Abstract
Janus kinase 3 (Jak3) is a cytoplasmic tyrosine (Tyr) kinase associated with the interleukin-2 (IL-2) receptor common gamma chain (γc) that is activated by multiple T-cell growth factors (TCGFs) such as IL-2, -4, and -7. Using human T cells, it was found that a recently discovered variant of the undecylprodigiosin family of antibiotics, PNU156804, previously shown to inhibit IL-2–induced cell proliferation, also blocks IL-2–mediated Jak3 auto-tyrosine phosphorylation, activation of Jak3 substrates signal transducers and activators of transcription (Stat) 5a and Stat5b, and extracellular regulated kinase 1 (Erk1) and Erk2 (p44/p42). Although PNU156804 displayed similar efficacy in blocking Jak3-dependent T-cell proliferation by IL-2, -4, -7, or -15, it was more than 2-fold less effective in blocking Jak2-mediated cell growth, its most homologous Jak family member. A 14-day alternate-day oral gavage with 40 to 120 mg/kg PNU156804 extended the survival of heart allografts in a dose-dependent fashion. In vivo, PNU156804 acted synergistically with the signal 1 inhibitor cyclosporine A (CsA) and additively with the signal 3 inhibitor rapamycin to block allograft rejection. It is concluded that inhibition of signal 3 alone by targeting Jak3 in combination with a signal 1 inhibitor provides a unique strategy to achieve potent immunosuppression.
Introduction
Complete activation of T cells requires 3 threshold-limited sequential signals.1 Signal 1, delivered by antigens that engage a specific T-cell receptor (TCR), is followed by signal 2, delivered by a B7-CD28 interaction. Within seconds to minutes of TCR engagement, the CD3ζ chain is tyrosine (Tyr) phosphorylated during the autoactivation of Zap70, Lck, and Fyn protein Tyr kinases.2-4 Concomitantly, calcium (Ca++) mobilization triggers the catalytic activation of calcineurin (CaN) phosphatase to dephosphorylate nuclear factor of activated T cell (NFAT)—a necessary step for NFAT to translocate to the nucleus and bind discrete DNA-binding elements within the promoter of the interleukin-2 (IL-2) gene.5 Signals 1 and 2 are critical for the synthesis and secretion of IL-2, which, in concert with other T-cell growth factors (TCGFs) such as IL-4, -7 , -9, -13, and -15, deliver signal 3 through cytokine receptors, a necessary step for driving the clonal expansion of T cells.6 These cytokine receptors share a common gamma chain (γc) that, when combined with a private α chain for each cytokine, delivers intracellular signals by Janus Tyr kinase 1 (Jak1) and Jak3 and that activates signal transducers and activators of transcription 1 (Stat1), Stat3, Stat5a/b, and Stat6.6-11
Current clinical immunosuppressive regimens are dominated by CaN inhibitors (cyclosporine [CsA] or FK50612) that block T-cell progression through the early G1 stages of the cell cycle.12,13 However, ubiquitous expression of CaN in many different tissues contributes to several adverse side effects, including nephrotoxicity and neurotoxicity.14,15 One therapeutic approach aimed at improving immunosuppression while simultaneously reducing CsA-induced toxicity is to develop combination therapy. Rapamycin (RAPA), an agent recently approved by the United States Food and Drug Administration for use in clinical transplantation, disrupts late G1 and early S cell-cycle progression by inhibition of the serine (Ser)–threonine (Thr) kinase, the mammalian target of rapamycin (mTOR).16 Our extensive preclinical and recent clinical studies have documented that a combination of CsA and RAPA produces potent synergistic interactions allowing for a 50% reduction of CsA trough blood levels.17,18 Although RAPA is not nephrotoxic alone, however, the ubiquitous tissue distribution of mTOR leads to other toxicities such as myelosuppression and hyperlipidemia.18-22 The current study explores the hypothesis that the inhibition of molecular targets unique to lymphocytes, particularly those activated by TCGFs, would provide a novel and selective means to block T-cell function and allograft rejection when used alone or in combination with CaN inhibitors. In particular, we seek to identify antagonists of Jak3, thereby inhibiting an entire family of TCGF-dependent pathways. Indeed, Jak3 (primarily expressed in T, B, and natural killer [NK] cells) is activated through the γc and plays a critical role in T-cell development and function.23,24 Humans or mice genetically deficient in this enzyme or unable to activate Jak3 because of genetic disruption of the γc manifest severe combined immunodeficiency disease.10,23,24 Recent evidence suggests that disruption of Jak3 results in the inactivation of 2 critical Jak3 substrates, Stat5a and Stat5b.25 Although double Stat5a/b gene-deficient mice are not as severely immunosuppressed as Jak3 or γc knockouts, their T cells fail to proliferate in the presence of TCGFs such as IL-2,26 presumably because of greatly reduced levels of several cell-cycle proteins, including the cyclin-dependent kinase-6 and cyclins A, D2, D3, and E.27
A recently published report28 revealed that PNU156804, an analogue of the toxic parent compound undecylprodigiosin, blocks IL-2–induced T-cell proliferation in the late G1 phase of the cell cycle. On the molecular level, these authors showed that PNU156804 inhibits the activation of NF-κB and AP-1 transcription factors, the expression of cyclins D2 and E and cyclin-dependent kinases 2 and 4, and the hyperphosphorylation of the retinoblastoma protein. In contrast, IL-2 receptor (IL-2R) messenger RNA (mRNA) expression of α and γ chains was not affected by PNU156804.28 However, the exact molecular target for PNU156804 was not identified in this earlier study. The current experiments investigate whether PNU156804 inhibits TCGF-induced T-cell proliferation through blockade of the Jak3, Stat5a/b, and mitogen-activated protein kinase (MAPK) pathways. We also examined whether PNU156804, alone or in combination with CsA or RAPA, can effectively block allograft rejection.
Materials and methods
Cell culture and treatment
The rat T-cell line Nb2-11c, originally developed by Dr Peter Gout (Vancouver, BC, Canada), was grown in RPMI 1640 with 10% fetal calf serum (FCS; catalog no. 1020-90; Intergen, Purchase, NY), 2 mM L-glutamine, 5 mM HEPES, pH 7.3, and penicillin-streptomycin (50 IU/mL and 50 μg/mL, respectively), at 37°C–5% CO2. Freshly explanted normal human T lymphocytes purified by isocentrifugation (Ficoll; EM Science, Gibbstown, NJ) were phytohemagglutinin (PHA) activated for 72 hours as previously described.29 T lymphocytes were made quiescent by washing and incubating for 24 hours in RPMI 1640 medium containing 1% FCS before exposure to cytokines. Next, cells were treated with varying concentrations of PNU156804 (generously provided by Dr Roberto D'Alessio, Department of Pharmacology, Pharmacia and Upjohn Research Center, Nerviano, Italy) as described in the figure legends. All cells were then stimulated with 100 nM recombinant human IL-2 (Hoffman-LaRoche, Basel, Switzerland), anti-CD3 (PharMingen, San Diego, CA), or ovine prolactin (PRL) supplied by the National Hormone and Pituitary Program (National Institute of Diabetes, Digestive, and Kidney Disease, Bethesda, MD) at 37°C. Cell pellets were frozen at −70°C.
Proliferation assays
Quiescent human primary T cells, human Jurkat cells, or rat T cells (5.0 × 104/well) were plated in flat-bottom, 96-well microtiter plates in 200 μL quiescent media in the absence or presence of 1 nM IL-2, -4, -7, or -15 (PeproTech, Rock Hill, NJ) or of PRL. Next, cells were treated for 16 hours with PNU156804 or the inactive control, PNU159744, and were pulsed for 4 hours with [3H]-thymidine (0.5 μCi [0.0185 MBq]/200 μL) and harvested onto fiberglass filters. [3H]-thymidine incorporation was analyzed by liquid scintillation counting as previously described.29
Solubilization of membrane proteins, immunoprecipitation, and Western blot analysis
Frozen cell pellets were thawed on ice and solubilized in lysis buffer (108 cells/mL) as previously described.29 For human T cells, supernates were incubated, rotating end-over-end for 2 hours at 4°C with either 5 μL/mL polyclonal rabbit antisera raised against peptides derived from the unique carboxyl group (COOH) termini of Jak3 (amino acid [aa] 1104-1124) or carboxyl termini of human Stat5a (aa 775-794) or Stat5b (aa 777-787), and phosphoserine Stat5 rabbit polyclonal antibodies (pAbs) were generated against a phosphopeptide surrounding S725 of human Stat5a.30 For rat Nb2-11cc and T cells, Jak3, Jak2, p56Lck, and antiphosphotyrosine antibodies were obtained from Upstate Biotechnology (Lake Placid, NY). Proteins bound to antibodies were captured by incubation for 30 minutes with Protein A–Sepharose beads (Pharmacia, Piscataway, NJ), sedimented for purification, and eluted by boiling in 2× sodium dodecyl sulfate (SDS) sample buffer (20% glycerol, 10% 2-mercaptoethanol, 4.6% SDS, 0.004% bromophenol blue in 0.125 M Tris, pH 6.8) for 4 minutes. For phospho-MAPK assays, approximately 25 μg total cell lysate was dissociated in SDS sample buffer and was separated on 10% (all others on 7.5%) SDS–polyacrylamide gel electrophoresis (PAGE) under reducing conditions. Proteins were transferred to polyvinylidene difluoride (PVDF) (Immobilon; catalog no. 1PVH 00010; Millipore, Bedford, MA) as previously described.11 Western blot analysis was performed with either pAbs, murine antiphosphotyrosine monoclonal antibodies (mAbs) (4G10; catalog no. 05-321; Upstate Biotechnology, Lake Placid, NY), or phospho p44/42 MAPK (catalog no. 9101; New England Biolabs, Beverly, MA). Western blots with the above antibodies, rabbit antiphospho-Erk1/2 (extracellular regulated kinase 1/2), and monoclonal pan-Erk (catalog no. E17120; PharMingen, San Diego, CA) were diluted 1:1000 in blocking buffer and were used as previously described.11
Rat heart transplantations
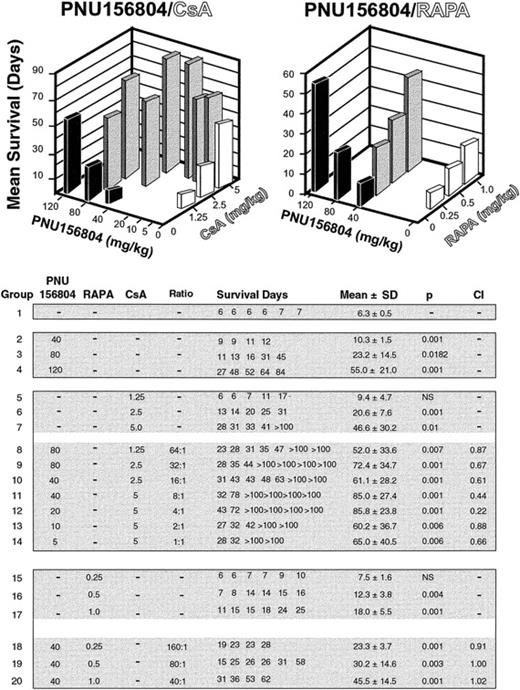
Wistar Furth (WF; RT1u) and Buffalo (BUF; RT1b) rats (each weighing 160-200 g) obtained from Harlan Sprague-Dawley (Indianapolis, IN) were cared for according to the guidelines of the Animal Welfare Committee. Rats were housed in light- and temperature-controlled quarters and given chow and water ad libitum. Heterotopic heart transplantation was performed using a standard microsurgical technique of end-to-side anastomoses to recipient aorta and vena cava.31 Cold ischemia times were less than 30 minutes. Graft survival time was defined as the last day of transabdominally palpable cardiac contractions. Recipients remained untreated or were treated by oral gavage every other day for 14 days (6 treatments) with 40, 80, or 120 mg/kg PNU156804 alone or in combination with daily oral gavage of 1.25, 2.5, or 5 mg/kg CsA or 0.25, 0.5, or 1 mg/kg RAPA. Some recipients were treated with CsA or RAPA alone. The results, presented as mean survival time ± standard deviation, were assessed for statistical significance by the Gehan survival test. In addition, the interaction between PNU156804 and CsA or RAPA was evaluated by the median effect analysis.32,33 Computer software was used to calculate combination index (CI) values: CI < 1 showed synergistic interactions, CI > 1 showed antagonistic interactions, and CI = 1 showed additive interactions.33
Histopathologic evaluation
WF recipients of BUF heart allografts were treated as described above with vehicle (n = 3) or vehicle containing 80 mg/kg PNU156804 (n = 3). At day 7 after transplantation, heart allografts were placed in Bouin fixative (Poly Scientific R&D, Bay Shore, NY). Each heart was sectioned in an identical fashion consisting of single horizontal cut followed by 3 consecutive incisions used to generate slides. Another dissection was made, and 3 more consecutive slices and slides were generated. Twelve slides per heart were stained with hematoxylin and eosin, as described previously.34Rejection was graded in accordance with the standards established by the International Society of Heart and Lung Transplantation35: grade 0, no evidence of rejection; grade 1A, focal perivascular infiltration; grade 1B, diffused interstitial infiltration; grade 2 for moderate infiltration with uni-focal myocyte damage; grade 3A, moderate infiltration with severe focal myocyte damage; grade 3B, moderate infiltration with severe diffused myocyte damage; and grade 4, severe infiltration with ongoing severe myocyte damage.
Results
PNU156804 blocks IL-2–mediated growth and Jak3 autophosphorylation
To investigate the effects of PNU156804 on T-cell proliferation, T cells that had been activated for 72 hours with PHA were then made quiescent and admixed with ascending PNU156804 concentrations (1-100 μM) for 16 hours in the absence or presence of 1 nM IL-2. As shown in Figure 1, PNU156804 completely abolished IL-2–induced [3H]-thymidine incorporation at 25 μM (IC50 of approximately 7.5 μM). In contrast, the inactive analog PNU159744 showed minimal effect on T-cell proliferation at similar concentrations of drug, with only 20% inhibition observed at 100 μM (Figure 1). This inhibitory effect was not caused by cell death from toxicity because cell viability was typically greater than 85% based on a trypan blue dye exclusion test at this time point (16 hours), which was measured at the completion of each experiment (data not shown). Moreover, the effect of the drug appeared to be confined to activated T cells because PNU156804 did not inhibit actively growing non-Jak3–expressing Jurkat cells, a model for unactivated T cells (Figure 1).
PNU156804 disrupts cell growth of activated, but not of unactivated, human T cells in a dose-dependent manner.
Proliferation of quiescent PHA-activated human T cells (5.0 × 104 cells/well) in the presence of 1 nM IL-2 was examined after treatment with increasing concentrations of PNU156804 (●) or inactive control PNU159744 (♦) for 16 hours at 37°C. Conversely, Jurkat cells were treated in an identical fashion with PNU156804 (▴). All cells were then pulsed with [3H]-thymidine (0.5 μCi [0.0185 MBq]/200 μL) for 4 hours, incorporated into a radiolabeled probe, and plotted on the abscissa expressed as total cpm (n = 6).
PNU156804 disrupts cell growth of activated, but not of unactivated, human T cells in a dose-dependent manner.
Proliferation of quiescent PHA-activated human T cells (5.0 × 104 cells/well) in the presence of 1 nM IL-2 was examined after treatment with increasing concentrations of PNU156804 (●) or inactive control PNU159744 (♦) for 16 hours at 37°C. Conversely, Jurkat cells were treated in an identical fashion with PNU156804 (▴). All cells were then pulsed with [3H]-thymidine (0.5 μCi [0.0185 MBq]/200 μL) for 4 hours, incorporated into a radiolabeled probe, and plotted on the abscissa expressed as total cpm (n = 6).
PNU156804 fails to inhibit anti-CD3 activation of p56Lck in primary human T cells
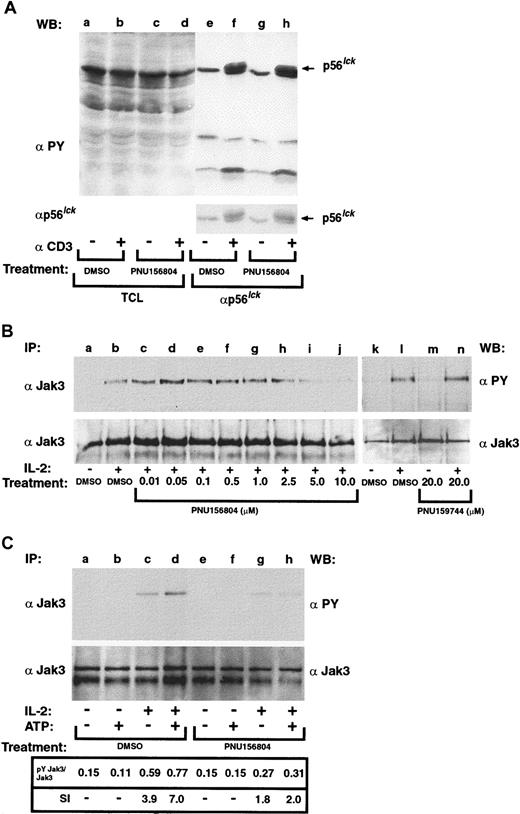
The above experiments suggest PNU156804 inhibits activated but not unactivated T cells by disrupting the signal 3 pathway. Earlier work by Mortellaro et al28 supports this model because the expression of IL-2 and its affinity-conferring α-chain, both dependent on signal 1 and 2, were not affected. To test this notion further, effects of PNU156804 on the TCR-activated pathways were investigated by stimulating T cells by anti-CD3 activation and measuring changes in p56Lck and other Tyr-phosphorylated proteins. For this assay, primary human T cells were treated with 20 mM active drug (PNU156804) for 16 hours and were stimulated without or with 5 μg/mL (μM) anti-CD3 antibody for 5 minutes at 37°C (Figure2A). Cell lysates were clarified, and 30 μg total cell lysate was separated on SDS-PAGE from vehicle (lanes a-b) or from PNU156804-treated samples (lanes c-d). Alternatively, lysates were immunoprecipitated with antibodies to p56Lck (lanes e-h). Proteins were Western blotted with antiphosphotyrosine antibodies. Overall, no changes were observed in total Tyr-phosphorylated protein or TCR activation of the p56Lck Tyr kinase. Striping and reprobing of the blot confirmed an equivalent loading of p56Lck.
PNU156804 inhibits the signal 3 mediator Jak3 but not the signal 1 transducer p56Lck.
(A) Antiphosphotyrosine (αPY) immunoblot of PHA-activated T cells stimulated with anti-CD3 in the presence of PNU156804. T cells (5.0 × 107 cells/lane) were treated with DMSO or with 20 μM PNU156804 for 16 hours and then were stimulated in the presence or absence of 5 μg anti-CD3 for 5 minutes and subjected to cell lysis and clarification. Total cell lysates were separated by SDS-PAGE (lanes a-d) or were immunoprecipitated with antibodies to p56Lck (lanes e-h) and separated on 10% SDS-PAGE and then subjected to antiphosphotyrosine Western blot (WB) analysis. The blot was then stripped and reblotted with anti-Lck to verify equivalent loading (indicated in the lower panel). Arrow denotes migration of p56Lck. (B) PHA-activated human T cells were treated with DMSO or increasing concentrations of PNU156804 (0-10 μM; lanes a-j) or inactive control PNU159744 (lanes k-n) and were stimulated in the absence (−) or presence (+) of IL-2 (100 nM) for 10 minutes at 37°C. Cells were lysed, clarified, and immunoprecipitated (IP) with anti-Jak3 (αJak3), separated on 10% SDS-PAGE, transferred to PVDF membrane, and Western blotted with antiphosphotyrosine. The blot was stripped and reprobed with Jak3 antibody. (C) For antiphosphotyrosine immunoblot of Jak3 autokinase assay, PHA-activated T cells were stimulated for 10 minutes with (+) or without (−) 100 nM IL-2 and then immunoprecipitated with anti-Jak3 and treated for 15 minutes on ice with DMSO control (lanes a-d) or 10 μM PNU156 804 (lanes e-h). Jak3 was incubated for 20 minutes at 37°C in the absence (−) or presence (+) of 15 μM unlabeled ATP, separated by SDS-PAGE, transferred to PVDF membrane, and Western blotted with antiphosphotyrosine antibodies (upper panel). The same blots were reblotted with α-Jak3 to verify equal loading of enzyme (lower panel). Ratio of Tyr-phosphorylated Jak3 to total Jak3 protein was analyzed by densitometry and plotted as the stimulatory index (SI) value in arbitrary units.
PNU156804 inhibits the signal 3 mediator Jak3 but not the signal 1 transducer p56Lck.
(A) Antiphosphotyrosine (αPY) immunoblot of PHA-activated T cells stimulated with anti-CD3 in the presence of PNU156804. T cells (5.0 × 107 cells/lane) were treated with DMSO or with 20 μM PNU156804 for 16 hours and then were stimulated in the presence or absence of 5 μg anti-CD3 for 5 minutes and subjected to cell lysis and clarification. Total cell lysates were separated by SDS-PAGE (lanes a-d) or were immunoprecipitated with antibodies to p56Lck (lanes e-h) and separated on 10% SDS-PAGE and then subjected to antiphosphotyrosine Western blot (WB) analysis. The blot was then stripped and reblotted with anti-Lck to verify equivalent loading (indicated in the lower panel). Arrow denotes migration of p56Lck. (B) PHA-activated human T cells were treated with DMSO or increasing concentrations of PNU156804 (0-10 μM; lanes a-j) or inactive control PNU159744 (lanes k-n) and were stimulated in the absence (−) or presence (+) of IL-2 (100 nM) for 10 minutes at 37°C. Cells were lysed, clarified, and immunoprecipitated (IP) with anti-Jak3 (αJak3), separated on 10% SDS-PAGE, transferred to PVDF membrane, and Western blotted with antiphosphotyrosine. The blot was stripped and reprobed with Jak3 antibody. (C) For antiphosphotyrosine immunoblot of Jak3 autokinase assay, PHA-activated T cells were stimulated for 10 minutes with (+) or without (−) 100 nM IL-2 and then immunoprecipitated with anti-Jak3 and treated for 15 minutes on ice with DMSO control (lanes a-d) or 10 μM PNU156 804 (lanes e-h). Jak3 was incubated for 20 minutes at 37°C in the absence (−) or presence (+) of 15 μM unlabeled ATP, separated by SDS-PAGE, transferred to PVDF membrane, and Western blotted with antiphosphotyrosine antibodies (upper panel). The same blots were reblotted with α-Jak3 to verify equal loading of enzyme (lower panel). Ratio of Tyr-phosphorylated Jak3 to total Jak3 protein was analyzed by densitometry and plotted as the stimulatory index (SI) value in arbitrary units.
Catalytically active Jak3 is required for IL-2–driven Tyr phosphorylation of Jak1 and Stat5a/b.11,26,27,36 37 To determine whether the blockade of T-cell proliferation was caused by a loss of Jak3 activity, PHA-activated T cells (72 hours) were treated with ascending concentrations of PNU156804 (0-10 μM) for 16 hours, followed by a 10-minute stimulation with 100 nM IL-2. Lysed cells were immunoprecipitated with Jak3 pAb and then Western blotted with antiphosphotyrosine mAb (Figure 2B). Tyr phosphorylation of Jak3 was notably reduced at 5 μM (lane i) and almost completely inhibited at 10 μM (lane j) concentrations. Five separate experiments confirmed the loss of Jak3 Tyr phosphorylation between 5 and 20 μM PNU156804. Inactive PNU159744 control was ineffective in blocking Jak3 activation (Figure 2B, lanes k-n). The same samples reblotted with anti-Jak3 pAb to measure total Jak3 confirmed equivalent protein levels (Figure 2B, lower panel). Thus, PNU156804 disrupts Tyr phosphorylation of Jak3.
Because an earlier study demonstrated that the lack of cell proliferation in the presence of PNU156804 was not attributed to the loss of IL-2R α or β chain expression,28 we examined whether the inhibition of Jak3 resulted from a direct effect of the drug on the Jak3 enzyme. In particular, several studies have shown a correlation between cytokine-induced Tyr phosphorylation and catalytic activation of Jak kinases.38-40 We have previously demonstrated that IL-2, -4, or -13 markedly increased the catalytic activity of Jak3 using an in vitro kinase assay based on antiphosphotyrosine immunoblotting and the addition of unlabeled adenosine triphosphate (ATP).11,41 42 Similar autokinase assays were performed herein on Jak3 immunoprecipitates obtained from T cells exposed to medium alone or with 100 nM IL-2 (3 minutes at 37°C). Affinity-purified Jak3 protein incubated with kinase buffer in the presence or absence of cold ATP (15 μM), either dimethyl sulfoxide (DMSO) or 10 μM PNU156804, and the auto-Tyr phosphorylation samples were separated on SDS-PAGE and subsequently blotted with antiphosphotyrosine mAbs. Representative data from 2 separate experiments measured Jak3 autophosphorylation normalized to nonphosphorylated protein (phosphorylated–nonphosphorylated) to calculate a stimulatory index (SI) value for in vitro treatment with either vehicle alone (Figure 2C, lanes a-d) or with 20 μM PNU156804 (Figure 2C, lanes e-h). Immunopurified Jak3 Tyr autophosphorylation was potently inhibited by PNU156804 because the addition of cold ATP failed to stimulate Jak3 Tyr autophosphorylation (lane h) in comparison with non–ATP-treated samples (lane g). Densitometric analysis of these sample points (SI, 1.8-2.0) showed that PNU156804 reduced Jak3 autophosphorylation by 90% in comparison with DMSO-treated control sets (SI, 3.9-7.0; Figure 1B, lanes c-d). Thus, these findings suggest that PNU156804 inhibits IL-2–mediated T-cell growth by ablating crucial Jak3-dependent signaling pathways.
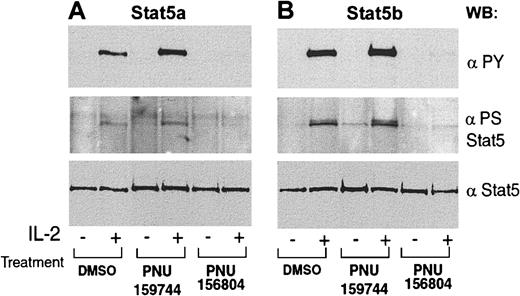
PNU156804 disrupts IL-2–mediated Stat5a/b Tyr/Ser phosphorylation
Given that both Stat5a and Stat5b are downstream of Jak3 and that T cells from Stat5a/b gene-deficient mice failed to proliferate in response to IL-2 stimulation,6 we examined whether PNU156804 inhibits IL-2–induced activation of Stat5a/b. For this assay, PHA-activated quiescent human T cells were treated with 20 μM PNU156804 or inactive control PNU159744 for 16 hours, followed by IL-2 stimulation. PNU156804 blocked Stat5a (Figure3) and Stat5b (Figure 3B) Tyr phosphorylation. In addition to Tyr phosphorylation, Stat5a/b transcription factors are (like other Stats) Ser phosphorylated.6 Indeed, interferon α/β (IFN-α/β) or IFN-γ–mediated Ser phosphorylation of Stat1α or IL-6 of Stat3 is believed necessary for its function, including maximal nuclear translocation, DNA binding, transcriptional activation, and cell cycle progression.43-45 We previously mapped a Ser phosphorylation site in Stat5a (Ser726) that is conserved in Stat5b (Ser731).30,46,47 As shown in Figure 3A-B, PNU156804 inhibited IL-2–induced Stat5a/b Ser kinase activity. In particular, neither site was inducibly Ser phosphorylated in the presence of cytokine and PNU156804, as measured by phosphoserine-specific Stat5a/b pAb (middle panels). The same samples reblotted with anti-Stat5a/b mAb confirmed equivalent protein levels (panels A and B). These results suggest that PNU156804 inhibits Jak3 from mediating Tyr and Ser phosphorylation of Stat5. In contrast, the inactive PNU159744 analogue showed no effect on either Tyr or Ser kinase activity. Because Jak-regulated Stat Tyr/Ser phosphorylation is required for dimerization, nuclear translocation, and gene transcription,25 we conclude that IL-2–Stat5a/b–mediated gene transcription critical for IL-2–mediated cell cycle progression is one explanation for the loss of IL-2–inducible T-cell proliferation observed in Figure 1.
PNU156804 inhibits IL-2–inducible Tyr phosphorylation of Stat5a/b in human T cells.
PHA-activated human T cells were pretreated with DMSO, 10 μM in active analogue PNU159744, or 10 μM PNU156804 for 16 hours and then stimulated for 10 minutes with (+) or without (−) 100 nM IL-2 for 10 minutes at 37°C. Cells were lysed and immunoprecipitated with anti-Stat5a (A) or anti-Stat5b (B) and were blotted with monoclonal antiphosphotyrosine Stat5 (Y701, upper panel), polyclonal phosphoserine Stat5 (S726-Stat5a or S731 Stat5b, middle panel), and reblotted with monoclonal pan-Stat5 (lower panel). WB indicates Western blot; PY, .
PNU156804 inhibits IL-2–inducible Tyr phosphorylation of Stat5a/b in human T cells.
PHA-activated human T cells were pretreated with DMSO, 10 μM in active analogue PNU159744, or 10 μM PNU156804 for 16 hours and then stimulated for 10 minutes with (+) or without (−) 100 nM IL-2 for 10 minutes at 37°C. Cells were lysed and immunoprecipitated with anti-Stat5a (A) or anti-Stat5b (B) and were blotted with monoclonal antiphosphotyrosine Stat5 (Y701, upper panel), polyclonal phosphoserine Stat5 (S726-Stat5a or S731 Stat5b, middle panel), and reblotted with monoclonal pan-Stat5 (lower panel). WB indicates Western blot; PY, .
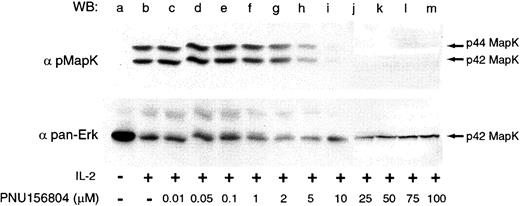
PNU156804 inhibits Erk1/2 Tyr/Thr phosphorylation
IL-2 potently activates the Shc/Ras/Raf/MAPK pathway by the adapter protein SHC, which binds to Tyr338 of the IL-2Rβ chain ultimately to drive T-cell proliferation.48 49 To investigate whether PNU156804 disrupts this signaling pathway, PHA-activated T cells were treated with vehicle alone (Figure4, lanes a-b) or with ascending PNU156804 concentrations. Total cell lysates separated on 10% SDS-PAGE were blotted with phospho-Erk1/2 pAb that recognize activated Thr202 and Tyr204 sites on both enzymes. As shown in the representative experiment, Erk1 and Erk2 were completely inhibited at 10 μM PNU156804 (lane i). Two additional experiments confirmed the loss of active Erk1/2 proteins observed at 10 to 20 μM PNU156804 concentrations. Immunoblotting with a pan-Erk1/2 (indicated beneath phosphorylation blots) verified equivalent loading, though with only weak p44 for Erk1. These data support the conclusion that the inhibition of Jak3 by PNU156804 disrupts Erk1/2 (p44/42) activity in IL-2–mediated signaling pathways.
PNU156804 disrupts IL-2–mediated p44/42 ERK1/2 phosphorylation.
Quiescent PHA-activated T cells were treated with DMSO (control; lanes a-b) or increasing concentrations of PNU156804 for 16 hours and were stimulated in the presence of 100 nM IL-2 at 37°C for 10 minutes. Cells were lysed, and total cell lysate was separated on 10% SDS-PAGE, transferred to PVDF membrane, Western blotted with antiphospho–p44/42 Erk1/2 (upper panel), stripped, and reprobed with pan-Erk antibody (lower panel). Arrows indicate the location of p44/42 Erk1/2.
PNU156804 disrupts IL-2–mediated p44/42 ERK1/2 phosphorylation.
Quiescent PHA-activated T cells were treated with DMSO (control; lanes a-b) or increasing concentrations of PNU156804 for 16 hours and were stimulated in the presence of 100 nM IL-2 at 37°C for 10 minutes. Cells were lysed, and total cell lysate was separated on 10% SDS-PAGE, transferred to PVDF membrane, Western blotted with antiphospho–p44/42 Erk1/2 (upper panel), stripped, and reprobed with pan-Erk antibody (lower panel). Arrows indicate the location of p44/42 Erk1/2.
PNU156804 preferentially inhibits Jak3- rather than Jak2-dependent cell proliferation
Because Jak3 is recruited by IL-2 and other TCGFs,6we tested the effect of PNU156804 on PHA-activated human T cells stimulated with either 1 nM IL-2, -4, -7, or -15 (Figure5). [3H]-thymidine incorporation, plotted as percentage inhibition of total incorporated radiolabel versus increasing concentrations of PNU156804, displayed similar efficacy in disrupting T-cell growth stimulated by various TCGFs (Figure 5A). Thus, γc–Jak-dependent T-cell proliferation is equally inhibited in response to IL-2, -4, -7, or -15.
PNU156804 selectively inhibits Jak3-γc-cytokine–mediated cell proliferation compared with Jak2 growth factors.
(A) Proliferation of quiescent PHA-activated human T cells (5.0 × 104 cells/well) were cultured in the absence or presence of 1 nM human IL-2 (●), IL-4 (○), IL-7 (▾), or IL-15 (▿) with increasing concentrations of PNU156804 (ordinate) for 16 hours at 37°C. Cells were then pulsed with [3H]-thymidine (0.5 μCi [0.0185 MBq]/200 μL) for 4 hours, and the incorporated radiolabeled probe plotted on the abscissa was expressed as percentage inhibition of total cpm from DMSO-treated sample sets (n = 6). (B) Quiescent rat T cells (5.0 × 104 cells/well) were cultured in the presence of the Jak2 activator (1 nM PRL [○]) or Jak3 activator (1 nM IL-2 [●]), with increasing concentrations of PNU156804 (ordinate, 0-100 μM) for 16 hours at 37°C. Cells were pulsed with [3H]-thymidine (0.5 μCi [0.0185 MBq]/200 μL) during the final 4 hours of the assay, and the DNA-incorporated radiolabeled probe was plotted on the abscissa and expressed as percentage inhibition of total cpm from DMSO-treated sample sets (n = 6). (inset) Antiphosphotyrosine immunoblot of Jak2-Jak3–activated signaling pathway. Nb2-11c cells treated with PNU156804 or DMSO (as described in Figure 1A) were stimulated with 100 nM IL-2 (lanes a-f) or 100 nM PRL (lanes g-l). Jak3 or Jak2 (upper panel), Stat5a (middle panel), and Stat5b (lower panel) were immunoprecipitated from lysates, separated by SDS-PAGE, transferred to PVDF membrane, and blotted with specific antiphosphotyrosine mAb.
PNU156804 selectively inhibits Jak3-γc-cytokine–mediated cell proliferation compared with Jak2 growth factors.
(A) Proliferation of quiescent PHA-activated human T cells (5.0 × 104 cells/well) were cultured in the absence or presence of 1 nM human IL-2 (●), IL-4 (○), IL-7 (▾), or IL-15 (▿) with increasing concentrations of PNU156804 (ordinate) for 16 hours at 37°C. Cells were then pulsed with [3H]-thymidine (0.5 μCi [0.0185 MBq]/200 μL) for 4 hours, and the incorporated radiolabeled probe plotted on the abscissa was expressed as percentage inhibition of total cpm from DMSO-treated sample sets (n = 6). (B) Quiescent rat T cells (5.0 × 104 cells/well) were cultured in the presence of the Jak2 activator (1 nM PRL [○]) or Jak3 activator (1 nM IL-2 [●]), with increasing concentrations of PNU156804 (ordinate, 0-100 μM) for 16 hours at 37°C. Cells were pulsed with [3H]-thymidine (0.5 μCi [0.0185 MBq]/200 μL) during the final 4 hours of the assay, and the DNA-incorporated radiolabeled probe was plotted on the abscissa and expressed as percentage inhibition of total cpm from DMSO-treated sample sets (n = 6). (inset) Antiphosphotyrosine immunoblot of Jak2-Jak3–activated signaling pathway. Nb2-11c cells treated with PNU156804 or DMSO (as described in Figure 1A) were stimulated with 100 nM IL-2 (lanes a-f) or 100 nM PRL (lanes g-l). Jak3 or Jak2 (upper panel), Stat5a (middle panel), and Stat5b (lower panel) were immunoprecipitated from lysates, separated by SDS-PAGE, transferred to PVDF membrane, and blotted with specific antiphosphotyrosine mAb.
To test the selectivity of the drug, PNU156804 was added to T cells undergoing Jak3-versus Jak2-dependent proliferation. For this assay, the rat Nb2-11c cell line was chosen because it responds to either PRL (Jak2) or IL-2 (Jak3) stimulation.50 As depicted in Figure5B, Nb2-11c cells treated with ascending concentrations of PNU156804 showed nearly 2-fold greater inhibition for the IL-2–Jak3-dependent pathway than for the PRL-Jak2–dependent pathway. Moreover, though 20 μM PNU156804 reduced cell proliferation to basal levels (≈20% of total [3H]-thymidine incorporation), PRL-mediated growth was retained by 2-fold or inhibited by less than 50% (Figure 5B). Using the same Nb2-11c cells, we compared the inhibitory effect of PNU156804 on Jak3- versus Jak2-mediated Stat5a/b activation. Nb2-11c cells treated with ascending concentrations of PNU156804 (as described in Figure 1) were stimulated with either IL-2 to activate Jak3 (lanes a-f) or PRL to activate Jak2 (lanes g-l). As shown by Western blot, the Tyr phosphorylation patterns of signaling proteins closely paralleled their proliferative response (Figure 5B; insert). Indeed, PNU156804 inhibited IL-2–mediated Tyr phosphorylation of Jak3, Stat5a, and Stat5b (maximum at 10 μM), whereas PRL activation of Jak2, Stat5a, and Stat5b showed minimal inhibition even at 25 μM (lane l). These experiments document that PNU156804 disrupts Jak3-dependent signaling molecules and proliferation driven by TCGFs in a similar fashion that is at least twice as efficacious as its effect on the Jak2-dependent signaling pathway(s).
In vivo effect of PNU156804 alone or in combination with CsA or RAPA on heart allograft survival
We also examined whether the Jak3 inhibitor PNU156804 displays immunosuppressive activity in vivo. As shown in Figure6, WF (RT1u) recipients of BUF (RT1b) heart allografts oral gavaged on alternate days for 14 days with DMSO alone showed a mean survival time of 6.3 ± 0.5 days (Figure 6). Alternate-day oral gavage of ascending doses of PNU156804 (40, 80, or 120 mg/kg) significantly extended heart allograft survival in a dose-dependent fashion (all P < .01). The signal 1 inhibitor CsA, delivered daily alone by oral gavage for 14 days (1.25, 2.5, or 5.0 mg/kg), produced in vivo effects similar to those produced by PNU156804. However, combinations of PNU156804 and CsA administered at ratios ranging from 64:1 to 1:1 exhibited much better effects than either drug alone, with many transplanted hearts surviving more than 100 days (Figure 6). To determine the quality of interaction between PNU156804 and CsA, results were evaluated by the median effect analysis to calculate CI values. Although all 2-drug ratios tested were synergistic (CI, 0.2-0.8), optimal results were produced by the PNU156804-CsA ratio of 4:1 (CI, 0.22).
PNU156804 prolongs the survival of heart allografts alone and acts synergistically with CsA but not RAPA.
WF (donor, RT11) to BUF (recipient, RT1a) rats received alternate-day oral gavage for 14 days (6 total) of 40, 80, or 120 mg/kg PNU156804 alone or in combination with CsA at ratios of 1:1 to 1:64 or in combination with RAPA at ratios of 40:1 to 160:1. Some recipients received various amounts of CsA or RAPA, alone as indicated within the table. Graft survival was evaluated daily; the last day of a heartbeat was considered the day of rejection. CI values were calculated by the median effect analysis: CI < 1 showed synergistic interactions, CI > 1 showed antagonistic interactions, and CI = 1 showed additive interactions. See “Materials and methods” for details.
PNU156804 prolongs the survival of heart allografts alone and acts synergistically with CsA but not RAPA.
WF (donor, RT11) to BUF (recipient, RT1a) rats received alternate-day oral gavage for 14 days (6 total) of 40, 80, or 120 mg/kg PNU156804 alone or in combination with CsA at ratios of 1:1 to 1:64 or in combination with RAPA at ratios of 40:1 to 160:1. Some recipients received various amounts of CsA or RAPA, alone as indicated within the table. Graft survival was evaluated daily; the last day of a heartbeat was considered the day of rejection. CI values were calculated by the median effect analysis: CI < 1 showed synergistic interactions, CI > 1 showed antagonistic interactions, and CI = 1 showed additive interactions. See “Materials and methods” for details.
Next, we examined the interaction of PNU156804 with another signal 3 inhibitor, RAPA, which targets the 256-kd Ser/Thr kinase and regulator of protein translation, mTOR. RAPA given alone daily by oral gavage for 7 days (0.25, 0.5, or 1.0 mg/kg) resulted in modest, albeit significant, prolongation of heart allograft survival. Combinations of PNU156804 and RAPA produced only additive effects (CI, 0.9-1.0). These results showed that selective inhibition of the Jak3-dependent signal 3 pathway blocks allograft rejection. This effect is synergistic with a signal 1 inhibitor but only additive with another signal 3 inhibitor.
Inhibition of Jak3 blocks graft damage and reduces leukocyte cell infiltration
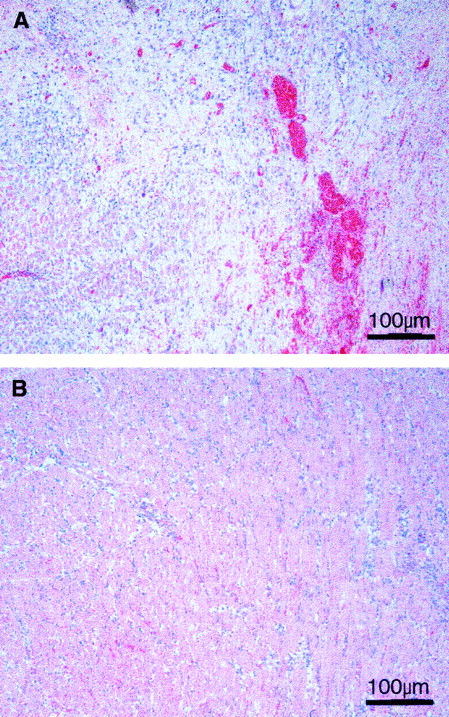
For histologic examination, heart allografts were obtained from recipients that had received alternate-day oral gavage of DMSO alone or DMSO combined with 80 mg/kg PNU156804. Each heart was uniformly cut horizontally, and 12 hematoxylin-eosin–stained sections per heart were scored as described in “Materials and methods.” Untreated heart allografts examined on day 7 after grafting displayed maximum grade 4 damage with extensive myocardial necrosis involving more than 50% of the complete cross-section. In the areas of necrosis, the myocardial fibers were totally destroyed up to the epicardium. The remaining areas of the samples without necrosis showed intense infiltration with polynuclear and mononuclear cells (Figure7A). In contrast, heart allografts from recipients treated with PNU156804 showed only grade 1B changes with mild infiltration of epicardium, myocardium, and endocardium, but without any evidence of myocyte damage (Figure 7B). Given that similar changes were observed on all sections in each group, we conclude that selective inhibition of Jak3 blocks graft damage and reduces infiltration of leukocytes.
PNU156804 blocks allograft damage to myocytes and reduces infiltration of polymorphonuclear cells.
BUF heart allografts were harvested and examined on day after grafting from WF recipients that received alternate-day oral gavage of DMSO vehicle alone (A) or vehicle with 80 mg/kg PNU156804 (B). The presented sections were obtained after uniform histologic analysis of 3 hearts for each experimental group, with 12 horizontal cuts made on each heart. Each section was scored using the Society of Heart and Lung Transplantation system. See “Materials and methods” for details. Original magnification, × 200.
PNU156804 blocks allograft damage to myocytes and reduces infiltration of polymorphonuclear cells.
BUF heart allografts were harvested and examined on day after grafting from WF recipients that received alternate-day oral gavage of DMSO vehicle alone (A) or vehicle with 80 mg/kg PNU156804 (B). The presented sections were obtained after uniform histologic analysis of 3 hearts for each experimental group, with 12 horizontal cuts made on each heart. Each section was scored using the Society of Heart and Lung Transplantation system. See “Materials and methods” for details. Original magnification, × 200.
Discussion
The current results demonstrate that PNU156804 inhibits TCGF-induced T-cell growth (IC50, approximately 7.5 μM; Figure 1A) by the disruption of Jak3 autokinase activity (Figure 2B-C). Consequently, PNU156804 (but not the inactive control compound PNU159744) blocks the activation of Jak3 substrates, namely, Stat5a and Stat5b, as assessed by phosphotyrosine and phosphoserine Western blots (Figure 3). In fact, PNU156804 completely disrupts not only Jak3-dependent downstream activation of Stat5a and Stat5b Ser kinases (Figure 3), it also disrupts the downstream Ser/Thr kinases, p44/Erk1 and p42/Erk2 (Figure 4). Although the inhibitory activity of PNU156804 was equally effective in blocking Jak3-driven T-cell proliferation by either IL-2, -4, -7, or -15 (Figure 5A), the drug was 2-fold less efficient in inhibiting growth by PRL through a closely homologous Tyr kinase, Jak2 (Figure 5B). Last, PNU156804 alone significantly extends cardiac allograft survival and acts synergistically in combination with CsA (CI, 0.2-0.8) and additively in combination with RAPA (CI, 1.0; Figure 6). Thus, PNU156804 represents a selective Jak3 inhibitor with sufficient potency to block allograft rejection.
Because presently used clinical immunosuppressants act in a ubiquitous fashion, they produce potent side effects. For example, an active metabolite of 6-mercaptopurine, 6-thioinosinic acid incorporates into nucleic acids causing DNA and RNA breakage in many cells, thereby causing severe bone marrow depression.51,52 Although glucocorticoids inhibit the production of many cytokines within different cell types, they produce multiorgan side effects, such as Cushingoid features, growth retardation, poor wound healing, and many others deleterious effects.52 CsA or FK506 can disrupt the Ser-Thr phosphatase activity of CaN in several non–T-cell types, thereby contributing to nephrotoxicity and neurotoxicity.53 Moreover, mTOR inhibition of RAPA ablates not only cytokine-mediated growth of T and B cells but many other cells, which can result in myelosuppression and hyperlipidemias.18,19,21 22 Given that the fundamental problem for all available immunosuppressants is the ubiquitous distribution of their targets, we must seek highly specific agents that inactivate molecules uniquely expressed in resting T cells (eg, Zap70) or in activated T and B cells (eg, Jak3).
Several recent studies have revealed that the Jak3 Tyr kinase enzyme is an essential signaling intermediate for the development and function of T and B cells and of NK cells.23,24 Indeed, the retroviral introduction of Jak3 enzyme into Jak3-deficient mice restores normal T-cell development.54 Although understanding of the signaling pathways activated by Jak3 (directly or indirectly) is incomplete, Jak3 signaling by Stat5a/b is necessary to regulate genes required for cellular proliferation.26 As shown here, PNU156804 abolishes IL-2–dependent T-cell proliferation by the inhibition of Jak3-mediated autokinase activity and Stat5a/b Ser/Tyr phosphorylation. Consequently, PNU156804 prevents Stat5a/b dimerization by their SH2 domains and by Stat5a/b translocation to the nucleus. Given the limited pattern of Jak3 expression, the γc-Jak3-Stat5 pathway is likely to represent a convergence point by which TCGFs drive T-cell clonal expansion, thereby making it a preferred pathway for novel and selective immunosuppression.
We have recently reported that AG-490 blocks T-cell proliferation by the inhibition of Jak3 autokinase activity and Stat5a/b Tyr phosphorylation.29,55 However, AG-490 is equally potent in inhibiting Jak2 activation, including eosinophils stimulated by granulocyte-macrophage colony-stimulating factor and in vascular smooth muscle cells and cardiac myocytes activated by angiotensin 2.56-58 Our most recent study showed that AG-490 inhibits IL-2–dependent proliferation of PHA-stimulated human T cells with an IC50 of approximately 20 μM.59 In contrast, PNU156804 is at least twice as effective in inhibiting human T cells when performed under identical experimental conditions by displaying an IC50 of approximately 7.5 μM for IL-2 and other T-cell growth factors that activate the Jak3 cascade. Furthermore, PNU156804 shows 2-fold greater specificity to block Jak3-dependent T-cell proliferation in response to IL-2 compared to Jak2-dependent cell proliferation (Figure 5). Whether in vivo comparison with both drugs will support the hypothesis that PNU156804 is more efficacious remains to be determined.
In addition to Tyr phosphorylation, Stats undergo Ser phosphorylation on a conserved Ser residue that plays a critical role in cytokine-induced nuclear translocalization, maximal gene transcription, and cell cycle progression.43-45,60-62 Earlier evidence has been presented that shows minichromosome maintenance-5 protein is required for full DNA replication by binding to phosphorylated Ser727 on Stat1α located within the transactivation domain.45 Interestingly, blockade of Jak3 by PNU156804 inhibits the phosphorylation of a similarly conserved proline-Ser-proline (Pro-Ser-Pro) motif in Stat5a and Stat5b. In contrast to the conserved Ser in Stat1, Stat3, and Stat4, which is located within a Pro-X-Ser-Pro motif and MAPK consensus phosphorylation site,25 the Ser phosphorylation site of Stat5a/b lies within a Pro-Ser-Pro motif lacking the invariant amino acid. This evidence suggests that Stat5a/b Ser kinase represents a unique molecular target for regulating T-cell activity to produce selective immunosuppressive effects.
A recently published report found that PNU156804 inhibits IL-2–induced proliferation of human T cells without affecting the expression of IL-2Rα and γc chains.28 As shown herein, PNU156804 disrupts not only IL-2–dependent T-cell proliferation by selective inhibition of the γc-Jak3-Stat5a/b–dependent signaling pathway, it also disrupts IL-4–, -7–, and -15–dependent T-cell proliferation. Although PNU156804 diminished B-cell proliferation in response to killed Staphylococcus aureusantigen, however, it also inhibited CD40-triggered activation of NF-κB.28 These latter results suggest a role for Jak3 Tyr kinase activity in the CD40-initiated signaling pathway. Indeed, it is readily established that CD40 signaling is critical for B-cell growth, survival, differentiation, and immunoglobulin class switching,63 yet the CD40-CD40 ligand complex is not thought to recruit γc. Jak3 and Stat3 can be directly activated by the CD40 receptor, activation events necessary for the subsequent expression of CD23, intercellular adhesion molecule-1, and lymphotoxin-α genes and for the production of immunoglobulin E.64,65 Another study reported that CD40 receptor activation by the CD40 ligand could also activate Jak3 and Stat5a in monocytes but not in B cells.66 It seems plausible to expect that PNU156804 may prove useful in determining whether Jak3 is an active participant in the CD40 signaling pathway.
Our current findings document that the blockade of Jak3 in vivo prevents allograft rejection (Figure 6). Furthermore, histologic examination of allografts from PNU156804-treated hosts showed reduced intragraft cellular infiltration of mononuclear cells without myocyte damage (Figure 7). Lack of clonal proliferation might limit the generation of a sufficient number of effector T cells—cytotoxic T cells and delayed-type hypersensitivity T cells—required to mediate allograft destruction. Whether the continuous delivery of PNU156804 would inhibit the CD40-Jak3 activation pathway, reducing B-cell responsiveness required for antibody synthesis, remains to be determined. Our in vivo results do, however, document for the first time that selective inhibition of the Jak3-Stat5 pathway, compared with Jak2-Stat5, is sufficient to block allograft rejection.
The combination of PNU156804 and CsA results in potent synergistic interaction (CI, 0.2-0.8), extending allograft survival beyond that produced by monotherapy with each drug alone (Figure 6). Similarly, CsA and RAPA act synergistically to prolong organ allograft survival in animal models17 and in humans.67 Rats treated intravenously with combinations of RAPA (0.04-0.8 mg/kg per day) and of CsA (0.5-2.0 mg/kg per day) displayed potent synergistic interactions on heart and kidney allograft survival, as documented by CI values of 0.001 to 0.2.67 We have shown in this model that even subtherapeutic CsA doses reduced the expression of IL-2 mRNA at the graft site, thereby facilitating inhibition by RAPA of IL-2–dependent growth T cells and producing synergistic interaction. A similar mechanism might explain the synergistic effects of CsA in combination with PNU156804 produced at all tested PNU156804-CsA ratios (1:1-64:1), as documented by CI values of 0.2 to 0.8. Optimal synergism was produced by a 4:1 PNU156804-CsA ratio (CI, 0.22). Oral delivery of CsA and RAPA resulted in synergism with an almost identical range of CI values between 0.1 and 0.6.18
In contradistinction, a combination of PNU156804 with RAPA yielded an additive effect (CI, 1). These results suggest that the sequential inhibition of signal 1 by CsA, followed by the inhibition of signal 3 by PNU156804 or RAPA, produces synergism, whereas the concomitant inhibition of signal 3 by PNU156804 and RAPA results only in an additive immunosuppressive effect. However, given that RAPA produces myelosuppressive and lipotoxic side effects because of the role of mTOR in non–cytokine-activated pathways,22 we postulate that a combination of CsA and PNU156804 would produce similarly potent immunosuppression without myelosuppressive and lipotoxic side effects. We propose that because mTOR lies downstream of Jak3 in T, B, and NK cells, TCGF signaling pathways could be selectively inhibited by PNU156804 without affecting mTOR signaling pathways in non–Jak3-expressing cell types.
In conclusion, PNU156804 preferentially disrupts Jak3 (compared with Jak2 autokinase activity), thereby selectively inhibiting γc-driven T-cell clonal expansion. Blockade of Jak3 should produce no adverse effects currently associated with RAPA. Moreover, synergy between CsA (blocking G0-G1transition) and PNU156804 (blocking G1-S progression) proffers a novel strategy for immunosuppression by blocking sequential activation signals.
We thank Natasha M. Teixeira, Dwavalon Young, and Scott Homes for skilled preparation of the manuscript and the figures.
Supported by grants from the National Institute of Diabetes and Digestive Kidney Diseases (NIDDK 38016-12), the National Institutes of Heart and Lung Diseases (NHL69723), and the Roche Foundation (862506002).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert A. Kirken, Department of Integrative Biology and Pharmacology, University of Texas Health Science Center at Houston, 6431 Fannin, MSB Rm 4.218, Houston, TX 77030; e-mail:robert.a.kirken@uth.tmc.edu.

![Fig. 1. PNU156804 disrupts cell growth of activated, but not of unactivated, human T cells in a dose-dependent manner. / Proliferation of quiescent PHA-activated human T cells (5.0 × 104 cells/well) in the presence of 1 nM IL-2 was examined after treatment with increasing concentrations of PNU156804 (●) or inactive control PNU159744 (♦) for 16 hours at 37°C. Conversely, Jurkat cells were treated in an identical fashion with PNU156804 (▴). All cells were then pulsed with [3H]-thymidine (0.5 μCi [0.0185 MBq]/200 μL) for 4 hours, incorporated into a radiolabeled probe, and plotted on the abscissa expressed as total cpm (n = 6).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/2/10.1182_blood.v99.2.680/6/m_h80222002001.jpeg?Expires=1769277378&Signature=rWS66pkJWo4lOvgO48pXjBnlapAlFUxtC1ybpr6zY9oZXnbJ4ZxTEHxMDfhMkLPJ5LDMuEUrLrfAXF7V~YS~cWdRfFsB53~5tLPjxJtjPdfbVMjzj2vVtKBKlaenyaQ~uJCNzvZkG0QXpyg~Q3APrC1AVsMB4K~u0nCewYpRMaAxrmocRK-MJcdebaMLlSDEvzNmJTxwDnQ5sN6vAbOX079HuuzkMbf~RRYzrRysgF7OuC0FPMURtxnLlAJ60bCxLmeokK2N9B4QkWIPQmavqjkElAYZbrIHfpNWYc06ddyVRPdYxoeZBknEkfmVSBeLW8Jv1acVb1YGqwMjQFpXKQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. PNU156804 selectively inhibits Jak3-γc-cytokine–mediated cell proliferation compared with Jak2 growth factors. / (A) Proliferation of quiescent PHA-activated human T cells (5.0 × 104 cells/well) were cultured in the absence or presence of 1 nM human IL-2 (●), IL-4 (○), IL-7 (▾), or IL-15 (▿) with increasing concentrations of PNU156804 (ordinate) for 16 hours at 37°C. Cells were then pulsed with [3H]-thymidine (0.5 μCi [0.0185 MBq]/200 μL) for 4 hours, and the incorporated radiolabeled probe plotted on the abscissa was expressed as percentage inhibition of total cpm from DMSO-treated sample sets (n = 6). (B) Quiescent rat T cells (5.0 × 104 cells/well) were cultured in the presence of the Jak2 activator (1 nM PRL [○]) or Jak3 activator (1 nM IL-2 [●]), with increasing concentrations of PNU156804 (ordinate, 0-100 μM) for 16 hours at 37°C. Cells were pulsed with [3H]-thymidine (0.5 μCi [0.0185 MBq]/200 μL) during the final 4 hours of the assay, and the DNA-incorporated radiolabeled probe was plotted on the abscissa and expressed as percentage inhibition of total cpm from DMSO-treated sample sets (n = 6). (inset) Antiphosphotyrosine immunoblot of Jak2-Jak3–activated signaling pathway. Nb2-11c cells treated with PNU156804 or DMSO (as described in Figure 1A) were stimulated with 100 nM IL-2 (lanes a-f) or 100 nM PRL (lanes g-l). Jak3 or Jak2 (upper panel), Stat5a (middle panel), and Stat5b (lower panel) were immunoprecipitated from lysates, separated by SDS-PAGE, transferred to PVDF membrane, and blotted with specific antiphosphotyrosine mAb.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/2/10.1182_blood.v99.2.680/6/m_h80222002005.jpeg?Expires=1769277378&Signature=4rC0NSmLFn96cA~pTmUs2Bxp92wuYKRxUI9iN0QeVpSKqoga41WqmI8x0l9uTIWdwLnHVRB77BBad~ORbcDtcVFZhOLFxruuZpTxGsjt8BIKJFV9Xxl4fwCeCK4ttOFW1ahSxNFQMBhLRosLQhVdtJUkYMbFIROa2tbxwz12C9qSAnuHMJ8X2YBlXZy2MdIWIZm4M4Q5-ee8uOeL-W-5nk1tGDEul4rfEXVkRHZVPBj~KdUz0CZc0fol8xBP9K2-Wb24dTQLtOzFhHAsQQ6ZlVtgLzlFxu19WfnlI5L9WbcbutAQHmf~Urp9PvsTEncyphY-AFpiKSMlmRJClDVz3g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)