Multiple myeloma (MM) is associated with severe normochromic/normocytic anemia. This study demonstrates that the abnormal up-regulation of apoptogenic receptors, including both Fas ligand (L) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), by highly malignant myeloma cells is involved in the pathogenesis of the ineffective erythropoiesis and chronic exhaustion of the erythroid matrix. By measuring Fas-L and TRAIL in plasma cells and the content of glycophorin A (GpA) in erythroblasts from a cohort of 28 untreated, newly diagnosed patients with MM and 7 with monoclonal gammopathy of undetermined significance (MGUS), selected in relation to their peripheral hemoglobin values, results showed that both receptors occurred at high levels in 15 severely anemic MM patients. Their marrow erythropoietic component was low and included predominantly immature GpA+dim erythroblasts, in contrast with the higher relative numbers of mature GpA+bright erythroid cells observed in the nonanemic patients and those with MGUS. In cocultures with autologous Fas-L+/TRAIL+ myeloma cells, the expanded GpA+dim erythroid population underwent prompt apoptosis after direct exposure to malignant plasma cells, whereas erythroblasts from nonanemic patients were scarcely affected. The evidence that Fas-L+/TRAIL+malignant plasma cells prime erythroblast apoptosis by direct cytotoxicity was also supported by the increase of FLICE in fresh immature GpA+dim erythroid cells, whereas ICE and caspase-10 increased in subsequent maturative forms. In addition, GATA-1, a survival factor for erythroid precursors, was remarkably down-regulated in fresh erythroblasts from the severely anemic patients. These results indicate that progressive destruction of the erythroid matrix in aggressive MM is due to cytotoxic mechanisms based on the up-regulation in myeloma cells of Fas-L, TRAIL, or both. It is conceivable that the altered regulation of these receptors defines a peculiar cytotoxic phenotype that drives the progression of aggressive MM.
Introduction
Defective erythropoiesis leading to chronic normochromic/normocytic anemia is common in the progression of multiple myeloma (MM).1,2 Its main features are hypoferremia in the presence of adequate iron stores and inappropriate erythropoietin (EPO) production.3 Several negative regulators of erythropoiesis are involved in the pathogenesis of defective erythroid maturation. The erythropoietic precursors are acted on by apoptosis inducers, namely, transforming growth factor (TGF)–β1, tumor necrosis factor (TNF)–α, interferon (IFN)–γ, and Fas ligand (Fas-L).4,5 These proapoptogenic factors are physiologically involved in modulating the maturation of erythroid cells and are up-regulated in the anemia of chronic disorders,6 as well as in many solid tumors and hematologic malignancies, including MM.7,8 Malignant plasma cells can also add to the progressive destruction of the erythroid matrix by direct Fas-L–mediated cytotoxicity.9
Recent work by independent groups has emphasized the role of the Fas/Fas-L system in restraining erythroid maturation.5,10Proerythroblasts at the prebasophilic/basophilic stage are susceptible to apoptosis because of their high sensitivity to Fas stimulation, whereas mature erythroblasts are refractory to the autotoxic lysis induced by excess Fas-L.10 That Fas exerts a prominent role in the control of erythropoiesis is also supported by additional studies, showing that erythroblasts treated with TNF-α or IFN-γ or both strongly trigger apoptosis in response to the CH11 anti-Fas monoclonal antibody (mAb) as prototypic agonist ligand of the receptor.11,12 Furthermore, mouse embryos lacking FADD, a cytoplasmic Fas-associated domain involved in the sequential activation of downstream caspases,13 undergo lethal erythroid accumulation,14 and TRAIL, the TNF-related apoptosis-inducing ligand,15 displays structural and functional similarities with Fas-L16 and acts through FADD in the homeostatic regulation of erythropoiesis.17However, in contrast with the inhibitory effect promoted by Fas-L in early erythroid precursors, TRAIL is apparently effective during the intermediate stage of erythroblast maturation when a mild to moderate presence of glycophorin A (GpA) is detectable in erythroid cells.17
We have recently shown that Fas-L overexpression by myeloma cells reflects their high degree of malignancy, as assessed by their proliferation in the absence of interleukin-6 (IL-6) as well as their unresponsiveness to Fas stimulation by the CH11 mAb.18These clones contribute to anemia in MM, because they exert a major cytotoxic effect on the marrow erythroid matrix by presenting Fas-L to immature erythroblasts.9 We hypothesize that erythropoietic failure also results from progressive depletion of mature erythroblasts as an effect of the inability of their precursors to proceed to mature forms in the presence of cytotoxic myeloma cells. This pathway then leads to a compensatory expansion of the early proerythroblast population within the bone marrow,9,19whose progression to the next maturation steps can also be inhibited by the persistent defect of EPO, particularly in patients with kidney failure.20
In this study we have evaluated the occurrence of the cytotoxic phenotype in malignant plasma cells from a cohort of patients with MM. In the majority of those with severe anemia and active disease, these cells greatly inhibited erythroid maturation.
Patients, materials, and methods
Patients
Twenty-eight patients with MM stages I to IIIB2 were selected from a larger population such that 2 groups were constructed: those with peripheral hemoglobin of 9 g/dL or less (group A) and those with a value of 11.5 g/dL or higher (group B), to compare those with severe anemia to those with borderline or normal erythropoiesis. All patients were newly diagnosed and were included in the study before receiving any chemotherapy, to avoid the potential interference of cytotoxic drugs in cellular responses. Seven patients with monoclonal gammopathy of undetermined significance (MGUS) and normal erythropoiesis were also included. The study was approved by the Ethical Committee of the University of Bari. Informed consent was obtained from all individuals.
Bone marrow phenotyping
Bone marrow cells were freshly typed by double-fluorescence analysis in a FACScan (Becton Dickinson, Mountain View, CA). Phenotypic differentiation of erythroblasts from plasma cells was first assessed by detection of GpA21 and CD38, respectively, using fluorescein isothiocyanate (FITC)–conjugated mAbs (Immunotech, Marseille, France, and Becton Dickinson, San Jose, CA). In addition, the expression of both Fas-L and TRAIL was measured in CD38+ cell populations. The biotinylated IgG1k from clone NOK-1 (Pharmingen, San Diego, CA) treated with phycoerythrin (PE)–conjugated streptavidin was used for Fas-L, whereas the expression of TRAIL was assessed by a rabbit polyclonal antiserum (Alexis, Vinci, Italy) in association with a goat PE-conjugated second antibody.
Erythroid cell maturation was investigated by measuring the fluorescence intensity of GpA expression.17 Enriched erythroblast populations (2.5-3 × 106) were obtained by magnetic cell sorting of bone marrow, using anti-GpA–conjugated microbeads, which resulted in purities for GpA+ cells of approximately 90% (Miltenyi Biotec, Bergisch Gladbach, Germany). Aliquots of these cells were then incubated with the PE-conjugated anti-GpA mAb (Immunotech) for semiquantitative flow cytometry evaluation of GpA. The analysis was completed with the Cell-Quest software (Becton Dickinson), which defines fluorescence intensity as dim, intermediate, and bright. These differential levels of GpA expression have been associated with the immature, semimature, and mature erythroblast stages of erythroid development.17
The expression of Fas, Fas-L, and TRAIL and its effector receptors DR4 and DR5 was also measured with respect to concurrent GpA content by double-fluorescence flow cytometry22 on isolated erythroblast populations. Fas was detected by the ZB4 IgG1 mAb (Immunotech), whereas rabbit and goat antisera were used for DR4 and DR5 molecules, respectively, and in all instances FITC-conjugated second antibodies were used for their measurement. In addition, erythroblasts were evaluated for the expression of GATA-1, namely, the transcription factor necessary for the terminal differentiation of the erythroid precursors.23 24 Aliquots of those cells were incubated with a rat anti-GATA-1 mAb (Santa Cruz Biotechnology, Santa Cruz, CA) and further treated with the FITC-conjugated second antibody prior to flow cytometry.
Cocultures of erythroblasts with myeloma cells
Mixed cultures of erythroblasts and plasma cells were set up to investigate the cytotoxic potential of both Fas-L+ and TRAIL+ myeloma cells. Similarly to the erythroblast preparations, malignant plasma cells were enriched by magnetic sorting with the anti-CD138 (syndecan-1)25,26 mAb (Miltenyi Biotech). This method was successful in providing excellent purity (> 96.5%) of the myeloma cells used for coculturing. The cultures were initiated using both EPO and IL-6, as reported.9Briefly, 2 to 5 × 105 fresh erythroblasts were incubated overnight in 24-well plates at 37°C in the presence of plasma cells at 1:1 ratio. Erythroblasts were cultured with autologous myeloma cells, and each coculture was prepared in parallel to measure the erythroblast apoptosis differentially induced by Fas-L, TRAIL, or both.
To measure the effect of Fas-L, myeloma cells were inhibited in their TRAIL expression by a 90-minute pretreatment with 10 μg/mL polyclonal anti-TRAIL antiserum (Alexis) and then added to the erythroblast preparations. Parallel functional assays were carried out to confirm the efficacy of the anti-TRAIL treatment, showing almost complete (91.3%) neutralization of the TRAIL-related cytotoxicity. This cytotoxicity was also assessed on further aliquots of myeloma cells, which were first incubated with 10 μg/mL of the recombinant Fas-Fc chimeric protein (Alexis) to inactivate Fas-L and then cocultured with the erythroblasts. The ability of this soluble isoform of Fas to saturate the binding site of Fas-L, leading to its functional impairment, had already been demonstrated.9 Subsequently, apoptosis of the erythroblast populations was measured.
Apoptosis
Apoptosis in cocultured erythroblasts was evaluated in comparison with control unstimulated cells from each patient, using a double-fluorescence assay that detects the outer leaflet exposure of phosphatidylserine by apoptotic erythroblasts.27 Thus, the cultured cells were washed and treated with the FITC-conjugated annexin-V (Boehringer Mannheim, Milan, Italy) and with the PE-labeled anti-GpA mAb (Immunotech). The extent of erythroblast apoptosis was then measured in the relevant GpA+ subset with the Cell-Quest software.
The occurrence of apoptosis in cocultured erythroblasts was also investigated by direct examination under a fluorescence microscope. Briefly, the cells were first treated with the PE-conjugated reagent to anti-TRAIL antiserum, and subsequently with 1 μg/mL 4,6-diamidino-2-phenylindole (DAPI) to visualize the morphologic changes of nuclei in apoptotic erythroblasts.
Caspase activity
Several experiments were performed to assess caspase expression and activation in fresh erythroblasts. The constitutive expression of FLICE (caspase-8), ICE (caspase-1), and caspase-10 was measured by double-fluorescence analysis. FLICE was detected by a mAb (Biosource, Camarillo, CA), and ICE and caspase-10 by rabbit polyclonal antisera (Biosource and Prosci, Poway, CA, respectively). Although most reagents had been optimized by their manufacturers for immunoblotting methods, their reliability was checked by flow cytometry assay.9 Cells were incubated with the PE-anti-GpA reagent, then fixed, permeabilized, and separately treated with specific anticaspase reagents. Finally, the assay was completed by staining with secondary FITC-conjugated antibody prior to flow cytometry.
Statistical analysis
Differences between means of groups of data were calculated using the nonparametric Mann-Whitney test.
Results
Expression of Fas-L and TRAIL and inhibition of erythroblast maturation in anemic patients
Table 1 shows the distribution of both anemic (group A) and nonanemic (group B) patients in parallel with the measurement of both plasma cells (CD38+) and erythroid precursors (GpA+) in the bone marrow. Fas-L and TRAIL expression by plasma cells as well as analysis of the distribution of GpA+ subsets of enriched erythroblast populations are also included and compared with the results from patients with MGUS.
Clinical data and phenotype characterization of plasma cells and erythroblasts from patients with MM or MGUS
| Patients, no. . | Stage . | Hb levels, g/dL . | Bone marrow aspirates . | Enriched erythroblast populations . | |||||
|---|---|---|---|---|---|---|---|---|---|
| CD38+ . | GpA+ . | CD38+ . | GpA+dim . | GpA+interm . | GpA+bright . | ||||
| Fas-L+ . | TRAIL+ . | ||||||||
| Myeloma patients | |||||||||
| Group A | |||||||||
| 01 | IIIB | 4.8 | 37.0 | 1.9 | 71.5 | 83.0 | 71.6 | 3.8 | 18.1 |
| 02 | IIIA | 6.5 | 29.2 | 4.5 | 44.1 | 55.6 | 56.7 | 12.4 | 20.3 |
| 03 | IIIA | 6.9 | 21.3 | 3.9 | 34.6 | ND | 35.5 | 41.1 | 13.8 |
| 04 | IIIB | 8.6 | 25.7 | 9.1 | 52.3 | 51.8 | 65.4 | 11.2 | 13.1 |
| 05 | II | 9.0 | 18.2 | 11.0 | 41.2 | 29.1 | 71.3 | 10.5 | 9.6 |
| 06 | IIIA | 7.8 | 45.1 | 2.6 | 73.0 | 90.0 | 68.4 | 9.3 | 11.0 |
| 07 | IIIA | 8.0 | 21.8 | 7.4 | 62.2 | 74.5 | 65.0 | 13.1 | 11.7 |
| 08 | IIIA | 7.6 | 48.0 | 2.3 | 29.0 | ND | 49.9 | 25.7 | 13.6 |
| 09 | IIIB | 6.5 | 39.7 | 5.5 | 37.0 | 52.2 | 47.1 | 16.0 | 26.3 |
| 10 | II | 8.6 | 61.4 | 6.1 | 47.5 | 31.0 | ND | ND | ND |
| 11 | II | 8.8 | 54.1 | 3.6 | 52.3 | 42.3 | 45.1 | 6.2 | 17.8 |
| 12 | IIIB | 8.1 | 35.3 | 2.4 | 50.7 | 66.0 | 59.5 | 21.3 | 9.1 |
| 13 | IIIA | 7.8 | 46.6 | 4.0 | 32.4 | 55.8 | 63.1 | 9.8 | 13.1 |
| 14 | IIIB | 6.7 | 41.8 | 3.1 | 88.6 | 98.0 | 70.1 | 2.9 | 19.4 |
| 15 | II | 8.8 | 30.5 | 5.2 | 43.5 | 61.5 | 44.5 | 23.0 | 21.1 |
| Mean ± SD | 37.0 ± 12.7 | 4.8 ± 2.6 | 50.6 ± 16.8 | 60.8 ± 21.1 | 58.0 ± 11.7 | 14.7 ± 10.2 | 15.5 ± 5.0 | ||
| Group B | |||||||||
| 01 | II | 12.1 | 14.2 | 10.5 | 23.1 | 6.5 | 23.4 | 17.1 | 47.9 |
| 02 | IIIA | 11.7 | 19.1 | 5.7 | 19.4 | 15.2 | 10.5 | 5.0 | 76.3 |
| 03 | I | 13.8 | 8.1 | 18.5 | 19.7 | ND | 17.2 | 28.1 | 43.7 |
| 04 | II | 12.6 | 9.4 | 13.0 | 11.4 | 21.5 | 18.1 | 9.1 | 62.0 |
| 05 | I | 13.5 | 24.7 | 4.3 | 17.3 | 18.5 | 33.5 | 21.8 | 39.2 |
| 06 | I | 14.1 | 18.0 | 9.0 | 8.5 | 12.1 | ND | ND | ND |
| 07 | IIIA | 12.8 | 31.6 | 4.8 | 30.5 | 11.0 | 13.3 | 16.1 | 60.0 |
| 08 | II | 13.1 | 17.0 | 9.1 | 23.1 | 10.6 | 28.8 | 11.1 | 49.5 |
| 09 | I | 15.0 | 18.3 | 11.4 | 5.6 | 11.4 | 15.6 | 13.6 | 58.8 |
| 10 | IIIA | 11.9 | 15.1 | 15.0 | 24.6 | 30.4 | 38.3 | 21.1 | 35.1 |
| 11 | I | 13.6 | 17.1 | 20.8 | 6.3 | 9.8 | 19.1 | 12.0 | 60.2 |
| 12 | IIIA | 12.5 | 22.4 | 9.4 | 41.0 | 33.1 | 16.8 | 4.6 | 68.3 |
| 13 | II | 13.1 | 12.0 | 11.4 | 8.0 | 20.3 | 21.3 | 25.4 | 53.0 |
| Mean ± SD | 17.4 ± 6.3 | 10.9 ± 4.9 | 18.3 ± 10.4 | 16.7 ± 8.3 | 21.3 ± 8.3 | 15.4 ± 7.6 | 54.5 ± 12.0 | ||
| MGUS patients | |||||||||
| 01 | 13.4 | 4.2 | 9.5 | 11.4 | 7.1 | 15.5 | 23.0 | 59.1 | |
| 02 | 11.9 | 10.2 | 4.7 | 18.4 | 3.4 | 9.3 | 18.8 | 67.0 | |
| 03 | 14.3 | 3.6 | 12.0 | 9.4 | 6.2 | 30.0 | 27.2 | 39.6 | |
| 04 | 15.1 | 5.5 | 18.1 | 6.5 | 11.0 | 11.6 | 7.7 | 74.0 | |
| 05 | 12.3 | 4.6 | 19.4 | 7.5 | 4.1 | 28.8 | 32.0 | 36.3 | |
| 06 | 13.1 | 6.8 | 11.3 | 14.1 | 8.6 | 13.9 | 12.5 | 70.2 | |
| 07 | 14.0 | 3.9 | 10.1 | 4.3 | ND | ND | ND | ND | |
| Mean ± SD | 5.5 ± 2.3 | 12.1 ± 5.0 | 10.2 ± 4.8 | 6.7 ± 2.8 | 18.1 ± 8.9 | 20.2 ± 9.0 | 57.7 ± 16.1 | ||
| Patients, no. . | Stage . | Hb levels, g/dL . | Bone marrow aspirates . | Enriched erythroblast populations . | |||||
|---|---|---|---|---|---|---|---|---|---|
| CD38+ . | GpA+ . | CD38+ . | GpA+dim . | GpA+interm . | GpA+bright . | ||||
| Fas-L+ . | TRAIL+ . | ||||||||
| Myeloma patients | |||||||||
| Group A | |||||||||
| 01 | IIIB | 4.8 | 37.0 | 1.9 | 71.5 | 83.0 | 71.6 | 3.8 | 18.1 |
| 02 | IIIA | 6.5 | 29.2 | 4.5 | 44.1 | 55.6 | 56.7 | 12.4 | 20.3 |
| 03 | IIIA | 6.9 | 21.3 | 3.9 | 34.6 | ND | 35.5 | 41.1 | 13.8 |
| 04 | IIIB | 8.6 | 25.7 | 9.1 | 52.3 | 51.8 | 65.4 | 11.2 | 13.1 |
| 05 | II | 9.0 | 18.2 | 11.0 | 41.2 | 29.1 | 71.3 | 10.5 | 9.6 |
| 06 | IIIA | 7.8 | 45.1 | 2.6 | 73.0 | 90.0 | 68.4 | 9.3 | 11.0 |
| 07 | IIIA | 8.0 | 21.8 | 7.4 | 62.2 | 74.5 | 65.0 | 13.1 | 11.7 |
| 08 | IIIA | 7.6 | 48.0 | 2.3 | 29.0 | ND | 49.9 | 25.7 | 13.6 |
| 09 | IIIB | 6.5 | 39.7 | 5.5 | 37.0 | 52.2 | 47.1 | 16.0 | 26.3 |
| 10 | II | 8.6 | 61.4 | 6.1 | 47.5 | 31.0 | ND | ND | ND |
| 11 | II | 8.8 | 54.1 | 3.6 | 52.3 | 42.3 | 45.1 | 6.2 | 17.8 |
| 12 | IIIB | 8.1 | 35.3 | 2.4 | 50.7 | 66.0 | 59.5 | 21.3 | 9.1 |
| 13 | IIIA | 7.8 | 46.6 | 4.0 | 32.4 | 55.8 | 63.1 | 9.8 | 13.1 |
| 14 | IIIB | 6.7 | 41.8 | 3.1 | 88.6 | 98.0 | 70.1 | 2.9 | 19.4 |
| 15 | II | 8.8 | 30.5 | 5.2 | 43.5 | 61.5 | 44.5 | 23.0 | 21.1 |
| Mean ± SD | 37.0 ± 12.7 | 4.8 ± 2.6 | 50.6 ± 16.8 | 60.8 ± 21.1 | 58.0 ± 11.7 | 14.7 ± 10.2 | 15.5 ± 5.0 | ||
| Group B | |||||||||
| 01 | II | 12.1 | 14.2 | 10.5 | 23.1 | 6.5 | 23.4 | 17.1 | 47.9 |
| 02 | IIIA | 11.7 | 19.1 | 5.7 | 19.4 | 15.2 | 10.5 | 5.0 | 76.3 |
| 03 | I | 13.8 | 8.1 | 18.5 | 19.7 | ND | 17.2 | 28.1 | 43.7 |
| 04 | II | 12.6 | 9.4 | 13.0 | 11.4 | 21.5 | 18.1 | 9.1 | 62.0 |
| 05 | I | 13.5 | 24.7 | 4.3 | 17.3 | 18.5 | 33.5 | 21.8 | 39.2 |
| 06 | I | 14.1 | 18.0 | 9.0 | 8.5 | 12.1 | ND | ND | ND |
| 07 | IIIA | 12.8 | 31.6 | 4.8 | 30.5 | 11.0 | 13.3 | 16.1 | 60.0 |
| 08 | II | 13.1 | 17.0 | 9.1 | 23.1 | 10.6 | 28.8 | 11.1 | 49.5 |
| 09 | I | 15.0 | 18.3 | 11.4 | 5.6 | 11.4 | 15.6 | 13.6 | 58.8 |
| 10 | IIIA | 11.9 | 15.1 | 15.0 | 24.6 | 30.4 | 38.3 | 21.1 | 35.1 |
| 11 | I | 13.6 | 17.1 | 20.8 | 6.3 | 9.8 | 19.1 | 12.0 | 60.2 |
| 12 | IIIA | 12.5 | 22.4 | 9.4 | 41.0 | 33.1 | 16.8 | 4.6 | 68.3 |
| 13 | II | 13.1 | 12.0 | 11.4 | 8.0 | 20.3 | 21.3 | 25.4 | 53.0 |
| Mean ± SD | 17.4 ± 6.3 | 10.9 ± 4.9 | 18.3 ± 10.4 | 16.7 ± 8.3 | 21.3 ± 8.3 | 15.4 ± 7.6 | 54.5 ± 12.0 | ||
| MGUS patients | |||||||||
| 01 | 13.4 | 4.2 | 9.5 | 11.4 | 7.1 | 15.5 | 23.0 | 59.1 | |
| 02 | 11.9 | 10.2 | 4.7 | 18.4 | 3.4 | 9.3 | 18.8 | 67.0 | |
| 03 | 14.3 | 3.6 | 12.0 | 9.4 | 6.2 | 30.0 | 27.2 | 39.6 | |
| 04 | 15.1 | 5.5 | 18.1 | 6.5 | 11.0 | 11.6 | 7.7 | 74.0 | |
| 05 | 12.3 | 4.6 | 19.4 | 7.5 | 4.1 | 28.8 | 32.0 | 36.3 | |
| 06 | 13.1 | 6.8 | 11.3 | 14.1 | 8.6 | 13.9 | 12.5 | 70.2 | |
| 07 | 14.0 | 3.9 | 10.1 | 4.3 | ND | ND | ND | ND | |
| Mean ± SD | 5.5 ± 2.3 | 12.1 ± 5.0 | 10.2 ± 4.8 | 6.7 ± 2.8 | 18.1 ± 8.9 | 20.2 ± 9.0 | 57.7 ± 16.1 | ||
The 28 patients with MM were selected in relation to their peripheral hemoglobin levels (group A, ≤ 9 g/dL; group B, ≥ 11.5 g/dL). Erythroblasts obtained by magnetic sorting were distributed in relation to the fluorescence intensity of GpA expression as dim, intermediate, and bright flow cytometric pattern, namely, immature, partially differentiated, and mature erythroblasts, respectively. Numbers are percent of positive cells.
Hb indicates hemoglobin; ND, not done.
As expected, patients with advanced stage III disease were prevalent in group A, compared with group B. Furthermore, the expression of GpA+ cells in bone marrow aspirates varied greatly in patients with MM. The erythroblast population (mean percent of GpA+ cells) was less than half in group A compared to group B (P < .001). Both Fas-L and TRAIL were variably expressed by CD38+ cells within each group, although their mean values were significantly higher in group A than in group B and MGUS (P < .0001 in all instances). Both receptors were concurrently overexpressed by myeloma cells, especially in patients A-01, A-06, and A-14. By contrast, CD38+ cells in both group B and MGUS patients had much lower expression of both receptors, and there were no significant differences between the 2 receptors (P ≥ .1).
These data corroborated the evidence9 that the expression of Fas-L (and TRAIL as well) by malignant plasma cells is inversely related to relative erythroblast numbers. Overall, the pattern in MGUS was similar to that of group B (P = .63).
Analysis of GpA intensity on enriched erythroblast populations revealed that group A patients had higher relative percentages of immature erythroblasts than group B and MGUS. Their mean GpA+dimpercentage was more than double (P < .001 in both instances), indicating that immature erythroblasts were greatly predominant. No difference was recorded for GpA+intermcells (P = .1666). By contrast, group A patients had significantly lower percentages of GpA+bright cells than group B and MGUS (P < .0001 in both instances), whereas no difference was found between these 2 groups (P = .61), in keeping with their intact erythropoiesis as assessed by normal blood hemoglobin levels.
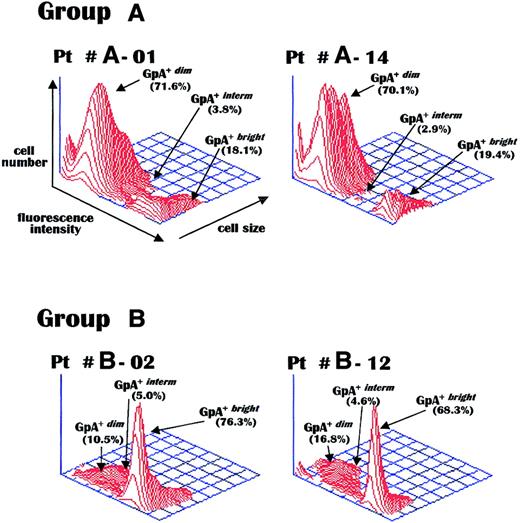
Figure 1 shows the GpA fluorescence intensity patterns of bead-purified erythroblasts from representative patients. As can be seen, the erythroid population from patients A-01 and A-14 was predominantly GpA+dim, whereas that of patients B-02 and B-12 was mostly composed of GpA+bright cells. This inverse pattern of the immature and mature erythroblast subsets correlated with the actual level of red cell production in these patient groups and suggested that the defective erythroblast maturation in group A was related to the greater expression of apoptogenic receptors by malignant plasma cells.
GpA measurement in erythroblasts.
Representative flow cytometry of GpA expression levels measured via a PE-conjugated antiserum in bone marrow erythroblasts purified from marrows of MM patients with active disease and severe anemia (group A) or with preserved erythropoiesis (group B). Group A patients display a preponderance of immature (GpA+dim) erythroblasts, with a lack of mature (GpA+bright) erythroblasts, as compared to the considerable peak of mature cells in both group B patients. Pt indicates patient.
GpA measurement in erythroblasts.
Representative flow cytometry of GpA expression levels measured via a PE-conjugated antiserum in bone marrow erythroblasts purified from marrows of MM patients with active disease and severe anemia (group A) or with preserved erythropoiesis (group B). Group A patients display a preponderance of immature (GpA+dim) erythroblasts, with a lack of mature (GpA+bright) erythroblasts, as compared to the considerable peak of mature cells in both group B patients. Pt indicates patient.
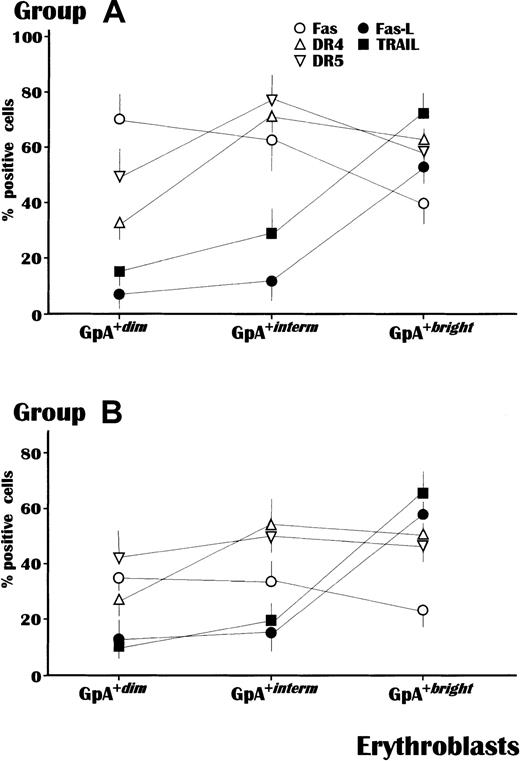
In the next set of experiments, the fluorescence intensity of GpA was correlated with the expression of a number of membrane receptors involved in the erythroblast apoptosis pathway. Fas-L and TRAIL are physiologically expressed by normal erythroblasts as their maturation progresses, presumably to control erythropoiesis, whereas their coreceptors, namely, Fas and DR molecules, are functional during early or intermediate maturation.10 17
This analysis was completed in all subjects from groups A and B, with the exception of patients A-10 and B-06 because of insufficient GpA+ cells, and showed a substantial difference in the mean percent cells expressing these receptors (Figure2). Apoptogen receptors, namely, Fas, DR4, and DR5, increased in expression during maturation in both groups. However, the levels of Fas were significantly higher in all subsets of GpA+ cells from group A (P < .05 in all instances). The highest Fas expression (73.4% ± 8%) occurred within the GpA+dim population from group A compared with only 37.1% ± 5% in group B. In addition, though at lower levels, Fas was also up-regulated in both GpA+interm and GpA+bright erythroblasts from group A patients as compared to those of group B. The expression of DR4 and DR5 was also different, in that GpA+interm erythroblasts from group A showed higher levels of both receptors than those from group B, whereas their expression was overall equivalent within the other cell populations.
Receptor expression in erythroblasts in relation to their maturative stage, measured by GpA intensity.
The mean levels of Fas were significantly higher in all erythroblast subsets (P < .05) from MM patients with severe anemia (A) compared to those from patients of the control group (B). Both DR4 and DR5, as agonist receptors for TRAIL, were also up-regulated in erythroblasts from anemic patients though prevalently in the GpA+interm subset. By contrast, Fas-L and TRAIL occurred at comparable values in both groups and exhibited their maximal intensity in GpA+bright cells. The analysis was completed by flow cytometry using the Cell-Quest software and values are expressed as mean ± SEM.
Receptor expression in erythroblasts in relation to their maturative stage, measured by GpA intensity.
The mean levels of Fas were significantly higher in all erythroblast subsets (P < .05) from MM patients with severe anemia (A) compared to those from patients of the control group (B). Both DR4 and DR5, as agonist receptors for TRAIL, were also up-regulated in erythroblasts from anemic patients though prevalently in the GpA+interm subset. By contrast, Fas-L and TRAIL occurred at comparable values in both groups and exhibited their maximal intensity in GpA+bright cells. The analysis was completed by flow cytometry using the Cell-Quest software and values are expressed as mean ± SEM.
By contrast, there were no differences between groups A and B in the occurrence of Fas-L and TRAIL, which were poorly expressed during the early maturative stages, but increased expression within the GpA+bright population.
These results suggested that progression through erythroblast maturation in MM is regularly associated with a variable expression of phenotypic markers, namely, GpA, Fas-L, TRAIL, and their coreceptors. However, abnormal up-regulation of functional molecules, such as Fas and the receptors to TRAIL, is apparently predominant during both the early and the intermediate maturative steps of erythropoiesis, especially in anemic patients.
Apoptosis of erythroblasts from patients with MM in the presence of Fas-L+/TRAIL+ myeloma cells
To resemble in vivo conditions, coculture experiments of erythroblasts with autologous myeloma cells were carried out to assess the susceptibility of erythroblasts to apoptosis in the presence of autologous Fas-L+/TRAIL+ plasma cells. To obtain separate measurements of the effect of Fas-L or TRAIL, each coculture was set up in triplicate to include untreated plasma cells to determine the cumulative efficacy, plasma cells first TRAIL disabled by saturation with anti-TRAIL mAb to evaluate the effect of Fas-L, and plasma cells first Fas-L inactivated by soluble Fas to verify TRAIL activity. The effect of these prenested cell preparations, named TRAIL- or Fas-L–inhibited plasma cells, was compared with that of control erythroblast suspensions cultured in their absence. After coculturing, the cells were washed, treated with the FITC-annexin-V and the PE-anti-GpA mAb, and then investigated by double-fluorescence flow cytometry. The analysis was completed in 8 cocultures using cells from group A patients (patients 01, 02, 04, 06, 07, 11, 12, and 14) and in 7 from group B (patients 02, 04, 05, 07, 10, 11, and 12).
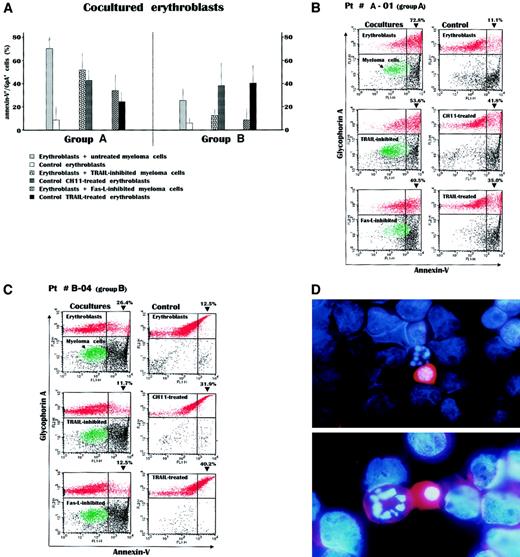
Figure 3A shows mean values for erythroblast apoptosis in these cultures. Apoptosis was significantly higher in cultures from group A using untreated as well as TRAIL- or Fas-L–inhibited myeloma cells (P < .02 in all instances). Cultures from group B had no significant cytotoxicity of autologous plasma cells. However, a decline of these values was observed in cultures using inhibited malignant plasma cells, which still maintained a significantly higher level of erythroblast apoptosis than control erythroblast suspensions (P < .02). Control erythroblasts treated with either anti-Fas CH11 MoAb or recombinant TRAIL (Alexis) to resemble the effect of both apoptogenic receptors showed an apoptosis extent similar to that induced by either TRAIL-inhibited or Fas-L–inhibited plasma cells, respectively, whereas in parallel experiments we assessed the ability of EPO to reduce the extent of Fas-induced erythroblast apoptosis (data not shown).
Measurement of apoptosis by annexin-V detection in erythroblasts after their overnight incubation with autologous myeloma cells.
(A) The experiment included 8 cocultures from group A patients and 7 from group B patients. A great increase in the annexin-V+/GpA+ cell population was recorded within the cocultures from the group A patients. This was particularly evident in cocultures incubating erythroblasts with untreated myeloma cells, whereas the selective disablement of either Fas-L or TRAIL in parallel cultures whose myeloma cells were inhibited by the soluble Fas-Fc or the anti-TRAIL antibody, respectively, revealed the effect separately induced by each apoptogenic receptor of myeloma cells. These effects were almost equivalent to those obtained in control erythroblasts directly stimulated by either the CH11 anti-Fas mAb or the recombinant TRAIL. On the contrary, the effect induced in the cocultures from group B patients by the autologous plasma cells was modest and lower than the respective values observed in group A patients (P < .02 in all instances). Values are mean ± SD of double-fluorescent (annexin-V+/GpA+) cells. (B) Flow cytometry analysis of cocultured cells from a patient with severe anemia. Incubation of erythroblasts (red) with myeloma cells (green) induced a dramatic increase of annexin-V+cells within the GpA+ population. This effect, presumably induced by the concurrent expression of both Fas-L and TRAIL by myeloma cells (top), was then separately measured by inhibiting either TRAIL (middle) or Fas-L (bottom) and was still higher than that recorded in control erythroblasts treated with the anti-Fas mAb or TRAIL (right). (C) Erythroblasts from a patient with preserved erythropoiesis were only moderately driven to apoptosis by their autologous plasma cells and the alternate inhibitions abolished the feeble cytotoxic effect in both cases. (D) Morphologic pattern in the coculture from patient A-1. The malignant myeloma cells among the erythroblasts whose nuclei are stained in blue by DAPI show a high content of TRAIL (red) detected by the PE-conjugated antibody in both cytoplasm and membrane and are adjacent to apoptotic erythroblasts characterized by nuclear fragmentation. Original magnification, × 50 (top), × 100 (bottom).
Measurement of apoptosis by annexin-V detection in erythroblasts after their overnight incubation with autologous myeloma cells.
(A) The experiment included 8 cocultures from group A patients and 7 from group B patients. A great increase in the annexin-V+/GpA+ cell population was recorded within the cocultures from the group A patients. This was particularly evident in cocultures incubating erythroblasts with untreated myeloma cells, whereas the selective disablement of either Fas-L or TRAIL in parallel cultures whose myeloma cells were inhibited by the soluble Fas-Fc or the anti-TRAIL antibody, respectively, revealed the effect separately induced by each apoptogenic receptor of myeloma cells. These effects were almost equivalent to those obtained in control erythroblasts directly stimulated by either the CH11 anti-Fas mAb or the recombinant TRAIL. On the contrary, the effect induced in the cocultures from group B patients by the autologous plasma cells was modest and lower than the respective values observed in group A patients (P < .02 in all instances). Values are mean ± SD of double-fluorescent (annexin-V+/GpA+) cells. (B) Flow cytometry analysis of cocultured cells from a patient with severe anemia. Incubation of erythroblasts (red) with myeloma cells (green) induced a dramatic increase of annexin-V+cells within the GpA+ population. This effect, presumably induced by the concurrent expression of both Fas-L and TRAIL by myeloma cells (top), was then separately measured by inhibiting either TRAIL (middle) or Fas-L (bottom) and was still higher than that recorded in control erythroblasts treated with the anti-Fas mAb or TRAIL (right). (C) Erythroblasts from a patient with preserved erythropoiesis were only moderately driven to apoptosis by their autologous plasma cells and the alternate inhibitions abolished the feeble cytotoxic effect in both cases. (D) Morphologic pattern in the coculture from patient A-1. The malignant myeloma cells among the erythroblasts whose nuclei are stained in blue by DAPI show a high content of TRAIL (red) detected by the PE-conjugated antibody in both cytoplasm and membrane and are adjacent to apoptotic erythroblasts characterized by nuclear fragmentation. Original magnification, × 50 (top), × 100 (bottom).
Figure 3B includes the flow cytometry results for a representative group A patient (A-01). As can be seen, the incubation of erythroblasts with myeloma cells promoted apoptosis, namely, the annexin-V+ cell peak, at levels as high as 72.8% of the entire GpA+ population with respect to control erythroblasts (11.1%). However, by separately measuring the effect of Fas-L on erythroblasts cocultured with TRAIL-inhibited myeloma cells, or alternatively the effect of TRAIL on those with Fas-L–inhibited plasma cells, we still found a variable induction of erythroblast apoptosis, though lower than that induced by untreated myeloma cells (left panels).
As expected, control erythroblast suspensions treated with CH11 mAb, or recombinant TRAIL, showed a variable though prompt increase in the annexin-V+ population (right panels). The flow cytometric patterns for a patient from group B (B-04; Figure 3C) show an increment in the annexin-V+/GpA+ cell subset in response to the presence of autologous plasma cells, though much lower than for patient A-01. However, the differential measurement of the Fas-L– or TRAIL-induced apoptosis revealed a weak effect for both receptors, whereas control erythroblasts were again responsive to stimulation of either Fas by CH11 mAb, or both DR4 and DR5 by recombinant TRAIL.
The morphologic pattern of the effect induced in the coculture of patient A-01 by exposing erythroblasts to malignant autologous myeloma cells is presented in Figure 3D. The double-fluorescence staining emphasizes that the malignant plasma cells have high expression of TRAIL in both the cytoplasm and cell membrane, as revealed by the PE-conjugated antibody (red). The erythroblast nuclei are stained by DAPI (blue), which reveals chromatin condensation and fragmented nuclear material in erythroblasts juxtaposed to the myeloma cells.
These analyses provide evidence that malignant plasma cells have an apoptogenic effect on autologous erythroblasts through their expression of Fas-L and TRAIL. This effect was prominent in cocultures from severely anemic patients, potentially as a result of both the increased expression of death receptors by their myeloma cells, and the prevalence in their cultures of immature erythroblasts highly susceptible to apoptosis.
In vivo activation of erythroblast apoptosis
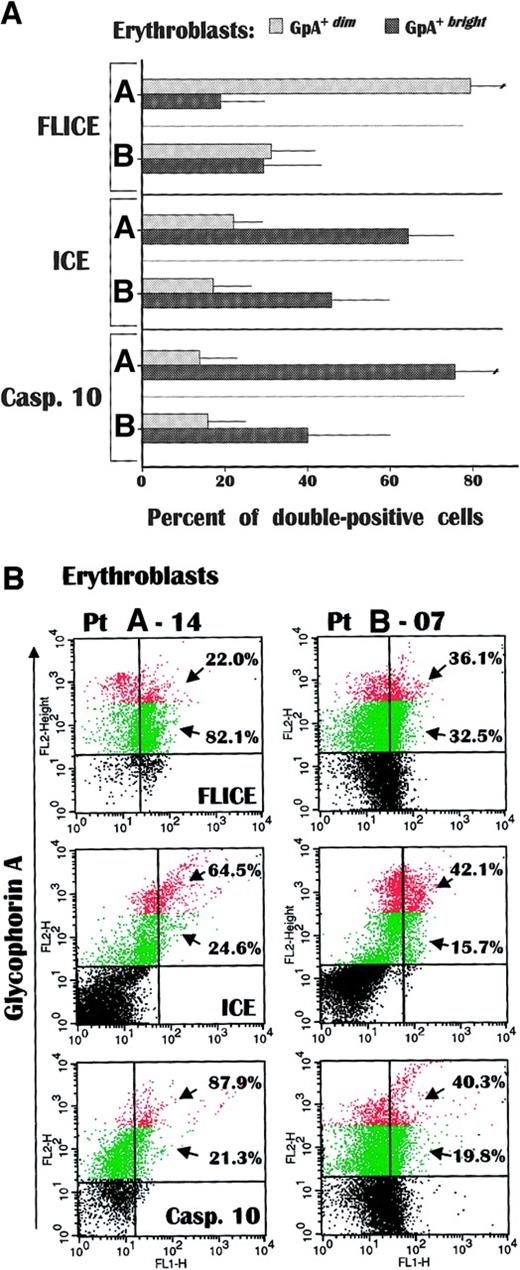
Further experiments were designed to determine whether erythroblasts can be activated in vivo to apoptosis following their putative stimulation by myeloma cells, by measuring the expression of cytoplasmic proteases in freshly derived erythroblasts from several patients (A-01, A-04, A-06, A-07, A-12, A-14 and B-04, B-05, B-07, B-11). FLICE, ICE, and caspase-10 were investigated by double-fluorescence analysis and correlated with GpA intensity. Their expression varied in relation to the concurrent state of erythropoiesis (Figure 4A). FLICE was predominantly expressed (> 82.0%) by the GpA+dim cells from group A patients, thus confirming the expansion of the immature erythroid subset.
Caspase expression in fresh GpA
+dim and GpA+brighterythroblasts. FLICE was greatly accumulated in GpA+dim cells from group A, whereas both ICE and caspase-10 occurred at significantly higher values in their GpA+brighterythroblasts than in group B. Although the analysis evaluated the extent of proenzyme caspases, the differential accumulation of these proteases in fresh erythroid cells suggested the occurrence in vivo of separate apoptotic stimuli through Fas in immature erythroblasts, and TRAIL receptors as the maturation progresses. Data are expressed as mean ± SD of double-fluorescence analysis. (B) Flow cytometric pattern of caspase expression in GpA+dim (green) and GpA+bright (red) erythroblasts in a representative anemic (A-14) and a nonanemic (B-07) patient. The percentages indicate the number of positive cells within each erythroblast subset.
Caspase expression in fresh GpA
+dim and GpA+brighterythroblasts. FLICE was greatly accumulated in GpA+dim cells from group A, whereas both ICE and caspase-10 occurred at significantly higher values in their GpA+brighterythroblasts than in group B. Although the analysis evaluated the extent of proenzyme caspases, the differential accumulation of these proteases in fresh erythroid cells suggested the occurrence in vivo of separate apoptotic stimuli through Fas in immature erythroblasts, and TRAIL receptors as the maturation progresses. Data are expressed as mean ± SD of double-fluorescence analysis. (B) Flow cytometric pattern of caspase expression in GpA+dim (green) and GpA+bright (red) erythroblasts in a representative anemic (A-14) and a nonanemic (B-07) patient. The percentages indicate the number of positive cells within each erythroblast subset.
Although we measured the expression of the native proenzyme forms of the caspases, their dramatic accumulation was interpreted as presumably induced in vivo because the GpA+bright cells from group A patients as well as the full erythroblast population from group B patients showed a moderate presence of FLICE+ cells (20%-35%) that reflected its constitutive expression.9By contrast, both ICE and caspase-10 were poorly expressed by GpA+dim erythroblasts from all patients, and again increased remarkably within the GpA+bright population of group A. The cellular content of both procaspases was significantly higher than in the group B GpA+bright subset (P < .05 in both instances).
Figure 4B depicts the relative enrichment of caspase-positive cells within fresh erythroblasts from patients A-14 and B-07 in relation to their GpA levels. The GpA+dim cells from patient A-14 showed the highest frequency of FLICE expression, suggesting that the biochemical transduction of death signals through this caspase was operative in vivo, in particular in immature erythroid cells (green). By contrast, both ICE and caspase-10 were largely expressed by the GpA+bright erythroblasts (red) from both patients, though their maximum increase occurred in mature cells from patient A-14. Therefore, the high level of caspase expression in fresh erythroid cells from anemic patients supports the hypothesis that activation of erythroblast apoptosis in vivo occurs via parallel transduction pathways of death signals in relation to their maturative steps; immature erythroblasts are more susceptible to Fas-transduced apoptosis as a result of FLICE overexpression, whereas the increase of both ICE and caspase-10 in GpA+bright cells emphasizes their sensitivity to TRAIL as maturation progresses.
Defective in vivo GATA-1 expression by erythroblasts from anemic MM patients
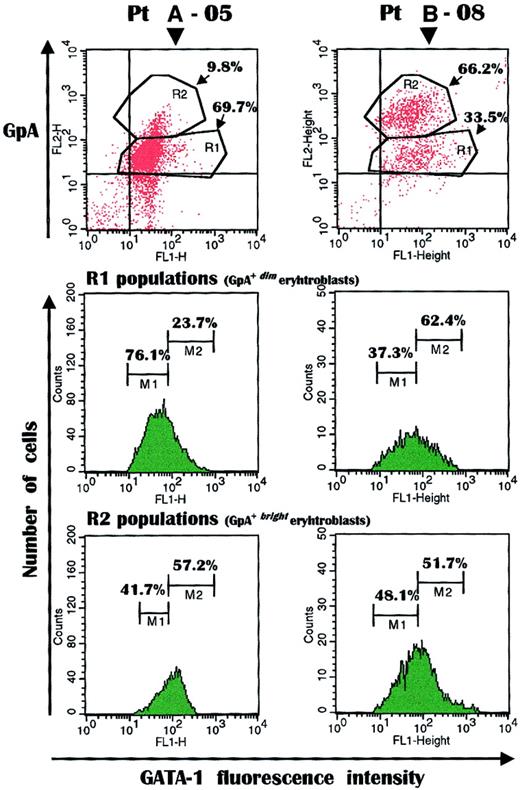
Because impaired erythropoiesis in severely anemic patients was associated with a maturative arrest of erythroid precursors by the malignant myeloma cells, experiments were carried out to assess whether the expression of GATA-1, one transcriptional factor that regulates erythroblast maturation, was concurrently disturbed. Thus, the cellular content of GATA-1 was measured by double-fluorescence analysis within each erythroblast population with respect to its maturation in a number of patients from both groups (A-02, A-05, A-09, A-11, A-14 and B-01, B-07, B-08, B-11).
Similar results were recorded within each group of patients. Figure5 depicts this analysis in representative patients (A-05 and B-08) and shows the expected discrepancy in the relative distribution of their GpA+dim and GpA+bright erythroblast populations. The immature erythroblast subset (R1) in patient A-05, in fact, was greatly expanded (70%) to the detriment (< 10%) of the mature cell population (R2), whereas a lower percentage of immature cells (33.5%) was detected in patient B-08. However, a substantial diversity in GATA-1 expression was recorded between the 2 GpA+dimpopulations. The immature erythroblast subset in patient A-05 was associated with a low fluorescence intensity of the transcriptional factor (M1 peak: 76.1% of positive cells), whereas a high fluorescence intensity (M2 peak) was detected in only a minority of the GpA+dim erythroblasts (23.7% of positive cells). By contrast, the immature erythroblasts from patient B-08 showed the opposite pattern of GATA-1 content; the M1 peak included 37.3% extent of positive cells, whereas the subset of immature cells with high intensity of GATA-1 expression was considerably elevated (62.4%).
GATA-1 measurement in fresh erythroblasts.
The transcription factor was detected in myeloma patients with severe (A-05) or without (B-08) anemia showing a discrepancy in their extent of GpA+dim (R1) and GpA+bright (R2) populations. The immature erythroblast subset expressing GATA-1 at very low intensity (M1) was considerably expanded in the anemic patient (76.1% versus 37.3% of patient B-08) to the detriment of the M2 population, which included immature erythropoietic cells with a regular to high content of GATA-1. Conversely, the M2 population was substantial (62.4%) in patient B-08. GATA-1 at either low or high fluorescence intensity was similarly expressed as percentage of positive cells in both patients, though the proportion of the mature erythroblast population was modest in the anemic patient.
GATA-1 measurement in fresh erythroblasts.
The transcription factor was detected in myeloma patients with severe (A-05) or without (B-08) anemia showing a discrepancy in their extent of GpA+dim (R1) and GpA+bright (R2) populations. The immature erythroblast subset expressing GATA-1 at very low intensity (M1) was considerably expanded in the anemic patient (76.1% versus 37.3% of patient B-08) to the detriment of the M2 population, which included immature erythropoietic cells with a regular to high content of GATA-1. Conversely, the M2 population was substantial (62.4%) in patient B-08. GATA-1 at either low or high fluorescence intensity was similarly expressed as percentage of positive cells in both patients, though the proportion of the mature erythroblast population was modest in the anemic patient.
However, by measuring GATA-1 fluorescence intensity in mature erythroblasts from both patients, a substantial equivalence of data was observed because both the M1 and the M2 peaks showed comparable results in both patients (M1, 41.8% versus 48.1%, and M2, 57.2% versus 51.7% in patients A-05 and B-08, respectively). These findings suggested that a major defect of GATA-1 expression was detectable in immature erythroblasts, in particular in patients with severe anemia, and we hypothesize this was associated with the cleavage of its native form induced by Fas-L or TRAIL or both.24
Discussion
The present study addresses a novel mechanism of anemia in MM and possibly also in other hematologic malignancies. Because severe anemia associated with bone lesions defines the clinical progression of MM, we explored the role of up-regulated apoptogenic receptors exposed by highly malignant myeloma cells in the destruction of the erythroid matrix. We measured the levels of both Fas-L and TRAIL expression by bone marrow plasma cells from a cohort of patients with untreated MM or MGUS, grouped in relation to the occurrence of anemia, and found that their highest intensity was detectable in severely anemic subjects with clinical signs of active disease. The erythroblast population in their marrow aspirates was constitutively depleted and mostly composed of GpA+dim cells. This relative GpA+dim expansion was not detected in myeloma patients without severe anemia, in that they exhibited both GpA+bright and GpA+dimcells and their erythropoiesis was comparable to that in MGUS patients.
In addition, coculturing these erythroblasts with autologous myeloma cells resulted in marked erythroblast cytotoxicity induced by either Fas-L or TRAIL or both presented by the malignant plasma cells from the severely anemic patients with MM. The initiator and executioner caspase28 content of fresh erythroblasts was also determined to assess whether these cytotoxic mechanisms were operative in vivo. FLICE was greatly expressed by immature erythroblasts in anemic patients and a concurrent increase of both ICE and caspase-10 was detectable in their mature erythroid cells. Erythropoiesis was also studied by measuring the content of GATA-1 in each erythroblast subset. This transcriptional factor of erythroid maturation was extremely down-regulated in the expanded GpA+dim population of the anemic patients.
Taken together, these findings strongly suggest that in the absence of any suppressive effect induced by chemotherapy, several cytotoxic mechanisms are exerted by myeloma cells and are responsible for chronic inhibition of erythropoiesis. Moreover, the abnormal overexpression of apoptogenic receptors such as Fas-L or TRAIL (or both) defines the major malignant phenotype associated with progression of MM.
A persistent derangement of the cytokine network is thought to contribute to the defective erythropoiesis in hematologic malignancies, leading to chronic normochromic/normocytic anemia. TNF-β inhibits the formation of erythroid colony-forming unit in mice29 and decreased hemoglobin values in metastatic cancer patients treated with its recombinant form in a phase I trial.30 Increased TNF-β serum levels in both MM31 and other malignancies32 are thus suspected to down-regulate the maturation of erythroblasts by direct cytotoxicity, or by enhancing the susceptibility of their apoptogen receptors. IFN-γ secretion is also up-regulated in hematologic malignancies. This proinflammatory cytokine is believed to contribute to the progression of anemia in MM31,33 and in other cancers34 by up-regulating Fas in GpA+dimerythroblasts,5,11 as well as TRAIL in solid tumors,35 and by direct activation of several caspases including ICE, CPP32, and FLICE.36 However, the pathogenic role of these cytokines in the progression of MM is still controversial, because no correlation between IFN-γ or TNF-α levels and several variables of MM clinical activity was apparent in a previous study.37 The great expansion of annexin-V+ erythroblasts cultivated with Fas-L+or TRAIL+ malignant plasma cells strongly suggests that the cytotoxic killing of erythroid cells was carried out via both Fas and the receptors to TRAIL, presumably up-regulated in vivo by those proinflammatory cytokines.
Our data indicate that the extensive damage to the erythropoietic process caused by Fas-L+/TRAIL+ myeloma cells and resulting in severe anemia occurs at different maturation phases. GpA+dim erythroblasts, particularly at the basophilic stage, show maximal Fas sensitivity despite their poor responsiveness to TRAIL,38 though they express both DR4 and DR5 molecules as agonist receptors of this ligand.39 Conversely, GpA+interm erythroblasts, obtained in vitro by maturation of CD34+ hematopoietic progenitors with EPO, are susceptible to TRAIL-mediated apoptosis.17Both subsets are driven to apoptosis by Fas-L+ and TRAIL+ GpA+brighterythroblasts, which operate a negative regulatory feedback to inhibit excess maturation, whereas they are resistant to most apoptogenic ligands.10 17 Therefore, the ultimate stage of erythroid precursors sensitive to those cell death activators includes GpA+interm erythroblasts.
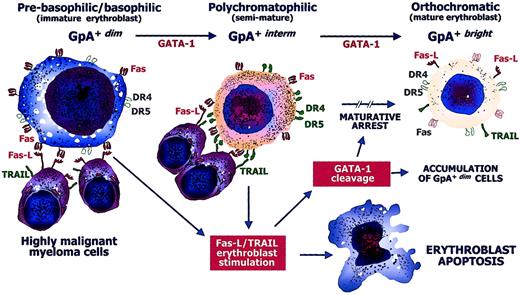
The expansion of GpA+dimerythroblasts in the severely anemic patients with MM suggests that the enhanced apoptosis during maturation is the major event leading to defective erythropoiesis. In the presence of an excess of both Fas-L and TRAIL provided by the accumulation of malignant myeloma cells, maturation is suppressed at either the GpA+dim or the GpA+interm stage. Accretion of GpA+dim erythroblasts in group A patients may thus be a consequence of maturative arrest, whereas the few erythropoietic precursors that escape the Fas-L/TRAIL cytotoxicity of malignant myeloma cells complete their differentiation and become GpA+bright erythroblasts. Figure6 illustrates the hypothetical events that possibly lead to the maturative arrest of erythropoiesis and to the gradual destruction of erythroid productive capacity by Fas-L+/TRAIL+ highly malignant myeloma cells.
Defective erythropoiesis in MM.
Hypothetical model of the pathogenic defective maturation of erythroid cells by malignant cytotoxic plasma cells in active MM. GpA+dim erythroblasts progress to the GpA+bright form by GATA-1. At their prebasophilic/basophilic stage (GpA+dim), erythroblasts are highly susceptible to Fas stimulation because they can be negatively regulated by Fas-L from mature (GpA+bright) erythroblasts just as GpA+interm erythroblasts are restrained by TRAIL. Fas and the TRAIL receptors, namely, DR4/DR5 molecules, are expressed during erythroid differentiation, though they are not functional in specific stages (empty symbols). Highly malignant myeloma cells express large amounts of both Fas-L and TRAIL and exert a direct erythroblast cytotoxicity by inducing apoptosis. The cytotoxic mechanism operates during either the immature or the semimature stage of differentiation by both Fas and DR4/DR5. The cytoplasmic caspases activated by these apoptogen receptors induce the cleavage of GATA-1, whose intracellular defect promotes an arrest in the maturative progression. This results in a relative increase of immature erythroblasts and systematic impairment of the erythroid matrix.
Defective erythropoiesis in MM.
Hypothetical model of the pathogenic defective maturation of erythroid cells by malignant cytotoxic plasma cells in active MM. GpA+dim erythroblasts progress to the GpA+bright form by GATA-1. At their prebasophilic/basophilic stage (GpA+dim), erythroblasts are highly susceptible to Fas stimulation because they can be negatively regulated by Fas-L from mature (GpA+bright) erythroblasts just as GpA+interm erythroblasts are restrained by TRAIL. Fas and the TRAIL receptors, namely, DR4/DR5 molecules, are expressed during erythroid differentiation, though they are not functional in specific stages (empty symbols). Highly malignant myeloma cells express large amounts of both Fas-L and TRAIL and exert a direct erythroblast cytotoxicity by inducing apoptosis. The cytotoxic mechanism operates during either the immature or the semimature stage of differentiation by both Fas and DR4/DR5. The cytoplasmic caspases activated by these apoptogen receptors induce the cleavage of GATA-1, whose intracellular defect promotes an arrest in the maturative progression. This results in a relative increase of immature erythroblasts and systematic impairment of the erythroid matrix.
This interpretation is supported by the caspase content of fresh erythroblasts. The largest FLICE+ population was found in GpA+dim erythroblasts from severely anemic patients, thus suggesting Fas-pathway activation in vivo,28 whereas ICE and particularly caspase-10, which have been associated with the TRAIL pathway,40 41 were increased in subsequent maturation phases. The low to moderate expression of these caspases in erythroblasts from myeloma patients with efficient erythropoiesis emphasizes the substantial defect of Fas-L/TRAIL-mediated cytotoxicity in those cells.
Another interesting point of this study is the defective content of GATA-1 in erythroid precursors from anemic patients. This transcription factor promotes survival and is essential for the terminal differentiation of erythroblasts, because it binds regulatory motifs in globin genes23 and promotes the activation of other functional genes committed to the expression of EPO receptors, GpA, and transferrin receptor.42,43 Elegant studies by De Maria and coworkers24 have demonstrated that GATA-1 is promptly cleaved by several caspases, including FLICE, in CD34+cell-derived erythroblasts, following their treatment with the agonist anti-Fas mAb from clone CH11, or with TRAIL. This cleavage may be supposed to lead to maturative arrest. Our evidence of a clear-cut defect of the native form of GATA-1 in the expanded GpA+dimpopulation (Figure 5) is in line with these studies and demonstrates that myeloma cells may also down-regulate GATA-1 by both Fas-L and TRAIL pathways, in addition to direct cytotoxic killing.
The median survival of patients with MM receiving conventional chemotherapy with melphalan plus prednisone is approximately 36 months.44 However, patients with aggressive disease are, or rapidly become, refractory to this treatment and show early expansion of myeloma cells. Despite years of intensive investigation, the molecular phenotype(s) of highly malignant plasma cells associated with the invasive evolution of MM have not yet been fully defined. Our studies emphasize the deregulated apoptosis in myeloma cells and correlate the occurrence of specific phenotypic and functional derangements with their ability to proliferate and expand within the bone marrow to the detriment of other cells. The apoptogenic phenotype of plasma cells,45 as identified by both the abnormal overexpression of Fas-L9 and TRAIL and the deficient response to Fas stimulation, together with IL-6 insensitivity,18 may well be a marker of a high degree of malignancy and unrestrainable progression.
Supported by the Ministry for the Universities and Scientific and Technological Research, the Italian Ministry of Health Istituto Superiore di Sanità, Rome, National AIDS Research Project, Italy, and by an AIRC (Associazione Italiana per la ricerca sul cancro) research grant, Milan, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Franco Silvestris, DIMO, University of Bari, Section of Internal Medicine and Clinical Oncology, Piazza G. Cesare, 11-70124 Bari, Italy; e-mail: f.silvestris@dimo.uniba.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal