sequence analysis and SSCP profiles of amplified exons 4 of GP IIIa DNAs from the mother Duv, the father, and their child.
(A) The genomic DNA sequences corresponding to the region 512 to 522 of the GP IIIa cDNA are shown. The mother is homozygous for the C517 (underlined), and the father and the child are C/T heterozygous (Y). The C>T base change results in a Thr (ACC) for an Ile (ATC) substitution at the amino acid 140 of the mature GP IIIa. The polymorphic position is indicated by an arrow. (B) The interesting part of the SSCP profiles of the exon 4–PCR products obtained for the reverse DNA single strand at 20°C is shown. The peak detected at 230 minutes in the mother's profile corresponds to the C517 allele of the GP IIIa. The peak at 220 minutes obtained for the father and the child is specific for the T517 form. SSCP confirms the father and the child are heterozygous for the C>T mutation.
Direct sequence analysis and SSCP profiles of amplified exons 4 of GP IIIa DNAs from the mother Duv, the father, and their child.
(A) The genomic DNA sequences corresponding to the region 512 to 522 of the GP IIIa cDNA are shown. The mother is homozygous for the C517 (underlined), and the father and the child are C/T heterozygous (Y). The C>T base change results in a Thr (ACC) for an Ile (ATC) substitution at the amino acid 140 of the mature GP IIIa. The polymorphic position is indicated by an arrow. (B) The interesting part of the SSCP profiles of the exon 4–PCR products obtained for the reverse DNA single strand at 20°C is shown. The peak detected at 230 minutes in the mother's profile corresponds to the C517 allele of the GP IIIa. The peak at 220 minutes obtained for the father and the child is specific for the T517 form. SSCP confirms the father and the child are heterozygous for the C>T mutation.
Link between the Thr140Ile mutation and the Duv alloimmunization
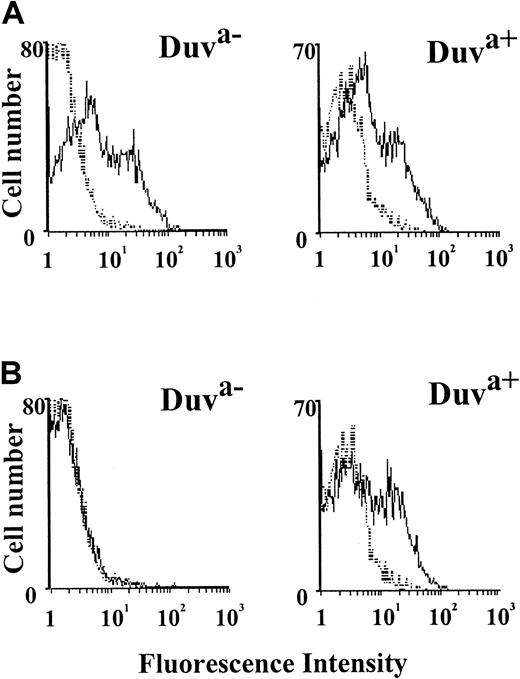
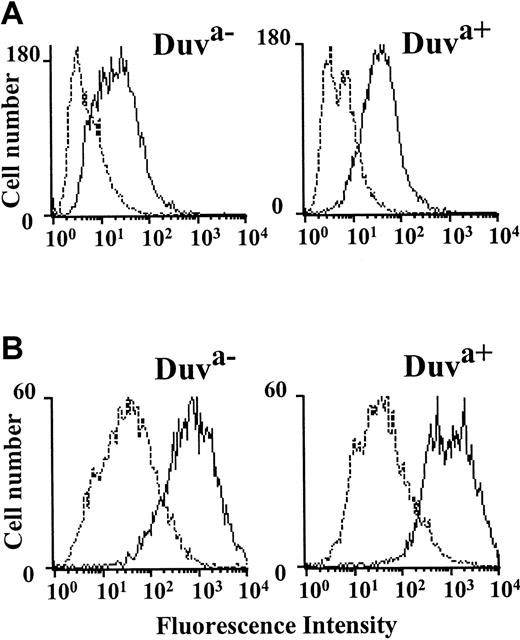
To study the role of the Thr140Ile polymorphism in neonatal thrombocytopenia, the allelic Duva+ form of the GP IIb-IIIa complex was reproduced in a mammalian cell transfection system. After transient transfection of GP IIb cDNA along with the wild-type (Duva−) or mutated (Duva+) GP IIIa cDNA, Cos cells were analyzed for the expression of the GP IIb-IIIa complex by flow cytometry using a panel of anti-GP IIb-IIIa Moabs (AP-2, AP-3, SZ 21, SZ 22). Both the Duva− and the Duva+allelic forms of the GP IIb-IIIa complex were expressed in approximately 30% of the transfected cells (data not shown). The flow cytometry histograms obtained with the Duva− and the Duva+ cells probed with an alloantiserum anti–HPA-1a (Figure 3A) or the maternal serum Duv (Figure 3B) showed that the maternal serum reacted selectively with the Duva+ cells, whereas anti–HPA-1a serum reacted with both cell types. Similar results were obtained by Western blot and immunoprecipitation assays (data not shown). These results demonstrated that the Thr140Ile substitution was responsible for the expression of the Duva+ epitope involved in the maternal alloimmunization.
Flow cytometric analysis of the maternal serum Duv binding with the Duva+ allelic form of the GP IIb-IIIa complex.
Cos cells expressing the Duva− or the Duva+form of the GP IIb-IIIa complex were incubated with a serum containing an anti–HPA-1a antibody (A) and with the maternal serum Duv (B) or an AB control serum (A and B, dotted line). Incubated cells were rinsed prior to incubation with an antihuman IgG conjugated to phycoerythrin. Washed cells were analyzed by a FACSCalibur cytometer. The maternal serum Duv reacts specifically with the cells expressing the Duva+ allelic form of the GP IIb-IIIa complex, whereas positive reaction was observed for both types of cells with anti–HPA-1a.
Flow cytometric analysis of the maternal serum Duv binding with the Duva+ allelic form of the GP IIb-IIIa complex.
Cos cells expressing the Duva− or the Duva+form of the GP IIb-IIIa complex were incubated with a serum containing an anti–HPA-1a antibody (A) and with the maternal serum Duv (B) or an AB control serum (A and B, dotted line). Incubated cells were rinsed prior to incubation with an antihuman IgG conjugated to phycoerythrin. Washed cells were analyzed by a FACSCalibur cytometer. The maternal serum Duv reacts specifically with the cells expressing the Duva+ allelic form of the GP IIb-IIIa complex, whereas positive reaction was observed for both types of cells with anti–HPA-1a.
Effect of the Duva+ polymorphism on the reactivity of anti–HPA-4a antibody
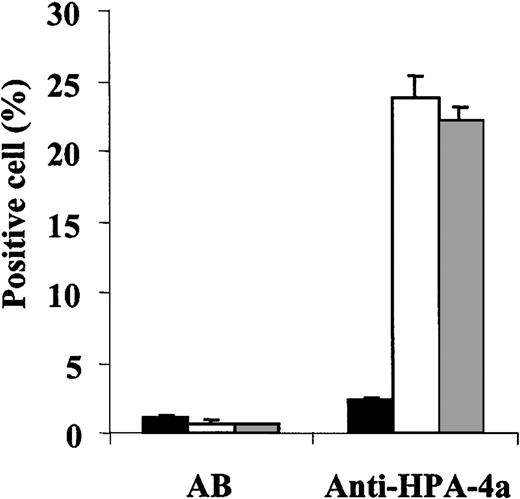
The Thr140Ile substitution is localized to 3 amino acids upstream from the Arg143 involved in the expression of the HPA-4a antigen. To determine whether the Duva+ polymorphism could modify the antigenicity of the HPA-4a epitope, the binding of an anti–HPA-4a antibody to GP IIb–mutated GP IIIa Cos cells was studied by flow cytometry (Figure 4). The anti–HPA-4a antibody containing sera tested reacted to the same extent with both the wild-type or mutated cells. These results show the Thr140Ile substitution does not significantly affect the reactivity of the anti–HPA-4a antibody tested.
Flow cytometric analysis of the binding of an anti–HPA-4a antibody to the Duva+ allelic form of the GP IIb-IIIa complex.
Cos cells expressing the Duva− (white bar) or the Duva+(gray bar) forms of the GP IIb-IIIa or nontransfected (black bar) were incubated with an AB serum as control and a serum containing an anti–HPA-4a antibody. Human bound IgGs were detected by flow cytometry as described in the legend to Figure 3. The anti–HPA-4a antibody reacted with both types of cells independently of the allelic forms of the GP IIb-IIIa. The serum AB did not show any significant reactivity whatever the cell type tested. Results correspond to a mean of 3 experiments ± SD.
Flow cytometric analysis of the binding of an anti–HPA-4a antibody to the Duva+ allelic form of the GP IIb-IIIa complex.
Cos cells expressing the Duva− (white bar) or the Duva+(gray bar) forms of the GP IIb-IIIa or nontransfected (black bar) were incubated with an AB serum as control and a serum containing an anti–HPA-4a antibody. Human bound IgGs were detected by flow cytometry as described in the legend to Figure 3. The anti–HPA-4a antibody reacted with both types of cells independently of the allelic forms of the GP IIb-IIIa. The serum AB did not show any significant reactivity whatever the cell type tested. Results correspond to a mean of 3 experiments ± SD.
Effect of the Thr140Ile mutation on the function of the integrin αIIbβ3
Fibrinogen and PAC-1 binding.
The Thr140Ile mutation is localized in the amino acid stretch Asp109 to Glu171 known to be a putative RGD binding site of the β3-subunit.30 To test the effect of this mutation on the integrin function—because no homozygous mutated platelets were available—stable cell lines expressing the Duva− and the Duva+ allelic forms of the GP IIb-IIIa complex were produced by cotransfection of GP IIb cDNA either with wild-type or mutated GP IIIa cDNA into CHO cells. Both allelic forms were able to bind to soluble fibrinogen (Figure5A) after DTT treatment in a similar manner, as shown by the increase in the mean fluorescence intensity, taking into account that the surface expression of the GP IIb-IIIa complexes on wild-type cells is slightly lower than for the mutated cells. This difference has been assessed by flow cytometry binding studies in saturation conditions of the Moabs directed against GP IIb-IIIa (AP-2) or GP IIIa (AP-3 and SZ 21) (data not shown). No binding occurred to nontransfected cells.
Analysis of the binding of soluble fibrinogen and of the Moab PAC-1.
CHO cells stably transfected and expressing the Duva− or Duva+ allelic forms of the GP IIb-IIIa were treated by 10 mM DTT (bold line) or buffer (dotted line) for 20 minutes prior to being incubated with 200 μg/mL fibrinogen-A488 (A) or with 1 μg/mL of the Moab PAC-1 (B). Washed cells were directly analyzed for the fibrinogen binding by flow cytometry or incubated with an IgG anti-IgM conjugated to phycoerythrin to detect PAC-1 binding and analyzed. The Duva+ form of GP IIIa did not impair fibrinogen or PAC-1 binding to the complex.
Analysis of the binding of soluble fibrinogen and of the Moab PAC-1.
CHO cells stably transfected and expressing the Duva− or Duva+ allelic forms of the GP IIb-IIIa were treated by 10 mM DTT (bold line) or buffer (dotted line) for 20 minutes prior to being incubated with 200 μg/mL fibrinogen-A488 (A) or with 1 μg/mL of the Moab PAC-1 (B). Washed cells were directly analyzed for the fibrinogen binding by flow cytometry or incubated with an IgG anti-IgM conjugated to phycoerythrin to detect PAC-1 binding and analyzed. The Duva+ form of GP IIIa did not impair fibrinogen or PAC-1 binding to the complex.
The binding of the ligand mimetic Moab PAC-131 was also analyzed in regard to the Duva+ polymorphism (Figure 5B). Results show that the PAC-1 antibody binds to both allelic forms of the GP IIb-IIIa complex in an activation-dependent manner. No binding was observed to nontransfected cells in the same conditions. Furthermore, the PAC-1 Moab bound similarly to GP IIb-IIIa activated by the anti-LIBS Moab D3 (not shown).
Aggregation.
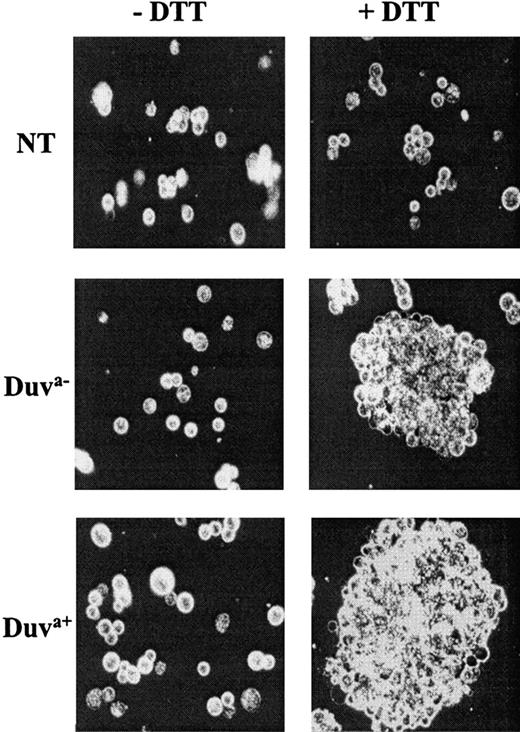
Soluble fibrinogen-dependent aggregation of both types of transfected cells was investigated following DTT treatment. Both cells expressing the Duva− and Duva+ allelic forms of the GP IIb-IIIa complex were able to form large aggregates (Figure6). Under the same experimental conditions, nontransfected cells did not aggregate.
Aggregation of transfected CHO cells.
Cells not transfected (NT) or expressing the Duva− or Duva+ allelic forms of the GP IIb-IIIa were treated by 10 mM DTT (+DTT) for 20 minutes at room temperature to activate the complex prior to being incubated with 100 μg/mL fibrinogen in the presence of 2 mM calcium with gentle shaking for 20 minutes. Only Duva− and Duva+ cells aggregated in the presence of fibrinogen when treated by DTT. No significant aggregation was observed for nontreated cells (−DTT) or nontransfected cells treated or not. The specimens were examined with a Leica-Orthoplan fluorescence microscope (Leica, Wetzlar, Germany) using a ×40 oil immersion objective. Microphotographs were taken using Ilford HP5 400 films (ILFORD Imaging, Mobberley, Cheshire, United Kingdom).
Aggregation of transfected CHO cells.
Cells not transfected (NT) or expressing the Duva− or Duva+ allelic forms of the GP IIb-IIIa were treated by 10 mM DTT (+DTT) for 20 minutes at room temperature to activate the complex prior to being incubated with 100 μg/mL fibrinogen in the presence of 2 mM calcium with gentle shaking for 20 minutes. Only Duva− and Duva+ cells aggregated in the presence of fibrinogen when treated by DTT. No significant aggregation was observed for nontreated cells (−DTT) or nontransfected cells treated or not. The specimens were examined with a Leica-Orthoplan fluorescence microscope (Leica, Wetzlar, Germany) using a ×40 oil immersion objective. Microphotographs were taken using Ilford HP5 400 films (ILFORD Imaging, Mobberley, Cheshire, United Kingdom).
Adhesion.
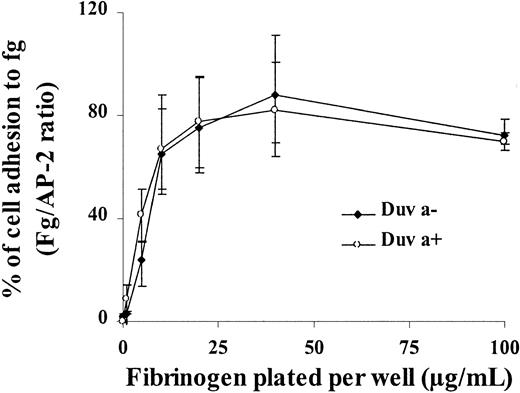
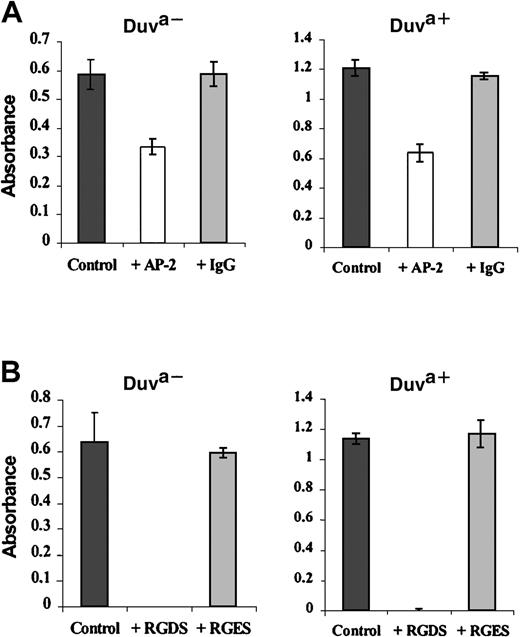
The functionality of the Duva+ allelic form of the GP IIb-IIIa complex was also analyzed in regard to its capacity to bind fibrinogen immobilized on plastic. Both wild-type or mutated GP IIIa CHO cells adhered to immobilized fibrinogen in a similar dose-dependent manner (Figure 7). This adhesion is mediated specifically by the GP IIb-IIIa complex because the incubation with anti–GP IIb-IIIa Moab AP-2 (20 μg/mL) prior to adhesion inhibited the cell attachment32 by 42% and 47% of their respective capacities (Figure 8A, white bars). A polyclonal mouse IgG used as control did not inhibit cell attachment (Figure 8A, gray bars).
Adhesion of transfected CHO cells onto immobilized fibrinogen.
Transfected cells expressing the Duva+ or the Duva− forms of the GP IIb-IIIa complex were incubated for 2 hours at 37°C in microtiter plates coated with different concentration of fibrinogen, BSA, or 20 μg/mL of the anti-GP IIb-IIIa Moab AP-2. Nonadherent cells were removed by washing, and adherent cells were fixed and stained in a crystal-violet solution for 30 minutes at room temperature. After washing and cell lysis in SDS, absorbance was measured at 560 nm. Cell adhesion was normalized to cell adhesion on AP-2 (fibrinogen/AP-2 ratio). Both cell types adhere onto fibrinogen in a dose-dependent manner. Results represent the mean ± SD of 3 experiments.
Adhesion of transfected CHO cells onto immobilized fibrinogen.
Transfected cells expressing the Duva+ or the Duva− forms of the GP IIb-IIIa complex were incubated for 2 hours at 37°C in microtiter plates coated with different concentration of fibrinogen, BSA, or 20 μg/mL of the anti-GP IIb-IIIa Moab AP-2. Nonadherent cells were removed by washing, and adherent cells were fixed and stained in a crystal-violet solution for 30 minutes at room temperature. After washing and cell lysis in SDS, absorbance was measured at 560 nm. Cell adhesion was normalized to cell adhesion on AP-2 (fibrinogen/AP-2 ratio). Both cell types adhere onto fibrinogen in a dose-dependent manner. Results represent the mean ± SD of 3 experiments.
Inhibition of the transfected cell adhesion by the Moab AP-2 or the peptide RGDS.
Transfected cells expressing the Duva+ or the Duva− forms of the GP IIb-IIIa complex were incubated in microtiter plates coated with 20 μg/mL fibrinogen. The incubation was performed in PBS-albumin (control) and in the presence of 20 μg/mL of either the Moab AP-2 or a nonrelevant polyclonal mouse IgG (A) or in the presence of 5 mM of the RGDS or RGES peptides (B). Quantification of bound cells was performed as described in the legend to Figure 7. The adhesion of CHO cells expressing the Duva+ GP IIIa form of the complex is inhibited by the Moab AP-2 or the peptide RGDS. Results represent the mean ± SD of 3 experiments.
Inhibition of the transfected cell adhesion by the Moab AP-2 or the peptide RGDS.
Transfected cells expressing the Duva+ or the Duva− forms of the GP IIb-IIIa complex were incubated in microtiter plates coated with 20 μg/mL fibrinogen. The incubation was performed in PBS-albumin (control) and in the presence of 20 μg/mL of either the Moab AP-2 or a nonrelevant polyclonal mouse IgG (A) or in the presence of 5 mM of the RGDS or RGES peptides (B). Quantification of bound cells was performed as described in the legend to Figure 7. The adhesion of CHO cells expressing the Duva+ GP IIIa form of the complex is inhibited by the Moab AP-2 or the peptide RGDS. Results represent the mean ± SD of 3 experiments.
The peptide RGDS (5 mM), a potent inhibitor of the platelet aggregation and adhesion mediated by the GP IIb-IIIa/fibrinogen interaction,33 inhibited the binding of both transfected cells (Figure 8B, white bars) to fibrinogen when compared with the results obtained either in the absence of peptide (black bars) or in the presence of 5 mM of the noninhibitory control peptide RGES (gray bars).
These results show that the Duva+ mutation does not impair the binding of the GP IIb-IIIa complex onto immobilized fibrinogen.
Outside-in signaling properties.
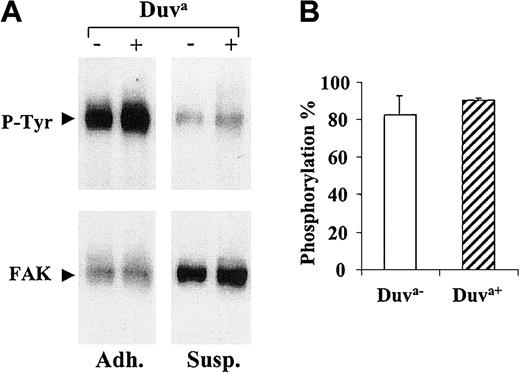
The oligomerization of GP IIb-IIIa complexes during platelet adhesion on immobilized fibrinogen leads to an outside-in signaling responsible for the autophosphorylation of pp125FAK. This outside-in signaling function documented in CHO cells expressing GP IIb-IIIa27 was evaluated in regard to the Duv polymorphism. As shown in Figure 9A, significant pp125FAK phosphorylation was observed in both adherent (Adh.) transfected CHO cell lines expressing either the wild-type or mutated GP IIIa but not in nonadherent (Susp.) cells. The phosphorylation levels expressed as phosphorylation percentage were similar for both cell types (Figure 9B).
Phosphorylation of pp125FAK.
CHO cells expressing the Duva+ or the Duva−forms of the GP IIb-IIIa complex were layered onto immobilized fibrinogen (Adh.) or maintained in suspension (Susp.) for 2 hours at 37°C before the cells were lysed and the pp125FAKimmunoprecipitated. (A) Blots were performed to detect phosphotyrosine (blots P-tyr) and the pp125FAK (blots FAK) by using the PY-20 and an anti-pp125FAK Moabs, respectively. Detection was performed by using an antimouse IgG conjugated to the peroxidase associated to a chemiluminescent detection system. (B) The histogram represents the mean phosphorylation percentage for each cell line, calculated from the band intensities measured by densitometric analysis (see “Materials and methods”). Results represent the mean ± SD of 3 experiments.
Phosphorylation of pp125FAK.
CHO cells expressing the Duva+ or the Duva−forms of the GP IIb-IIIa complex were layered onto immobilized fibrinogen (Adh.) or maintained in suspension (Susp.) for 2 hours at 37°C before the cells were lysed and the pp125FAKimmunoprecipitated. (A) Blots were performed to detect phosphotyrosine (blots P-tyr) and the pp125FAK (blots FAK) by using the PY-20 and an anti-pp125FAK Moabs, respectively. Detection was performed by using an antimouse IgG conjugated to the peroxidase associated to a chemiluminescent detection system. (B) The histogram represents the mean phosphorylation percentage for each cell line, calculated from the band intensities measured by densitometric analysis (see “Materials and methods”). Results represent the mean ± SD of 3 experiments.
Effect of the anti-Duva+ antibody on cell adhesion onto fibrinogen
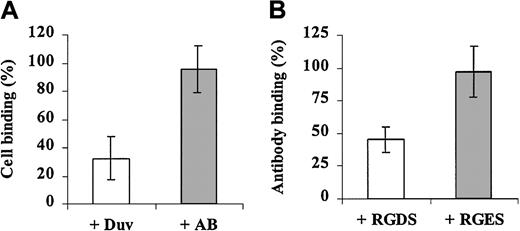
The effect of the anti-Duva+ antibody on adhesion was studied by using transfected cells in an adhesion test because no homozygous Duva+ platelets were available for aggregation studies. CHO cells expressing the Duva+ form of the GP IIb-IIIa complex were incubated with a saturating amount of the anti-Duva+ antibody and analyzed for their adhesion capacity onto fibrinogen (Figure 10A). The anti-Duva+ antibody inhibited cell adhesion to 67.5%, whereas the AB serum used as a control did not show a significant inhibitory effect (4.5%).
Cell adhesion inhibition and the anti-Duva+antibody.
(A) CHO cells expressing the Duva+ form of the complex were incubated with the anti-Duva+ (open bar) or a control (gray bar) sera. Washed cells were then layered onto immobilized fibrinogen (250 ng per well) for 2 hours at 37°C. Quantification of bound cells was performed as described in the legend to Figure 7. (B) CHO cells expressing the Duva+ form of the complex were incubated with RGDS (open bar) or a control RGES (gray bar) peptides prior to adding 10 μL anti-Duva+ sera corresponding to a half-saturation amount. After a washing, bound human antibodies were detected by flow cytometry as described in the legend to Figure 3. Results represent the mean ± SD of 3 experiments.
Cell adhesion inhibition and the anti-Duva+antibody.
(A) CHO cells expressing the Duva+ form of the complex were incubated with the anti-Duva+ (open bar) or a control (gray bar) sera. Washed cells were then layered onto immobilized fibrinogen (250 ng per well) for 2 hours at 37°C. Quantification of bound cells was performed as described in the legend to Figure 7. (B) CHO cells expressing the Duva+ form of the complex were incubated with RGDS (open bar) or a control RGES (gray bar) peptides prior to adding 10 μL anti-Duva+ sera corresponding to a half-saturation amount. After a washing, bound human antibodies were detected by flow cytometry as described in the legend to Figure 3. Results represent the mean ± SD of 3 experiments.
According to the localization of the Duv polymorphism in the RGD binding domain of GP IIIa, the inhibition of the anti-Duva+antibody binding by the RGDS peptide was analyzed by flow cytometry (Figure 10B). The anti-Duva+ antibody binding was inhibited to 54.7% in the presence of 25 mM RGDS. No significant inhibition was observed with the RGES control peptide in similar experimental conditions.
Discussion
We report here the characterization of a new platelet alloantigen of low frequency involved in a case of severe neonatal thrombocytopenia. The MAIPA technique revealed a strong selective binding of the maternal serum to the paternal GP IIb-IIIa complex. The serum failed to react with phenotyped platelets from a panel despite a maternofetal incompatibility in the HPA-3 system. Western blot study showed the epitope was localized on the GP IIIa subunit of the platelet GP IIb-IIIa complex. Sequencing analysis of the paternal gene coding for GP IIIa indicated a single base substitution 517C>T (cDNA) in a heterozygous state that resulted in a Thr140Ile polymorphism on mature GP IIIa. The same results were obtained in the child's DNA. Transient expression of the mutated GP IIIa in Cos cells cotransfected with GP IIb did not prevent the GP IIb-IIIa complex from being normally expressed on the cell surface and confirmed this point mutation was sufficient to induce the formation of the epitope recognized by the antibody present in the maternal serum. By using the SSCP technique, the frequency of this new allele was estimated to be less than 1% in our series.
Chymotryptic treatment of GP IIIa resulted in the loss of the reactivity of the anti-Duva+ antibody. Furthermore, the molecular conformation of GP IIIa was important for the expression of the epitope, because disruption of disulfide bonds by DTT treatment impaired the binding of the antibody. This is also the case with the HPA-1a and HPA-4a epitopes where synthetic linear peptides encompassing the antigenic polymorphisms are not recognized by the respective alloantibodies.5,34 As for these other well-characterized alloantibodies,5,35 36 the Thr140Ile polymorphism is necessary, but not sufficient, to express the epitope recognized by the anti-Duva+ antibody.
The effect of the anti-Duva+ antibody on platelet aggregation has not been tested because we were not able to find any Duva+ homozygous platelets. Nevertheless, its ability to impair the binding of CHO cells expressing the Duva+ form of the complex on fibrinogen strongly suggests that it may inhibit platelet aggregation similarly to anti–HPA-4a antibody.37In these conditions attention must be paid to the increased risk of bleeding due to a potential platelet dysfunction induced by the anti-Duva+ antibody. However, it has been difficult to predict hemorrhagic tendency on the basis of the in vitro experiments. Although it has been shown that anti–HPA-1a antibody induces a thrombastheniclike platelet dysfunction of normal HPA-1a platelets regardless of zygosity,38 this was not observed in other reports.37 39 In our case the infant, heterozygous for the Duva+ antigen, did not exhibit any severe bleeding episodes.
The Thr140Ile mutation is localized in a GP IIIa domain shown to be critical for both the surface expression and the function of the complex. Biochemical data on the structural/functional relationship30,40-43 and several amino acid substitutions, observed in patients with Glanzmann phenotypes or obtained by site-directed mutagenesis, stress the importance of this domain of GP IIIa.44-49 The Thr140Ile mutation could alter the local 3-dimensional structure of this GP IIIa domain and affect the adhesive and signaling properties of the integrin. The function of the complex IIb–mutated IIIa was analyzed by using cotransfected CHO stable cell lines. Fibrinogen, as did the ligand mimetic antibody PAC-1, bound the Duva+ allelic form of the complex once activated by DTT. The Thr140Ile substitution did not alter the fixation of the anti-LIBS D328 and the consequent activation of GP IIb-IIIa leading to the binding of PAC-1. CHO cells expressing the Duva+form of the complex can aggregate following activation by DTT and adhere to adsorbed fibrinogen in a dose-dependent manner. The use of the Moab AP-2 or the peptide RGDS as inhibitors confirms the specificity of this adhesion process. Binding of fibrinogen, PAC-1, and RGDS to the Duva+ form of GP IIb-IIIa shows that none of the 2 putative ligand binding sites50 on the complex seemed to be affected by the substitution. Normal cell spreading and pp125FAK phosphorylation show that the Duva+mutation did not affect the outside-in signaling property of the GP IIb-IIIa complex. Our results indicate that the Thr140Ile substitution had little or no effect on either the expression or the function of the GP IIb-IIIa complex. Similar results have been obtained with the Arg143Gln substitution51 that is proximal to the Duv polymophic site.
The 517C>T substitution did not impair HPA-4 genotyping by the PCR–sequence-specific primer technique we used. Moreover, we observed a normal reactivity of an anti–HPA-4a antibody (Pen) with the Duva+ form of GP IIb-IIIa.
To summarize, in the context of severe neonatal thrombocytopenia, we have characterized a new low-frequency antigen carried by platelet GP IIIa. A 517C>T substitution has been shown to result in a Thr140Ile polymorphism. For routine diagnosis, this substitution cannot be detected by the PCR–restriction fragment length polymorphism technique because no known restriction site is created or abolished. A mutation proximal to the Duv substitution has not yet been shown to be associated with the Glanzmann phenotype, and the present study is consistent with this, indicating as it does that the Thr140Ile substitution does not significantly affect the expression or the function of the complex.
The authors thank Drs Peter J. Newmann and Ronggang Wang for the kind gift of the anti–HPA-4a serum and the plasmids pcDNA3-IIb and pcDNA3-IIIa, Dr Lisa Jennings for the kind gift of the Moab D3, and Prof Alan Waters for reading the manuscript. We also thank Mr P. Gane for his technical help in flow cytometry studies.
Supported by a grant from the SESEP (Société d'Etudes et de Soins pour les Enfants Paralysés et Polymalformés), Paris, France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
C. Kaplan, Laboratoire d'Immunologie Plaquettaire, INTS, 6 rue A. Cabanel, 75 015 Paris, France; e-mail:cecile.kaplan@teaser.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal