The antimetastatic effect of the CD1d-binding glycolipid, α-galactosylceramide (α-GalCer), is mediated by NK1.1+T (NKT) cells; however, the mechanisms behind this process are poorly defined. Although it has been shown to involve NK cells and interferon-γ (IFN-γ) production, the way these factors collaborate to mediate effective tumor rejection and the importance of other factors characteristic of NKT cell and NK cell activation are unknown. Using gene-targeted mice and antibody treatments, the critical need for interleukin 12 (IL-12), IFN-γ, and NK cells has been shown in the antimetastatic activity of α-GalCer in the lungs and the liver. By contrast, in lung and liver metastasis models, cytotoxic molecules expressed by NK cells and NKT cells (perforin, Fas ligand, and tumor necrosis factor-related apoptosis-inducing ligand) and an NKT cell-secreted cytokine, IL-4, were not necessary for the antitumor activity of α-GalCer. Like IL-12, IL-18 was required for optimal serum IFN-γ induction and control of lung metastases by α-GalCer. IL-18 was unnecessary for α-GalCer–related suppression of liver metastases. Most importantly, after adoptive transfer of α-GalCer–reactive NKT cells or NK cells into NKT cell-deficient, IFN-γ–deficient, or RAG-1–deficient mice, it was demonstrated that the sequential production of IFN-γ by NKT cells and NK cells was absolutely required to reconstitute the antimetastatic activity of α-GalCer.
Introduction
NK1.1+ T (NKT) cells constitute a distinct subpopulation of mature T cells that in the mouse are characterized by the expression of a single invariant T-cell receptor α (TCRα) chain encoded by Vα14-Jα281, in conjunction with Vβ8.2, Vβ7, or Vβ2 TCR-β chains.1-3 Recent studies have revealed that NKT cells recognize glycolipid antigens presented by the major histocompatibility complex class Ib-like molecule, CD1d.4-7 Disruption of the invariant Vα14-Jα281 TCR results in a selective loss of Vα14 NKT cells, leaving other immune cells intact.8 In response to TCR ligation, NKT cells promptly produce large amounts of proinflammatory T-helper (Th)1 cytokines (eg, interferon [IFN]-γ, tumor necrosis factor [TNF]) and anti-inflammatory Th2 cytokines (eg, interleukin [IL]-4, IL-10, IL-13).4,9-11 NKT cells have also been reported to exhibit direct cytotoxicity against tumor target cells,8,12-14which has made it difficult to predict the consequences of their activation in vivo but nonetheless has caused much speculation that they play a central role in immunoregulation. Accumulated experimental evidence has supported their role in promoting innate antitumor immunity8,14-17 while paradoxically suppressing acquired antitumor immunity18 and autoimmunity19 and maintaining some forms of tolerance.20-22 These results have ensured continued debate and confirmed the central role these cells play in the immune system.23 24
The α-galactosylceramide (α-GalCer) is chemically and functionally analogous to natural glycolipids first purified from marine sponges on the basis of their antitumor properties against B16 melanoma.25,26 α-GalCer is presented by CD1d, leading to specific stimulation of NKT cells.4,10,11,27 The antitumor effect of α-GalCer is observed against various tumor cells of different origins, including melanomas, lung, colon, and renal cell carcinomas, erythroleukemias, and other hematopoietic malignancies.25,26,28-30 In vivo, the antitumor activities of α-GalCer and IL-12 are similar, whereas in vitro it has been demonstrated that the production of IFN-γ by NKT cells in response to α-GalCer requires IL-12 produced by dendritic cells (DCs)31 and direct contact between NKT cells and DCs through CD40–CD40 ligand interactions. Both α-GalCer and low doses of IL-12 are strong inducers of NKT cell activity and will not exert their antitumor activities in the absence of NKT cells.8 15-17 Despite these findings, little is known about the precise sequence of events and factors involved in α-GalCer–induced tumor suppression.
We know that recognition of the α-GalCer–CD1d complex leads to NKT cell activation.10,11,27 This results in rapid production (within hours) of Th1 and Th2 cytokines by NKT cells,32rapid elimination of NKT cells after they produce cytokines,33-36 proliferation and activation of NK cells4,10,32,37 and subsequent IFN-γ production,38 and bystander activation of immune responses mediated by conventional T cells and B cells.32,33,39-43NK cell activation was at least partly dependent on IFN-γ.32,33,39 Although α-GalCer has been shown to induce perforin-dependent cytotoxicity by NKT and NK cells, the significance of this pathway in vivo is less clear. Our previous study30 and more recently that of Hayakawa et al38 have demonstrated that the antimetastatic effect of α-GalCer was impaired in NK cell-depleted or IFN-γ–deficient mice. Collectively, these results indicate an important role for NKT and NK cells and the cytokine IFN-γ after α-GalCer administration. Despite these clues, none of the previous studies have defined the sequence of events after α-GalCer treatment, nor was it known whether IFN-γ production by NKT cells, NK cells, or both was key to its antimetastatic activity. The only means to address these outstanding questions was to use adoptive transfer techniques to examine the role of candidate effector molecules selectively expressed by or absent from either NKT or NK effector cell populations. In so doing, this is the only study to formally show that IFN-γ production by NKT cells and subsequent IFN-γ production by NK cells are critical for α-GalCer–mediated tumor protection.
Materials and methods
Mice
Inbred C57BL/6 and BALB/c wild-type (WT) mice were purchased from The Walter and Eliza Hall Institute of Medical Research (Melbourne, Australia) and Clear Japan (Tokyo, Japan). The following gene-targeted mice were bred at the Peter MacCallum Cancer Institute: C57BL/6 perforin-deficient (B6 pfp−/−) (from Dr Karupiah, John Curtin School of Medical Research, Canberra, Australia)44; C57BL/6 Fas ligand mutant (B6 gld); C57BL/6 TNF-deficient (B6 TNF−/−) (kindly provided by Dr Sedgwick and originally from the Centenary Institute of Cancer Medicine and Cell Biology, Sydney, Australia)45; C57BL/6 IFN-γ–deficient (B6 IFN-γ−/−) (from Genentech, San Francisco, CA)46; C57BL/6 IL-12p40–deficient (B6 IL-12−/−) (from Hoffmann-La Roche, Nutley, NJ)47; C57BL/6 IL-18–deficient (B6 IL-18−/−) (kindly provided by Dr Akira, Osaka University, Japan)48; C57BL/6 RAG-1–deficient (B6 RAG-1−/−) (from Dr Corcoran, The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia); C57BL/6 TCR Jα281-deficient (B6 Jα281−/−) (kindly provided by Dr Taniguchi, Chiba University Graduate School of Medicine, Japan)8,14; C57BL/6 CD1d-deficient (B6 CD1d−/−) (kindly provided by Dr Van Kaer, Vanderbilt University School of Medicine, Nashville, TN)49; BALB/c IFN-γ−/− (from Genentech); BALB/c pfp−/−; and BALB/c CD1d−/− (kindly provided by Dr Van Kaer, Vanderbilt University School of Medicine). All mice originally generated on a 129 background were backcrossed 9 to 20 times onto the C57BL/6 or BALB/c background. Mice of 6 to 12 weeks of age were used in all experiments performed according to animal experimental ethics committee guidelines.
Isolation of liver lymphocyte subsets
Mononuclear cells (MNCs) were prepared from the liver and spleen as previously described.50,51 To avoid the nonspecific binding of antibodies to FcγR, cells were preincubated with antimouse CD16/32 (2.4G2) monoclonal antibody (mAb) before staining. Flow cytometric sorting of cells was performed after staining with fluorescein isothiocyanate–conjugated anti-αβTCR (clone H57-597) and phycoerythrin-conjugated anti-NK1.1 (clone PK-136). All flow cytometry reagents were purchased from PharMingen (San Diego, CA), unless otherwise indicated. Liver NKT cells and liver or spleen NK cells were isolated, gated, analyzed, and sorted as previously described.50 After washing with phosphate-buffered saline twice, the labeled cells were sorted using a dual-laser FACStar Plus (Becton Dickinson, San Jose, CA). Sorting purity was always greater than 95%.
Tumor cell lines
The following standard experimental mouse tumor cell lines were used in vitro and in vivo. B16F10 melanoma (perforin-sensitive, FasL- and TNF-related apoptosis-inducing ligand [TRAIL]-insensitive, H-2b), 3LL Lewis lung carcinoma (perforin- and FasL-sensitive, TRAIL-insensitive, H-2b), RM-1 prostate carcinoma (perforin- and FasL-sensitive, TRAIL-insensitive, H-2b), and DA3 mammary carcinoma (perforin-, TRAIL-, and FasL-sensitive, H-2d). The maintenance of all of these cell lines and their sensitivities to various cytotoxic molecules have been described.17,30 51
Cytotoxicity assay
Cytolytic activity of MNCs from various mice was tested against tumor target cells by a standard 20-hour chromium 51Cr release assay as previously described.30 Hepatic MNC effectors were isolated from the α-GalCer- or vehicle-treated mice 3 hours or 24 hours after injection. In some experiments, the assay was performed in the presence of neutralizing rat anti-mIFN–γ mAb (R4-6A2) (10 μg/mL) or control rat immunoglobulin G1 (IgG1) (10 μg/mL). Recombinant mouse IFN-γ was provided by the Biological Resources Branch (National Cancer Institute, Frederick, MD).
Tumor models in vivo
B16F10, 3LL, RM-1, and DA3 cell lines were inoculated intravenously or intrasplenically, as indicated, at a dose previously shown in WT, gene-targeted, or antibody-treated mice to result in similar numbers of lung or liver metastases, respectively.14,17,51 Effector function was examined in all these models, and transfer experiments were performed in the B16F10 tumor model. For all experimental metastasis models, mice were injected intravenously or intrasplenically with tumor cells and were killed 14 days later, the lungs or livers were removed, and surface metastases were counted with the aid of a dissecting microscope.17 In all metastasis models, the data were recorded as the mean number of metastases ± SEM. Significance was determined by a Mann-WhitneyU rank sum test.
NK cell depletion and cytokine neutralization
NK cells were specifically depleted in B6 mice using 200 μg intraperitoneal rabbit anti-asialoGM1 (anti-asGM1) antibody (Wako Chemicals, Richmond, VA) on days 0, 1, and 7 after tumor inoculation as described.52 Some groups of B6 mice were treated with either rat anti–mouse IFN-γ mAb (500 μg), rat anti–mouse TRAIL (N2B2) mAb (500 μg), rat anti–mouse IL-4 mAb [11B.11, kindly provided by the Biological Resources Branch Repository (NCI)] (1 mg), or control rat IgG1 (1 mg) on days 0, 4, 7, and 10 after tumor inoculation. Protocols that have used similar concentrations of these mAbs and conditions have been shown to effectively inhibit TRAIL, IFN-γ, or IL-4 activity in vivo.30,51 53
α-GalCer treatment protocols
α-GalCer was kindly provided by the Pharmaceutical Research Laboratories (Kirin Brewery, Gumna, Japan) and was prepared as described.31 α-GalCer was suspended in saline supplemented with 0.5% polysorbate-20 (wt/vol), and the control vehicle was saline supplemented with 0.5% polysorbate-20 (wt/vol). Mice received 2 μg α-GalCer intraperitoneally 3 hours after tumor inoculation and liver lymphocyte transfer and on days 4 and 8. This protocol had previously been shown to be therapeutic in the B16F10 and Renca tumor models.30 38 Serum was collected from treated mice as indicated and was measured by a mouse IFN-γ–specific enzyme-linked immunosorbent assay according to the manufacturer's protocol (PharMingen).
Results
NKT and NK cells mediate the antimetastatic effect of α-GalCer
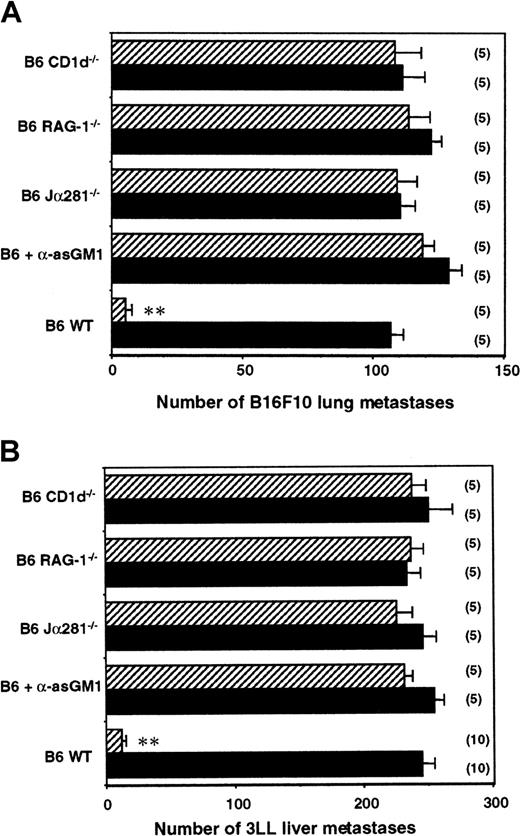
α-GalCer is presented by CD1d and recognized by NKT cells.4,10,11,27 The antimetastatic effect of α-GalCer is observed against a variety of tumor cells, including B16F10 melanoma and 3LL lung carcinoma.15,25 The potent antimetastatic effects of α-GalCer are associated with the proliferation and activation of NK cells in vivo. An inoculum of 5 × 105B16F10 tumor cells metastasized equivalently in B6 WT mice and in various gene-targeted mice (Figure 1A). Therapeutic administration of α-GalCer markedly reduced the numbers of B16F10 lung metastases in B6 WT mice (from 107 ± 5 to 5 ± 2) (Figure 1A). This antimetastatic activity was completely abolished in Jα281−/−, CD1d−/−, and RAG-1−/− mice, all of which lack NKT cells, or by anti-asGM1 antibody in WT mice—specifically depleted of NK cells but not of NKT cells52—indicating that α-GalCer acted through NKT and NK cell effector function. A similar pattern of activity was observed in mice inoculated intrasplenically with 3LL tumor cells in which liver metastasis was also controlled by NKT and NK cells (Figure 1B).
Key role of NKT cells and NK cells in the antimetastatic effect of α-GalCer.
Groups of B6 WT, B6 RAG-1−/−, B6 CD1d−/−, B6 Jα281−/− mice, or B6 mice treated with rabbit anti-asGM1 antibody on days −1, 0 (the day of tumor inoculation), and 7 were inoculated (A) intravenously with 5 × 105 B16F10 tumor cells or (B) intrasplenically with 5 × 105 3LL tumor cells. Some groups of mice were treated intraperitoneally with 2 μg α-GalCer (▨) or vehicle control (▪) on days 0, 4, and 8 after tumor inoculation, as indicated. In all experiments, 14 days after tumor inoculation the lungs (B16F10) or livers (3LL) of these mice were harvested, and tumor colonies were counted and recorded as the mean number of colonies ± SE. The number of mice in each group is indicated in parentheses, and asterisks indicate the groups in which α-GalCer treatment significantly reduced that group's number of lung or liver metastases (Mann-Whitney U: *P < .05; **P < .001).
Key role of NKT cells and NK cells in the antimetastatic effect of α-GalCer.
Groups of B6 WT, B6 RAG-1−/−, B6 CD1d−/−, B6 Jα281−/− mice, or B6 mice treated with rabbit anti-asGM1 antibody on days −1, 0 (the day of tumor inoculation), and 7 were inoculated (A) intravenously with 5 × 105 B16F10 tumor cells or (B) intrasplenically with 5 × 105 3LL tumor cells. Some groups of mice were treated intraperitoneally with 2 μg α-GalCer (▨) or vehicle control (▪) on days 0, 4, and 8 after tumor inoculation, as indicated. In all experiments, 14 days after tumor inoculation the lungs (B16F10) or livers (3LL) of these mice were harvested, and tumor colonies were counted and recorded as the mean number of colonies ± SE. The number of mice in each group is indicated in parentheses, and asterisks indicate the groups in which α-GalCer treatment significantly reduced that group's number of lung or liver metastases (Mann-Whitney U: *P < .05; **P < .001).
Key role of IFN-γ in the antimetastatic effect of α-GalCer
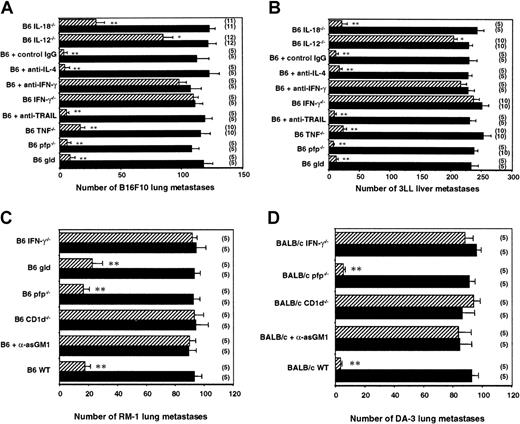
Using gene-targeted mice and neutralizing mAbs, we next examined the relative role of a number of NKT and NK cell molecules in the antimetastatic activity stimulated by α-GalCer. Th1 cytokine IFN-γ was critical to α-GalCer–mediated control of B16F10 lung and 3LL liver metastases (Figure 2A-B). In B6 IFN-γ−/− mice and in B6 WT mice treated with antimouse IFN-γ mAb, α-GalCer was without effect. TNF, another major Th1 cytokine reported to be produced by NKT cells,54 appeared to play a minor role in the antitumor activity of α-GalCer (fewer B16F10 metastases in B6 WT + α-GalCer group than in the B6 TNF−/− + α-GalCer group; P < .05). By contrast, IL-4, the major Th2 cytokine produced by NKT cells, was not required for α-GalCer–mediated antimetastatic function in either model (Figure 2A-B). Because α-GalCer stimulates NKT cell and NK cell cytotoxicity32,33 and each subset can express perforin and FasL,55 we also examined the activity of α-GalCer in perforin-deficient or FasL-mutant mice. Neither cytotoxic pathway was required for α-GalCer–mediated antimetastatic function (Figure 2A-B). Not surprisingly, TRAIL, which has been shown to be expressed on liver NK cells and to be up-regulated after α-GalCer stimulation,30 was not required for α-GalCer–mediated inhibition of TRAIL-resistant B16F10 or 3LL liver metastasis (Figure 2A-B). We have previously demonstrated that TRAIL-sensitive Renca tumor cells were controlled by α-GalCer in an IFN-γ– and a TRAIL-dependent (partially) manner.30 To further explore the importance of IFN-γ in the antimetastatic activity of α-GalCer, we examined 2 other tumor models, RM-1 (FasL sensitive) (Figure 2C) and DA3 (FasL sensitive) tumors (Figure 2D). These data are the first to demonstrate that α-GalCer has antimetastatic activity in the RM-1 and DA3 tumor models, and these models further confirmed a critical role for IFN-γ, but not for perforin or FasL, in the antimetastatic activity of α-GalCer.
Key role of IL-12 and IFN-γ in the antimetastatic effect of α-GalCer.
Groups of B6 WT, B6 gld, B6 pfp−/−, B6 TNF−/−, B6 IL-12−/−, B6 IL-18−/−, and B6 IFN-γ−/− mice or B6 WT mice treated with antimouse IFN-γ, antimouse TRAIL, antimouse IL-4, or control IgG1 mAb on days −1, 0 (the day of tumor inoculation), 4, 7, and 10 were inoculated (A) intravenously with 5 × 105 B16F10 tumor cells, (B) intrasplenically with 5 × 105 3LL tumor cells, or (C) intravenously with 1 × 105 of RM-1 tumor cells. Groups of BALB/c WT, BALB/c pfp−/−, BALB/c.CD1d−/−, BALB/c IFN-γ−/− mice or BALB/c WT mice treated with rabbit anti-asGM1 antibody on days −1, 0 (the day of tumor inoculation), and 7 were inoculated (D) intravenously with 1 × 106 DA3 tumor cells. Some groups of mice were treated intraperitoneally with 2 μg α-GalCer (▨) or vehicle control (▪) on days 0, 4, and 8 after tumor inoculation, as indicated. In all experiments, 14 days after tumor inoculation the lungs (B16F10, RM-1, DA3) or livers (3LL) of these mice were harvested, and tumor colonies were counted and recorded as the mean number of colonies ± SE. The number of mice in each group is indicated in parentheses, and asterisks indicate the groups in which α-GalCer treatment significantly reduced that group's number of lung or liver metastases (Mann-Whitney U: *P < .05; **P < .001).
Key role of IL-12 and IFN-γ in the antimetastatic effect of α-GalCer.
Groups of B6 WT, B6 gld, B6 pfp−/−, B6 TNF−/−, B6 IL-12−/−, B6 IL-18−/−, and B6 IFN-γ−/− mice or B6 WT mice treated with antimouse IFN-γ, antimouse TRAIL, antimouse IL-4, or control IgG1 mAb on days −1, 0 (the day of tumor inoculation), 4, 7, and 10 were inoculated (A) intravenously with 5 × 105 B16F10 tumor cells, (B) intrasplenically with 5 × 105 3LL tumor cells, or (C) intravenously with 1 × 105 of RM-1 tumor cells. Groups of BALB/c WT, BALB/c pfp−/−, BALB/c.CD1d−/−, BALB/c IFN-γ−/− mice or BALB/c WT mice treated with rabbit anti-asGM1 antibody on days −1, 0 (the day of tumor inoculation), and 7 were inoculated (D) intravenously with 1 × 106 DA3 tumor cells. Some groups of mice were treated intraperitoneally with 2 μg α-GalCer (▨) or vehicle control (▪) on days 0, 4, and 8 after tumor inoculation, as indicated. In all experiments, 14 days after tumor inoculation the lungs (B16F10, RM-1, DA3) or livers (3LL) of these mice were harvested, and tumor colonies were counted and recorded as the mean number of colonies ± SE. The number of mice in each group is indicated in parentheses, and asterisks indicate the groups in which α-GalCer treatment significantly reduced that group's number of lung or liver metastases (Mann-Whitney U: *P < .05; **P < .001).
Relative role of IL-12 and IL-18 in the antimetastatic effect of α-GalCer
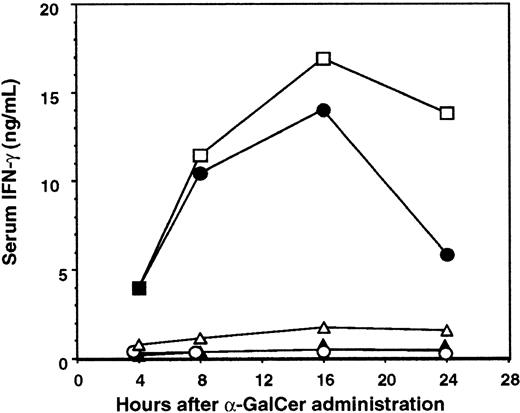
It has been shown that NKT cell expression of IFN-γ and IL-12 receptor in response to α-GalCer required direct contact with DC and IL-12 production by DCs.31 Furthermore, it was recently demonstrated that neutralization of IL-12 activity abrogated the therapeutic value of α-GalCer treatment in a mouse liver metastasis model.56 We therefore examined the antimetastatic activity of α-GalCer in IL-12−/− mice using B16F10 lung and 3LL liver metastasis models (Figure 2A-B). α-GalCer only demonstrated a minor antimetastatic effect in the lungs and livers of IL-12−/− mice, indicating that endogenous IL-12 was important for optimal activity in both organs. In addition, IL-18 alone or in combination with IL-12 has been shown to sustain NKT cell IFN-γ production independently of TCR stimulation,57 whereas IL-18 in combination with α-GalCer preferentially stimulated NKT cell IL-4 production.58 Interestingly, the antimetastatic activity of α-GalCer was retained in the liver (Figure 2B), but was impaired in the lungs (Figure 2A), of IL-18−/− mice (fewer B16F10 lung metastases in the B6 WT + α-GalCer group than in the B6 IL-18−/− + α-GalCer group;P < .05). Overall, the data indicate that in both these organs, IL-12 is more critical than IL-18 in the antimetastatic activity of α-GalCer. This requirement for IL-12 was also noted in the periphery, where α-GalCer–mediated induction of serum IFN-γ was completely abolished in IL-12−/− mice (Figure3). As shown here (Figure 3), NKT cells were required for early serum IFN-γ induction, whereas NK cells contributed to later sustained serum IFN-γ levels in α-GalCer–treated mice. Interestingly, serum IFN-γ induction was also markedly reduced in IL-18−/− mice (Figure 3), suggesting that though IL-18 was not required for the antimetastatic effect of α-GalCer in the liver, it was critical for optimal IFN-γ secretion in the serum in response to α-GalCer.
Key role of IL-12 and IL-18 in serum IFN-γ induction by α-GalCer.
Serum was collected from B6 WT (■) or anti-asGM1–treated B6 WT mice (●), B6 Jα281−/− mice (▴), B6 IL-12−/− mice (○), or B6 IL-18−/− mice (▵) after α-GalCer (2 μg intraperitoneally) administration at the times indicated. Serum IFN-γ levels were measured by a specific enzyme-linked immunosorbent assay, and data represent the mean ± SE (ng/mL) of duplicate samples from 2 different mice. Serum of all untreated mice contained less than 100 pg/mL IFN-γ.
Key role of IL-12 and IL-18 in serum IFN-γ induction by α-GalCer.
Serum was collected from B6 WT (■) or anti-asGM1–treated B6 WT mice (●), B6 Jα281−/− mice (▴), B6 IL-12−/− mice (○), or B6 IL-18−/− mice (▵) after α-GalCer (2 μg intraperitoneally) administration at the times indicated. Serum IFN-γ levels were measured by a specific enzyme-linked immunosorbent assay, and data represent the mean ± SE (ng/mL) of duplicate samples from 2 different mice. Serum of all untreated mice contained less than 100 pg/mL IFN-γ.
Critical contribution of perforin to α-GalCer–induced cytotoxicity by NK cells
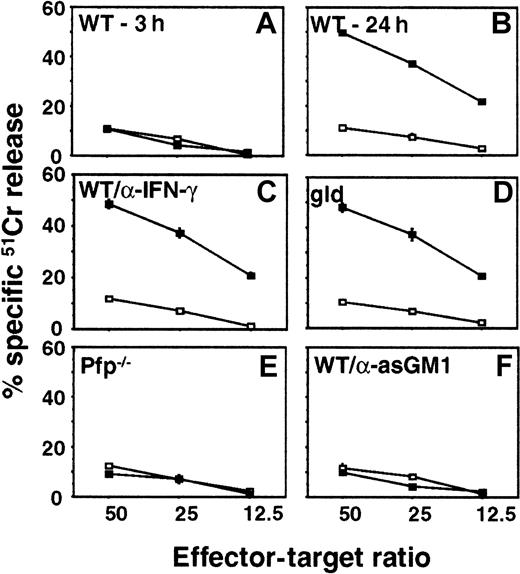
It has been shown that α-GalCer administration promotes NK cell cytotoxicity in an IFN-γ and an NKT cell-dependent manner.32 38 To examine whether IFN-γ, perforin, or FasL were responsible for direct cytolytic activity of NK cells against the tumor cells used in our study, we evaluated the cytotoxicity of hepatic MNCs isolated from pfp-deficient, FasL mutant, or WT mice (untreated or anti-asGM1–treated to deplete NK cells) against B16F10 (Figure4), 3LL, or RM-1 (data not shown) tumor target cells in a 20-hour cytotoxicity assay. Three hours after α-GalCer administration there was no significant induction of WT liver MNC cytotoxicity (Figure 4A). However, 24 hours after α-GalCer administration, the cytotoxic activity of WT MNCs was substantially induced against B16F10 (Figure 4B), 3LL, and RM-1 (data not shown) target cells. MNCs from FasL mutant mice (Figure 4C) or those from WT mice incubated with neutralizing anti-mIFN–γ mAbs (Figure4D) were as cytotoxic as WT MNCs (Figure 4B). By contrast, the α-GalCer–induced cytotoxic activity was completely abolished in MNCs from the perforin-deficient mice (Figure 4E) and the NK cell-depleted mice (Figure 4F). Similar data for each experimental group were obtained for 3LL (perforin-sensitive) and RM-1 (perforin- and FasL-sensitive) tumor targets. These data confirmed that NK cell expression of perforin was essential for optimal α-GalCer–induced cytotoxicity in vitro and that FasL and IFN-γ were not important in vitro for the direct cytotoxic effects of liver MNCs on the tumor target cells. Additional experiments in which B16F10, 3LL, RM-1, and DA3 tumor cells were exposed to increasing concentrations of mIFN-γ (up to 1000 U/mL for 24 hours) did not yield any detectable cytotoxicity and their proliferation was unaffected (data not shown).
Critical contribution of perforin to α-GalCer–induced cytotoxicity by NK cells.
Hepatic MNCs were obtained from B6 WT mice 3 hours (A) or 24 hours (B) after intraperitoneal injection of α-GalCer (▪, 2 μg/200 μL) or vehicle (■, 200 μL), as indicated. Liver MNCs were also harvested after 24 hours from B6 gld mice (D), B6 pfp−/− mice, (E) or B6 WT mice that were depleted of NK cells with anti-asGM1 antibody (F). Cytotoxicity against B16F10 melanoma targets was tested in a 20-hour 51Cr release assay at the indicated effector–target ratios. Some MNCs from B6 WT mice were also incubated in the presence of 10 μg/mL neutralizing anti-mIFN–γ mAb (C) as described.30 Data are represented as the mean ± SE of triplicate samples and are representative of 2 experiments.
Critical contribution of perforin to α-GalCer–induced cytotoxicity by NK cells.
Hepatic MNCs were obtained from B6 WT mice 3 hours (A) or 24 hours (B) after intraperitoneal injection of α-GalCer (▪, 2 μg/200 μL) or vehicle (■, 200 μL), as indicated. Liver MNCs were also harvested after 24 hours from B6 gld mice (D), B6 pfp−/− mice, (E) or B6 WT mice that were depleted of NK cells with anti-asGM1 antibody (F). Cytotoxicity against B16F10 melanoma targets was tested in a 20-hour 51Cr release assay at the indicated effector–target ratios. Some MNCs from B6 WT mice were also incubated in the presence of 10 μg/mL neutralizing anti-mIFN–γ mAb (C) as described.30 Data are represented as the mean ± SE of triplicate samples and are representative of 2 experiments.
NKT cells producing IFN-γ can transfer the antimetastatic effect of α-GalCer
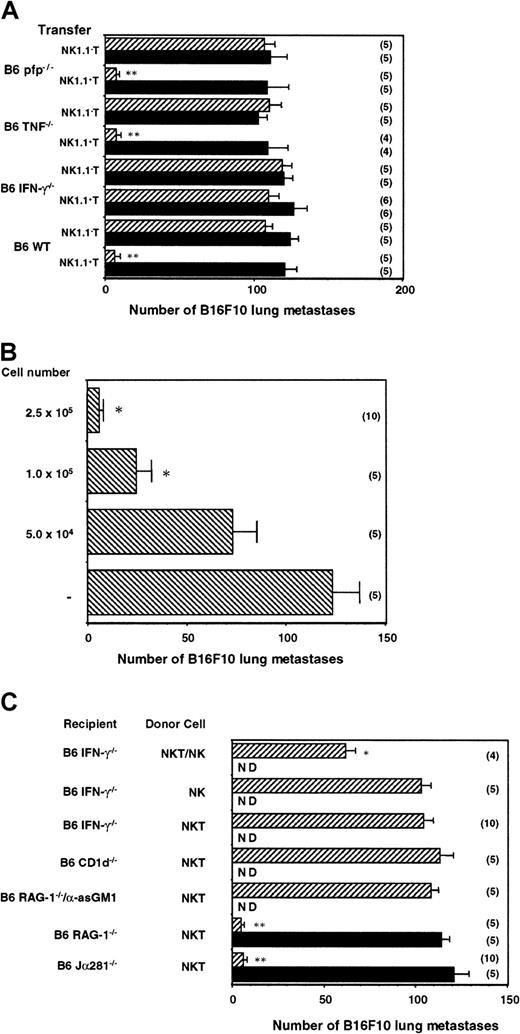
The data in Figure 2 demonstrated the key role that IFN-γ plays in α-GalCer–mediated antimetastatic activity; however, it was unclear which cells produced the key IFN-γ. To be able to dissect the specific contribution of NKT cell molecules in the antimetastatic function of α-GalCer, it was necessary to adoptively transfer protective NKT cells into mice that could not otherwise respond to α-GalCer treatment. Therefore, MNCs from B6 WT mice were isolated and sorted into NK1.1+ TCRαβ+ and NK1.1− TCRαβ+ cells. The liver was selected as a source of donor-derived cells because of the high proportion of NKT cells in this organ. Each of these populations (2.5 × 105 cells) was then adoptively transferred into Jα281−/− mice that had been inoculated with B16F10 melanoma. Starting 3 hours later, mice were treated with vehicle or α-GalCer as above. As observed in Figure5A, Jα281−/− mice that received α-GalCer and B6 WT NK1.1+ T cells, but not NK1.1− T cells, exhibited complete protection from B16F10 tumor. Transfer of NK1.1+ T cells alone (vehicle treated) was insufficient to confer protection, indicating that the protection transferred was not simply an artifact of any potential nonspecific activation of NK1.1+ T cells in their isolation or transfer. At least 1 × 105 NK1.1+ T cells transferred significant protection from B16F10 in α-GalCer–treated Jα281−/− mice (Figure 5B).
NK cell IFN-γ mediates the antimetastatic effect of α-GalCer initiated by NKT cell IFN-γ secretion.
(A) Groups of B6 Jα281−/− mice were inoculated intravenously with 5 × 105 B16F10 tumor cells. Three hours later, these mice received intravenous adoptive transfer of 2.5 × 105 sorted liver NK1.1+ T cells or NK1.1− T cells from B6 WT, B6 pfp−/−, B6 TNF−/−, or B6 IFN-γ−/− mice. Some groups of mice were treated intraperitoneally with 2 μg α-GalCer (▨) or vehicle control (▪) on days 0 (3 hours after tumor inoculation), 4, and 8 after tumor inoculation, as indicated. (B) Groups of B6 Jα281−/− mice were inoculated intravenously with 5 × 105 B16F10 tumor cells. Three hours later, these mice received intravenous adoptive transfer of sorted liver NK1.1+ T cells (between 0 and 2.5 × 105cells) from B6 WT mice, as indicated. All groups of mice were treated intraperitoneally with 2 μg α-GalCer on days 0 (3 hours after tumor inoculation), 4, and 8 after tumor inoculation. (C) Groups of B6 Jα281−/−, B6 IFN-γ−/−, B6 CD1d−/−, B6 RAG-1−/− mice, or B6 RAG-1−/− mice treated with anti-asGM1 antibody (as above) were inoculated intravenously with 5 × 105 B16F10 tumor cells. Three hours later, these mice received intravenous adoptive transfer of 2.5 × 105 sorted NK1.1+ T cells from B6 WT mice, 4.0 × 105 purified NK1.1+TCRαβ− NK cells, or both. Some groups of mice were treated intraperitoneally with 2 μg α-GalCer or vehicle control on days 0 (3 hours after tumor inoculation), 4, and 8 after tumor inoculation, as indicated. ND, not determined. In all experiments, 14 days after tumor inoculation the lungs of these mice were harvested, and B16F10 tumor colonies counted and recorded as the mean number of colonies ± SE. The number of mice in each group is indicated in parentheses, and asterisks indicate the groups in which α-GalCer treatment significantly reduced the number of lung metastases compared with untreated or vehicle control (Mann-Whitney U: *P < .05; **P < .001).
NK cell IFN-γ mediates the antimetastatic effect of α-GalCer initiated by NKT cell IFN-γ secretion.
(A) Groups of B6 Jα281−/− mice were inoculated intravenously with 5 × 105 B16F10 tumor cells. Three hours later, these mice received intravenous adoptive transfer of 2.5 × 105 sorted liver NK1.1+ T cells or NK1.1− T cells from B6 WT, B6 pfp−/−, B6 TNF−/−, or B6 IFN-γ−/− mice. Some groups of mice were treated intraperitoneally with 2 μg α-GalCer (▨) or vehicle control (▪) on days 0 (3 hours after tumor inoculation), 4, and 8 after tumor inoculation, as indicated. (B) Groups of B6 Jα281−/− mice were inoculated intravenously with 5 × 105 B16F10 tumor cells. Three hours later, these mice received intravenous adoptive transfer of sorted liver NK1.1+ T cells (between 0 and 2.5 × 105cells) from B6 WT mice, as indicated. All groups of mice were treated intraperitoneally with 2 μg α-GalCer on days 0 (3 hours after tumor inoculation), 4, and 8 after tumor inoculation. (C) Groups of B6 Jα281−/−, B6 IFN-γ−/−, B6 CD1d−/−, B6 RAG-1−/− mice, or B6 RAG-1−/− mice treated with anti-asGM1 antibody (as above) were inoculated intravenously with 5 × 105 B16F10 tumor cells. Three hours later, these mice received intravenous adoptive transfer of 2.5 × 105 sorted NK1.1+ T cells from B6 WT mice, 4.0 × 105 purified NK1.1+TCRαβ− NK cells, or both. Some groups of mice were treated intraperitoneally with 2 μg α-GalCer or vehicle control on days 0 (3 hours after tumor inoculation), 4, and 8 after tumor inoculation, as indicated. ND, not determined. In all experiments, 14 days after tumor inoculation the lungs of these mice were harvested, and B16F10 tumor colonies counted and recorded as the mean number of colonies ± SE. The number of mice in each group is indicated in parentheses, and asterisks indicate the groups in which α-GalCer treatment significantly reduced the number of lung metastases compared with untreated or vehicle control (Mann-Whitney U: *P < .05; **P < .001).
We next isolated NKT cells from several gene-targeted strains in which α-GalCer failed to have optimal antimetastatic activity. Each of the strains used displayed similar proportions and numbers of NKT cells to B6 WT mice (data not shown). Notably, NK1.1+ T cells from B6 IFN-γ−/− mice did not demonstrate any α-GalCer–mediated antitumor activity when transferred into Jα281−/− mice (Figure 5A). These data are the first to define the critical importance of NKT cell-derived IFN-γ in the antimetastatic function of α-GalCer. Significantly, the inhibition of α-GalCer activity with antimouse IFN-γ mAb (Figure 2) suggested that NKT cells from IFN-γ−/− mice were not simply inactive. By contrast, liver NK1.1+ T cells from B6 pfp−/− or B6 TNF−/− mice were as effective as NKT cells transferred from B6 WT mice (Figure 5A). Thus, despite the ability of α-GalCer to activate NKT cell perforin-mediated lysis15 and the detectable involvement of TNF in α-GalCer antitumor function (Figure 2A-B), NKT cell expression of these molecules did not contribute to the antimetastatic function of α-GalCer.
NK cell-derived IFN-γ is also required for the antimetastatic effect of α-GalCer initiated by NKT cells
Because B6 WT, but not B6 IFN-γ−/− NKT, cells could transfer protection against B16F10 lung metastasis in NKT cell-deficient Jα281−/− mice, we next addressed whether IFN-γ production by NKT cells was sufficient to mediate the antimetastatic effect of α-GalCer. The transfer of B6 WT NKT cells into CD1d−/− mice did not enable tumor protection (Figure5C), indicating that recipient antigen-presenting cells expressing CD1d (and not B16F10 or NKT cells themselves) were required for α-GalCer loading. Similar transfer of WT NKT cells into RAG-1−/−mice deficient in NKT cells, T cells, and B cells, but not NK cells, suggested that NK cells were sufficient to mediate the antimetastatic effect of α-GalCer (Figure 5C). A corroborating fact is that the depletion of NK cells with anti-asGM1 antibody from the RAG-1−/− mice completely abrogated the protective effect of the B6 WT NKT cell transfer into these mice, essentially confirming the role of NK cells (Figure 5C). Given that adoptive transfer of NKT cells into RAG-1−/− mice mediated tumor suppression in response to α-GalCer, donor NKT cells and recipient NK cells were the only known producers of IFN-γ that could contribute to the antitumor effect of α-GalCer in these mice. When B6 WT NKT cells were also transferred into B6 IFN-γ−/− recipients, these mice were not protected from B16F10 tumor metastasis on α-GalCer treatment (Figure 5C). These results suggested that NK cell production of IFN-γ, downstream of NKT cell activation and IFN-γ secretion, was also essential for the antimetastatic function of α-GalCer. To definitively prove that NKT and NK cells producing IFN-γ were required for the antimetastatic activity of α-GalCer, we cotransferred purified NKT cells and NK cells into α-GalCer–treated B6 IFN-γ−/− mice. Despite the competing endogenous NK cells in the B6 IFN-γ−/− mice, the additional transfer of B6 WT NK cells (4 × 105) was sufficient to partially restore the antimetastatic activity of α-GalCer (Figure 5C). The transfer of purified NK cells alone was without effect. A similar cotransfer of a lower dose of NK cells (2 × 105) with NKT cells (2.5 × 105) into B6 IFN-γ−/−mice treated with α-GalCer had a minor, but significant, effect on B16F10 metastasis (data not shown). We were unable to restore full protection, which was possibly limited by the high ratio of resident IFN-γ−/− NK cells to transferred WT NK cells. It was technically beyond our methods, however, to purify higher numbers of fresh NK cells. Collectively, these data represent the first completely definitive demonstration that early IFN-γ production by NKT cells and later IFN-γ secretion by NK cells are necessary and sufficient for the antimetastatic activity of α-GalCer.
Discussion
Previous studies have demonstrated in mice that the marine sponge glycolipid, α-GalCer, can activate NKT cells, rapidly trigger their cytokine secretion including IFN-γ and IL-4, and subsequently stimulate downstream NK cells, T cells, and B cells.32-34,36,38 43 From these studies, it was clear that NKT cells, NK cells, and IFN-γ were primary to α-GalCer–mediated tumor protection. It must be stressed, however, that the way these factors interacted, the key source of the IFN-γ, the effector function of NK cells, and the role of IL-4 and other cytolytic molecules characteristic of NKT and NK cell activation remained undefined. Indeed, in the absence of conditional gene targeting, adoptive transfer experiments are the only definitive way of ascribing a particular effector function to a given subset of cells. Herein, we have used adoptive transfer techniques and various gene-targeted mice and neutralizing mAbs to formally define the importance of key factors associated with NKT–NK cell activation in α-GalCer–mediated tumor suppression.
For the first time we have demonstrated that NKT cell-derived IFN-γ and NK cell-derived IFN-γ are both essential for the antimetastatic activity of α-GalCer. Surprisingly, despite the ability of transferred NKT cells to confer protection in T- and B-cell–deficient or NKT-cell–deficient mice treated with α-GalCer, the expression of IFN-γ in recipient mice was critical. The potent production of IFN-γ by NKT cells alone was not sustained, nor sufficient, for tumor suppression. These data suggested that, despite the ability of α-GalCer to trigger NKT cells to release multiple Th1 and Th2 cytokines and to acquire cytotoxic potential, it is the ability of NKT cell IFN-γ to trigger NK cell IFN-γ production that provides α-GalCer its antimetastatic function. This hypothesis was confirmed with the depletion of NK cells from RAG-1−/− recipients of NKT cells and the restoration of α-GalCer antimetastatic activity in B6 IFN-γ−/− mice that received purified NKT cells and NK cells.
It remains unclear how NK cell IFN-γ contributes to the antimetastatic activity of α-GalCer and, in particular, which cell types are the targets of IFN-γ produced by NK cells. All in vitro efforts to demonstrate a direct cytotoxic effect of IFN-γ on the tumor cell lines used have indicated that IFN-γ is not directly cytotoxic and that these cells continue to proliferate when cultured in high doses of IFN-γ. In particular, NK cell IFN-γ may be key to the local proliferative capacity of NK cells or their further recruitment of NK cells and other leukocytes to the tumor site. It is possible that other as yet unappreciated effector cells may act downstream of NK cells, though no evidence of the involvement of other nonlymphoid cell types has ever been presented for these experimental tumors. Significantly, IFN-γ has potent antiangiogenic activity,59-61 and IFN-γ–inducible factors produced by various cells, such as IFN-inducible protein 10 (IP-10) and monokine induced by IFN-γ,62 may indirectly inhibit tumor neovascularization and induce tumor hypoxia.
NK cells activated by NKT cells might sometimes make use of other effector mechanisms that are IFN-γ dependent. Recently, we have shown that NK cells express functional TRAIL after α-GalCer administration,30 and TRAIL expression was IFN-γ dependent. Another study indicated that human NKT cells, cultured with α-GalCer–pulsed DCs, could themselves express TRAIL.63However, B16F10 and 3LL are not TRAIL-sensitive tumors (data not shown), and α-GalCer demonstrated complete antimetastatic function in anti-TRAIL mAb-treated mice. Although mouse NKT cells can express perforin15,28 and can mediate perforin-dependent tumor cell lysis in vitro,14 it is unknown whether NKT cells use perforin-mediated cytotoxicity in vivo. Others have already demonstrated that NKT cells may exhibit FasL-mediated cytotoxicity55 and that FasL may be a mechanism by which NKT cells undergo activation-induced cell death36after exposure to α-GalCer. However, Fas–FasL interactions also appeared to be dispensable for α-GalCer–induced tumor suppression. Regardless, NK cell IFN-γ clearly has a profound impact on the growth of metastases after α-GalCer administration, and the end effector mechanisms resulting in the suppression of metastasis undoubtedly warrant greater study.
The antimetastatic activity of α-GalCer is an example of NKT cells providing immune-activating function; thus, perhaps it is not surprising that IFN-γ is a critical factor in its activity. However, the importance of various effector molecules in the biologic effects caused by α-GalCer administration may depend considerably on the downstream function examined. For example, in contrast to our tumor metastasis models, α-GalCer–mediated abortion through the degeneration of embryonic trophoblasts was not observed in Jα281−/−, IFN-γ−/−, TNF−/−, or perforin−/− mice.54It should be noted that decidual NKT cells specifically express Vβ7 rather than Vβ8.2 and produce IFN-γ and TNF, but not IL-2 and IL-4, on α-GalCer stimulation.54 By contrast, α-GalCer induces spleen or liver NKT cell IL-4 secretion. NKT cells participate in several models of immune suppression,20-22 and a few recent studies19-21,64 have begun to define the possible mechanisms by which NKT cells may exert immune suppression. Interestingly, in the model of allograft tolerance, NKT cell transfer experiments also indicated that NKT cell IFN-γ production was critical for long-term transplant survival, whereas IL-4 was not essential.21 We have found that IL-4 was not required for α-GalCer–mediated antimetastatic function. It remains to be tested whether other Th2 cytokines produced by NKT cells, such as IL-6, IL-10, IL-13, and TGF-β, may influence the antitumor activity of α-GalCer. One report has described the increased activity of antitumor CTL after α-GalCer administration,42 but it remains to be determined whether these activities depend strictly on NKT cell IFN-γ or other effector mechanisms.
The outcome between the NKT cell and the DC appears of central importance in the downstream antitumor activities of α-GalCer. It has been demonstrated with mAbs that direct contact of the NKT cell with DC and that IL-12 production by DC contributed to NKT cell IL-12R expression and production of IFN-γ.31 Our data using IL-12−/− and IL-18−/− mice has shown that IL-12 p40 is required for the optimal antitumor activity of α-GalCer, whereas IL-18 is important for optimal protection from lung, but not liver, metastasis. Our data using α-GalCer treatment were reminiscent of a model in which IL-18 was necessary for the induction of serum, but not liver, IFN-γ levels after mouse cytomegalovirus infection.65 Although it is possible to determine whether ablation of both host IL-12 and IL-18 production completely inhibits the antitumor activity of α-GalCer, this experiment is less informative given that the doubly deficient IL-12−/−IL-18−/− mice have severely impaired NK cell function.48
An important question that remains is whether other CD1d-restricted effector cells can mediate the activity of α-GalCer. Recently, CD1d-restricted, α-GalCer–reactive NK1.1− T cells have been described35,66,67 that exhibit characteristics similar to those of NK1.1+ T cells. Indeed, their ability to rapidly produce immunoregulatory cytokines and their dependence on TCR Jα28135,66 suggest that they are closely related to NKT cells, though they differ in their ability to auto-present α-GalCer by their own cell surface CD1d and by reduced production of IFN-γ compared to the NK1.1+ fraction.67 Our study has not evaluated the activity of this population, though it was probably present within the NK1.1− T-cell fraction used as a control population in these experiments. The fact that these cells did not offer α-GalCer–mediated protection may simply reflect the fact that CD1d-restricted cells are only a minor subset of this population. The future use of the adoptive transfer system described in this study will allow us to evaluate the antitumor potential of these cells. In particular, CD40L and CD28 expressed on the surface of NKT cells appear important in the antitumor function of NKT cells,68 and other molecules such as NK1.1 and CD4 will be of future interest.
The clear potency of small numbers of adoptively transferred liver NK1.1+ T cells after α-GalCer administration in these experimental models is a promising indication of the therapeutic potential of these effector cells against NK cell-sensitive tumors. The combined antitumor effects of small numbers of transferred NKT cells and NK cells were particularly encouraging. It will be interesting to determine whether NKT cells isolated from other organs (thymus, spleen, bone marrow) are comparable in their α-GalCer–mediated antitumor capacity on adoptive transfer. Transfer of CFSE-labeled liver MNCs revealed that NKT cells and NK cells migrated to the liver of recipient WT mice and persisted for at least 7 days (data not shown). Additional studies are required to assess the migration of NKT cells to other lymphoid sites. Despite the reported death of most NK1.1+ T cells after activation,33-36 it may be possible to follow the fate of transferred NKT cells and other host bystander leukocytes in models in which we have previously shown that NKT cells contribute to the natural immunosurveillance of methylcholanthrene-induced sarcoma.14
We thank Shayna Street and Christine Hall for genotyping the gene-targeted mice, Nicole Haynes for technical assistance, and Elise Randle-Barrett for flow cytometric sorting. We also thank Dr Koezuka of Kirin Brewery for kindly providing α-GalCer.
Supported by grants-in-aid from the National Health and Medical Research Council of Australia (NHMRC), the Human Frontier Science Program, the Ministry of Education, Science and Culture, Japan, and donations from Rothschild Australia. M.J.S. is supported by an NHMRC Research Fellowship. N.Y.C. is supported by an Australian Postgraduate Research Award. D.I.G. is supported by an ADCORP Diabetes Australia Research Fellowship, and the NHMRC.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mark Smyth, Cancer Immunology, Peter MacCallum Cancer Institute, Locked Bag 1, A'Beckett St, East Melbourne 8006, Victoria, Australia.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal