The association of hepatitis viruses with non-Hodgkin lymphomas (NHL) is not rare. Several authors have reported an exceeding prevalence of hepatitis C virus (HCV)1-3 or of hepatitis B virus (HBV)4,5 infection in patients affected by NHL. A sustained increase of alanine aminotransferase (ALT) associated with high levels of HBV viremia (HBV-DNA) 1 to 2 months after the suspension of chemotherapy has been described in patients suffering from NHL and infected by HBV.7,8 Recently, a nucleotide analogue (lamivudine) was shown to be beneficial in HBV-infected patients with signs of active replication.6 The aim of this study was to investigate the role of lamivudine to treat or to prevent hepatitis reactivation in HBV-infected subjects suffering from NHL and undergoing chemotherapy.
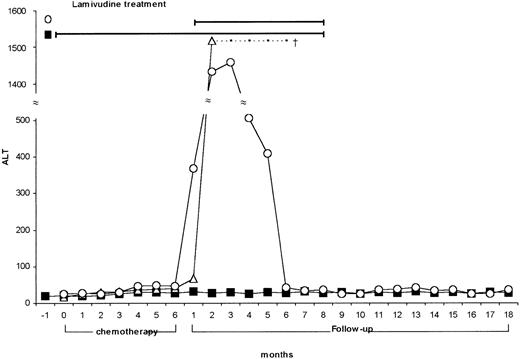
We screened 550 patients (271 males and 279 females) affected by NHL, ranging in age from 38 to 67 years (median 52 years), and 148 control subjects suffering from metabolic disorders, attending our institutions during the last 10 years. Of 550 NHL patients, 108 had signs of HBV contact (19%) and 21 were HBsAg-positive (3.5%), while 86 (15.6%) were HCV-Ab positive with detectable HCV-RNA in their serum (Table1). HCV prevalence in NHL was higher than in the control group (15.6% vs 2.02% P < .000), while HBsAg-positive prevalence was not significantly different (3.5% vs 1.3%; ns). No age or sex distribution differences were found in the various groups (Table 1). NHL patients were treated according to different therapeutic protocols, which were chosen on the basis of the specific hystological type and clinical stage of the disease. Evaluation and follow-up of the patients included medical history, physical examination, complete blood count, ALT, and markers of viral hepatitis. Among the 21 HBV-positive NHL patients, 12 had hepatitis reactivation soon after chemotherapy. Of them, 9 were treated with lamivudine (Zeffix; Glaxo-Wellcome 100 mg/die), and 3 were not (prelamivudine era). All patients treated recovered from the viral reactivation and are still followed up, whereas the 3 patients who did not receive lamivudine died of acute liver failure. In 3 patients lamivudine was given prophylactically during and after chemotherapy, and the treatment was discontinued 2 months after the last chemotherapy course. These 3 patients did not show any sign of acute B hepatitis recurrence (Figure 1). These data confirm the efficacy of lamivudine, as it has been recently reported in the literature.9-11 It is noteworthy that, at variance with “spontaneous” HBV reactivation in carriers of chronic hepatitis, which may relapse at lamivudine interruption, the halting of viral replication in NHL HBV–infected subjects persists even after lamivudine discontinuation. A possible explanation for such a different behavior is that viral replication in NHL patients is caused by the immunosuppressive activity of chemotherapy; the replication is followed by liver attack at postchemotherapy immunological recovery, leading to acute or even fulminant hepatitis. The use of lamivudine, by blocking B virus replication, interrupts the sequence of events; then, the drug is no more needed when the recovered immune surveillance re-establishes tolerance to a small viral load. In our series of NHL patients, while a high percentage of HBsAg-positive cases (12/21 = 57%) had signs of viral reactivation, no HCV-infected patient (n = 86) had ALT increase during or after chemotherapy. This seems to support the idea that the immune system might play a different role in the pathogenic mechanism(s) underlying HCV- and HBV-related chronicity.
Epidemiological characteristics of NHL patients and control subjects
| . | NHL . | Controls . | ||||
|---|---|---|---|---|---|---|
| Total . | HBV . | HCV . | Total . | HBV . | HCV . | |
| Patients/subjects | 550 | 21 (3.8%) | 86 (15.6%) | 148 | 2 (1.3%) | 3 (2.02%) |
| Age (y) | ||||||
| Median | 52 | 45 | 58 | 56 | 51 | 63 |
| (range) | (38-67) | (38-64) | (42-67) | (44-72) | (44-58) | (49-72) |
| Sex (m/f) | 271/279 | 11/10 | 35/51 | 78/70 | 2/0 | 2/1 |
| HBeAg/HBeAb | 0/21 | 0/21 | 0/1 | 0/2 | 0/2 | — |
| HBcAb | 108 | 21 | 26 | 13 | 2 | — |
| HBsAg | 21 | 21 | 1 | 2 | 2 | — |
| HBV-DNA+/HBV-DNA−* | — | 0/21 | 0/1 | 0/2 | 0/2 | — |
| HBV-DNA+/HBV-DNA−† | — | 15/0* | 1/0 | — | — | — |
| HCV-Ab/HCV-RNA | 86/86 | 1/1 | 86/86 | 3/3 | 0 | 3/3 |
| ALT* mean (SD) | 35.73 ± 21.3 | 22.27 ± 9.4 | 25.68 ± 12.4 | 33.4 ± 15.7 | 66 ± 16.9 | 62 ± 18.5 |
| ALT† mean (SD) | 31.67 ± 28.5 | 1051.28 ± 898.5‡ | 32.8 ± 28.7 | — | — | — |
| . | NHL . | Controls . | ||||
|---|---|---|---|---|---|---|
| Total . | HBV . | HCV . | Total . | HBV . | HCV . | |
| Patients/subjects | 550 | 21 (3.8%) | 86 (15.6%) | 148 | 2 (1.3%) | 3 (2.02%) |
| Age (y) | ||||||
| Median | 52 | 45 | 58 | 56 | 51 | 63 |
| (range) | (38-67) | (38-64) | (42-67) | (44-72) | (44-58) | (49-72) |
| Sex (m/f) | 271/279 | 11/10 | 35/51 | 78/70 | 2/0 | 2/1 |
| HBeAg/HBeAb | 0/21 | 0/21 | 0/1 | 0/2 | 0/2 | — |
| HBcAb | 108 | 21 | 26 | 13 | 2 | — |
| HBsAg | 21 | 21 | 1 | 2 | 2 | — |
| HBV-DNA+/HBV-DNA−* | — | 0/21 | 0/1 | 0/2 | 0/2 | — |
| HBV-DNA+/HBV-DNA−† | — | 15/0* | 1/0 | — | — | — |
| HCV-Ab/HCV-RNA | 86/86 | 1/1 | 86/86 | 3/3 | 0 | 3/3 |
| ALT* mean (SD) | 35.73 ± 21.3 | 22.27 ± 9.4 | 25.68 ± 12.4 | 33.4 ± 15.7 | 66 ± 16.9 | 62 ± 18.5 |
| ALT† mean (SD) | 31.67 ± 28.5 | 1051.28 ± 898.5‡ | 32.8 ± 28.7 | — | — | — |
Before chemotherapy.
1 to 2 months after chemotherapy.
Data on 15 patients, 6 patients not collectable.
Lamivudine nontreated, treated, and pretreated NHL patients.
ALT values of HBV-infected NHL patients nontreated (Α) (n = 3), treated with lamivudine after chemotherapy (○) (n = 9), and pretreated with lamivudine (during and after chemotherapy) (▧) (n = 3).
Lamivudine nontreated, treated, and pretreated NHL patients.
ALT values of HBV-infected NHL patients nontreated (Α) (n = 3), treated with lamivudine after chemotherapy (○) (n = 9), and pretreated with lamivudine (during and after chemotherapy) (▧) (n = 3).
In conclusion, our study, while confirming the high prevalence of HCV infection in NHL patients in specific geographic areas, points out that HCV reactivation after chemotherapy is not a clinical problem. By contrast, life expectancy in carriers of hepatitis B virus may be negatively influenced by the viral infection. Lamivudine has proven to be a useful drug to prevent or treat HBV reactivation in patients undergoing chemotherapy for NHL. This study may represent the basis for a prospective multicenter trial aimed to assess lamivudine efficacy in avoiding reactivation of HBV-related hepatitis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal