Antibodies against CD20 can activate complement and induce antibody-dependent cellular cytotoxicity (ADCC) in B lymphocytes. In B-cell lines, such antibodies also induce apoptosis. In this study, the expression and function of CD20 on B-cell chronic lymphocytic leukemia (B-CLL) cells were analyzed. Flow cytometric analysis demonstrated that B-CLL cells express CD20 with a fluorescence intensity that is significantly weaker than that of normal CD5+ and CD5− B cells and that of malignant CD5− low-grade non-Hodgkin lymphoma cells. A small population of cells from healthy donors that have an expression pattern of CD5 and CD20 identical to that of B-CLL cells were identified, and this population was confirmed to be of T lineage, not B lineage. Culture of freshly isolated B-CLL cells in the presence of the chimeric anti-CD20 antibody rituximab and a cross-linking F(ab)2 fragment, resulted in dose- and time-dependent induction of apoptosis. The induction of apoptosis occurred under conditions in which the influence of complement activation and ADCC was negligible. Cross-linking of rituximab induced strong and sustained phosphorylation of the 3 mitogen activated protein (MAP) kinases c-Jun NH2-terminal protein kinase, extracellular signal–regulated kinase, and p38. Introduction of the p38 inhibitor SB203580 into the system completely blocked signaling downstream of p38, as evidenced by the absence of MAPKAP K2 activity, and significantly reduced the degree of anti-CD20–induced apoptosis. These results demonstrate that cross-linking of rituximab bound to CD20 on freshly isolated B-CLL cells induces apoptosis through a signaling pathway that is dependent on p38 MAP-kinase activation.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is the most common form of leukemia in the Western world, with an annual incidence exceeding 5 new cases per 100 000 individuals, and the disease must still be regarded as incurable.1,2 The pathogenesis of B-CLL is poorly understood, but deregulated control of apoptosis is believed to be critical for the characteristic accumulation of B lymphocytes in this disease.1,3 B-CLL cells are long-lived, small, mature CD5+CD19+CD23+ B cells that appear to be arrested early in the G1-phase of the cell cycle.1-5
CD20 is a 33- to 37-kd membrane protein of unknown function, expressed on mature B cells and their B-lineage bone marrow precursors.6-8 Because CD20 neither sheds from the cell surface nor internalizes upon antibody binding, sustained binding of anti-CD20 antibodies can activate effector mechanisms, such as complement-mediated lysis and antibody-dependent cellular cytotoxicity (ADCC), and immunotherapy with anti-CD20 antibodies has demonstrated significant efficacy in the treatment of B-cell neoplasms.9-14 However, in B-cell lines, cultured under conditions that preclude activation of these effector mechanisms, anti-CD20 antibodies also induce apoptosis.15-17CD20-induced apoptosis in these cell lines has been shown to be partially dependent on protein tyrosine kinase activity, Ca++ mobilization, and effector caspase activation.16
In this study, we have used freshly isolated primary leukemia cells from patients with B-CLL to analyze the functional consequences of binding of a mouse/human chimeric anti-CD20 antibody (rituximab) to CD20 expressed in the membrane of the malignant cells. We demonstrate that rituximab activates a CD20-mediated signaling pathway that results in apoptosis and is dependent on p38 mitogen activated protein (MAP)–kinase activation.
Patients, materials, and methods
Patients
Fresh blood samples were obtained from patients previously diagnosed with CD19+CD5+CD23+B-CLL, who had been off all treatment, including corticosteroids, for at least 30 days. The mean percentage of CD5+CD20+ cells in the mononuclear fraction was 96.1% (range, 90%-99%). All patients signed informed consent. The study was approved by the regional committee on scientific ethics in Copenhagen.
Flow cytometry
Mononuclear cells were isolated from fresh blood by Lymphoprep separation (Nycomed Pharma, Oslo, Norway), washed, and stained with fluorochrome-conjugated antibodies against CD20 and CD5 (Becton Dickinson, Erembodegem, Belgium). Buffy coats from 16 healthy donors and tumor samples from 11 patients with low-grade non-Hodgkin lymphoma (NHLs) were used as controls. For analysis of CD20dim cells in normal buffy coats, directly conjugated antibodies against CD3, CD4, CD8, CD13, CD14, CD19, CD22, CD23, CD33, CD34, CD38, CD56, CD95, kappa or lambda immunoglobulin light chains (all from Becton Dickinson) or FMC7, immunoglobulin (Ig)–M or IgG (DAKO, Glostrup, Denmark) were applied. We acquired 5000 to 30 000 ungated events and analyzed them on a FACsort flow cytometer (Becton Dickinson) with the use of the CellQuest software.
Cell culture and reagents
Mononuclear cells were cultured at 2 × 106/mL in RPMI 1640 containing 10% heat inactivated fetal calf serum (Harlan Sera-Lab, Sussex, England) and antibiotics, in a humidified atmosphere containing 5% CO2. Cell suspensions were supplemented with 4 μg/mL, unless otherwise indicated, of chimeric anti-CD20 antibody rituximab (kindly provided by IDEC Pharmaceuticals, San Diego, CA) or a control human IgG-preparation (SSI-IVIG, a kind gift from Dr Flemming Balstrup, Statens Seruminstitut, Copenhagen, Denmark), containing greater than 95% IgG with 61% IgG1. For cross-linking, the cultures were further supplemented with anti–human IgG1F(ab)2 fragment (AO407, DAKO) at concentrations 5 times that of the primary antibody. For inhibition of p38 MAP-kinase activity, cells were preincubated for 60 minutes in the presence of 2.5 to 10 μM SB203580, an inhibitor of the p38 pathway, or 10 to 25 μM UO126, an inhibitor of the extracellular signal–regulated kinase (ERK) pathway (Alexis Biochemicals, San Diego, CA) and subsequently supplemented with rituximab or the control preparation.
Detection of apoptosis
For morphology studies, cytospin preparations were stained with May-Grünwald-Giemsa. To quantitate the extent of apoptosis, cells were harvested, washed in phosphate-buffered saline (PBS), and gently resuspended in 0.4 mL hypotonic fluorochrome solution (0.05 mg/mL propidium iodide (PI) in 0.1% sodium citrate, 0.37% NP40, and 0.02 mg/mL RNase A), as previously described.18-20There were 5000 events, at a rate of 100 to 200 events per second acquired on a FACSort flow cytometer, with the use of a threshold of 20 in FL-2 linear mode. For phosphatidylserine exposure and membrane integrity analysis, cells were double-stained with fluorescein isothiocyanate (FITC)–labeled annexin-V and PI with the Apoptosis Detection Kit I (6693KK; PharMingen, San Diego, CA) according to the manufacturer's instructions.21 There were 30 000 ungated events acquired on a FACsort flow cytometer; these were analyzed by means of the CellQuest software.
Western blot analysis
Cells were washed once in ice-cold PBS and lysed in ice-cold lysis buffer (10 mM Hepes [pH 7.3], 0.1% Triton X-100, 400 mM KCl, 2 mM EDTA, 1 mM ethyleneglycotetraacetic acid, 1 mM dithiothreitol [DTT], 1 mM Na3VO4, 1 mM phenylmethyl sulfonyl fluoride, 5 μg/mL leupeptin, 5 μg/mL aprotinin, 5 μg/mL pepstatin). Sodium dodecyl sulfate–polyacrylamide gel electrophoresis was performed with the use of equal amounts of whole cell lysate, followed by transfer to nitrocellulose filters. After blocking with 5% milk and overnight incubation with the primary antibodies (c-Jun NH2-terminal protein kinase [JNK] [sc-571], ERK [sc-94-G], p38 [sc-535] (Santa Cruz Biotechnology, Santa Cruz, CA) and pY-JNK (9255S), pY-ERK (9106S), and pY-p38 (9211S) (New England BioLabs, Beverly, MA), the nitrocellulose sheet was further processed by means of horseradish peroxidase-conjugated secondary antibody (PO260, PO448, or PO160) (DAKO) and developed by means of an enhanced chemiluminescence system (Amersham Pharmacia Biotech, Little Chalfont, England).
In vitro kinase assay
First, 1 × 108 cells were lysed for 30 minutes on ice in 1 mL lysis buffer (50 mM Tris-base, 0.5% NP-40, 150 mM NaCl, 10 mM NaF, 10 mM sodium pyrophosphate, 1 mM EDTA, 10 μg/mL leupeptin, 10 μg/mL aprotinin). Cellular debris was removed by centrifugation at 12 000g for 10 minutes at 4°C. Total MAPKAP K2 was immunoprecipitated by addition of 3 μg primary antibody (06-534) (Upstate Biotechnology, Lake Placid, NY) overnight, followed by 10 μL protein G sepharose for an additional hour. The immunoprecipitates were washed 3 times in wash buffer (50 mM Tris base, 0.1% NP-40, 150 mM NaCl, 10 mM NaF, 10 mM sodium pyrophosphate, 1 mM EDTA, 10 μg/mL leupeptin, 10 μg/mL aprotinin) and once in kinase assay buffer (25 mM Hepes [pH 7.4], 25 mM β-glycerophosphate, 25 mM MgCl2, 0.1 mM Na3VO4, 2 mM DTT). Then, 50 μL beads in assay buffer were left as a 1:1 suspension, and 15 μL MAPKAP K2 substrate (12-240) (Upstate Biotechnology) was added. In vitro kinase reactions were initiated by the addition of 5 μCi (1.85 × 105 Bq) [γ32-P]adenosine triphosphate and incubated at 30°C for 20 minutes. Reactions were spotted on P81 Whatman paper. The samples were washed 3 times for 5 minutes in 75 mM phosphoric acid and once in acetone and then air dried; their radioactivity was determined in a β-counter.
Statistical analysis
CD20 expression was analyzed with the unpaired nonparametrical Mann-Whitney test, and statistical analysis was carried out by means of GraphPad Prism software (San Diego, CA).
Results
CD20 expression on normal and malignant lymphocytes
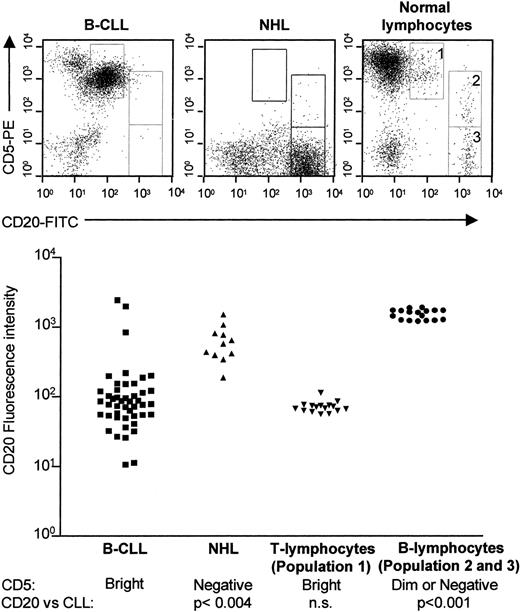
CD5+ B-CLL cells have been reported to express CD20 with weaker fluorescence intensity in their plasma membrane than B cells of CD5− B-cell malignancies.20 We examined the expression of CD20 on B-CLL cells and compared this expression with normal CD5+ and CD5− B cells and CD5− low-grade NHL cells (Figure1). Using dual-color staining and flow cytometry, we were able to define 3 separate CD20+populations in healthy donors: (1) CD5brightCD20dim, (2) CD5dimCD20bright, and (3) CD5−CD20bright (Figure 1, upper panel, right top). All B-CLL samples (n = 44) expressed CD20 but in intensities varying from CD20dim to CD20bright (Figure 1, left, bottom). CD20 expression on B-CLL cells was significantly weaker than on the CD20brightCD5dim or negative healthy populations (P < .001, Mann-Whitney), and the CD20bright NHL cells (P < .004, Mann-Whitney). However, the intensity of CD20 staining on B-CLL cells was not significantly different from that of the healthy CD5brightCD20dim population (population 1 in Figure 1, top). Surprisingly, further analysis of this healthy CLL-like population demonstrated that these cells did not express B-cell markers such as CD19, CD22, CD23, or surface membrane immunoglobulin; they did not express myeloid markers such as CD13, CD14, or CD33; and they did not express the NK-cell marker CD56 (data not shown) (n = 6). However, the CD5brightCD20dim cells stained positive for the pan–T-cell marker CD3; they had an equal distribution between CD4+ and CD8+ cells; and they expressed predominantly the αβ-chain of the T-cell receptor although γδ–positive cells were also identified in this population. Thus, although normal CD5brightCD20dim cells express CD5 and CD20 with intensities very comparable to those of B-CLL cells, the healthy population appears to be of T lineage, not of B lineage (Figure1).
CD5/CD20 expression on B-CLL cells, low-grade NHL cells, and normal lymphocytes.
Mononuclear cells were isolated from peripheral blood of B-CLL patients (n = 44); from blood, bone marrow, or fine-needle lymph node aspirates from patients with low-grade NHL (n = 11), and from buffy coats from healthy volunteer blood donors (n = 16). The mononuclear cells (1 × 106/mL) were stained simultaneously with FITC-conjugated anti-CD20 and phycoerythrin-conjugated anti-CD5 monoclonal antibodies, or isotype-matched control antibodies, and analyzed on a FACSort flow cytometer. Normal lymphocytes can be divided into 3 populations: (1) CD5brightCD20dim; (2) CD5dimCD20bright; and (3) CD5−CD20bright populations (top, right panel). In B-CLL samples, one population of CD5brightCD20dim cells can be identified (top, left panel). In NHL samples, one population of CD5−CD20bright cells can be identified (top, middle panel). The mean CD20 fluorescence intensity on B-CLL cells is significantly weaker than that of normal CD5dim or CD5− B cells or CD5− NHL cells, but not statistically different from that of normal CD20dimCD5bright cells, which express CD3, CD4, or CD8, in the absence of B cells, natural-killer (NK) cells, and myeloid-lineage markers (bottom).
CD5/CD20 expression on B-CLL cells, low-grade NHL cells, and normal lymphocytes.
Mononuclear cells were isolated from peripheral blood of B-CLL patients (n = 44); from blood, bone marrow, or fine-needle lymph node aspirates from patients with low-grade NHL (n = 11), and from buffy coats from healthy volunteer blood donors (n = 16). The mononuclear cells (1 × 106/mL) were stained simultaneously with FITC-conjugated anti-CD20 and phycoerythrin-conjugated anti-CD5 monoclonal antibodies, or isotype-matched control antibodies, and analyzed on a FACSort flow cytometer. Normal lymphocytes can be divided into 3 populations: (1) CD5brightCD20dim; (2) CD5dimCD20bright; and (3) CD5−CD20bright populations (top, right panel). In B-CLL samples, one population of CD5brightCD20dim cells can be identified (top, left panel). In NHL samples, one population of CD5−CD20bright cells can be identified (top, middle panel). The mean CD20 fluorescence intensity on B-CLL cells is significantly weaker than that of normal CD5dim or CD5− B cells or CD5− NHL cells, but not statistically different from that of normal CD20dimCD5bright cells, which express CD3, CD4, or CD8, in the absence of B cells, natural-killer (NK) cells, and myeloid-lineage markers (bottom).
Rituximab induces apoptosis in freshly isolated B-CLL cells
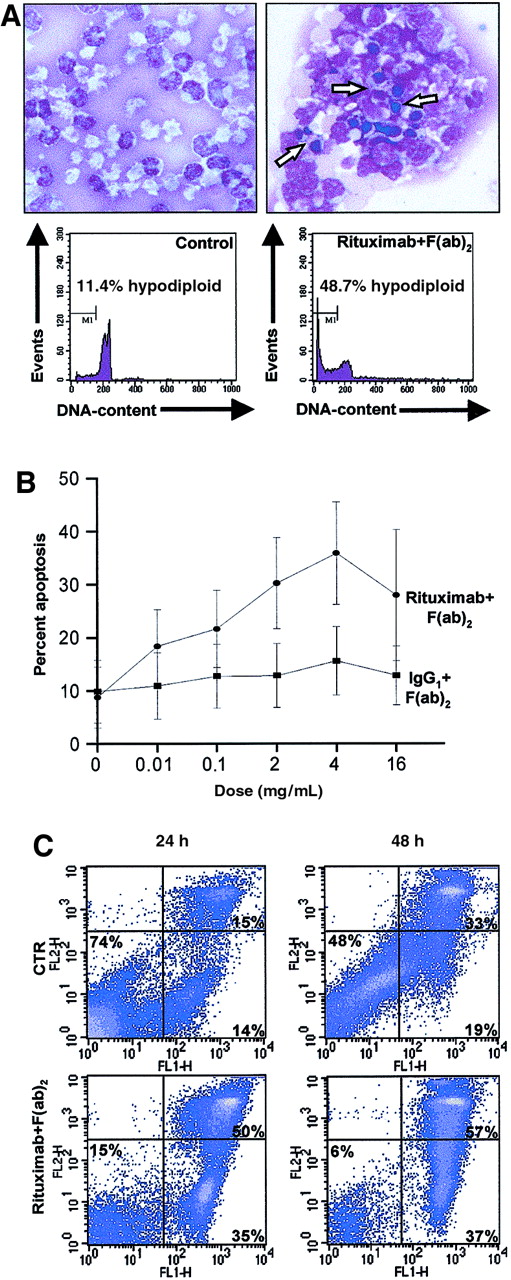
We studied the functional effect of rituximab in primary leukemia cells from patients with B-CLL, using 3 corroborative apoptosis assays: morphology analysis, PI staining of cell DNA, and detection of phospholipid asymmetry and plasma membrane integrity, by double staining with annexin V–FITC and PI. We cultured freshly isolated B-CLL cells in the presence or absence of rituximab. When cross-linked, rituximab induced morphological changes typical of apoptosis and significantly increased the fraction of hypodiploid nuclei (observed in 25 independent experiments) (Figure2A). This induction of apoptosis was dose and time dependent, with the maximal response at a dose of 4 μg rituximab per milliliter medium (Figure 2B-C).
Effect of rituximab on freshly isolated B-CLL cells.
(A) First, 2 × 106/mL freshly isolated B-CLL cells were cultured for 24 hours in the presence (right panel) or absence (left panel) of 4 μg/mL rituximab and 20 μg/mL mouse anti–human IgG1 F(ab)2 fragment. Cytospin slides were prepared from 2 × 105 cells and stained with May-Grünwald-Giemsa (top panels). Apoptotic cells are indicated by arrows. Original magnification × 100. For quantification of the degree of apoptosis, isolated nuclei from 5 × 105cells from the same experiment were lysed, stained with PI, and analyzed for DNA content on a FACSort flow cytometer. Several condensed and fragmented apoptotic nuclei appear in the cells cultured in the presence of rituximab (top, right panel); concomitantly, apoptotic nuclei appear within the hypodiploid region (M1) of the DNA histogram (bottom, right panel). (B) Freshly isolated B-CLL cells from 7 patients were cultured for 24 hours in triplicate with the indicated concentrations of rituximab and secondary antibody in 5-fold excess. Following harvest and washing, the cells were subsequently analyzed for DNA content as above. Dots and squares indicate the mean percentages of apoptotic cells at the indicated concentrations; error bars indicate the estimated 95% confidence intervals of the means. (C) Freshly isolated B-CLL cells were cultured as described in panel A, for 24 or 48 hours. After harvest, cells were incubated with FITC-conjugated annexin V and counterstained with PI in order to detect early apoptotic and late apoptotic/necrotic cells by flow cytometry. The lower left quadrants of each panel show the viable cells, which exclude PI and are negative for annexin V–FITC binding. The lower right quadrants represent the early apoptotic cells, annexin V–FITC+ and PI−, demonstrating cytoplasmic membrane integrity. The upper right quadrants contain the nonviable, late apoptotic/necrotic cells, positive for annexin V–FITC binding and for PI uptake.
Effect of rituximab on freshly isolated B-CLL cells.
(A) First, 2 × 106/mL freshly isolated B-CLL cells were cultured for 24 hours in the presence (right panel) or absence (left panel) of 4 μg/mL rituximab and 20 μg/mL mouse anti–human IgG1 F(ab)2 fragment. Cytospin slides were prepared from 2 × 105 cells and stained with May-Grünwald-Giemsa (top panels). Apoptotic cells are indicated by arrows. Original magnification × 100. For quantification of the degree of apoptosis, isolated nuclei from 5 × 105cells from the same experiment were lysed, stained with PI, and analyzed for DNA content on a FACSort flow cytometer. Several condensed and fragmented apoptotic nuclei appear in the cells cultured in the presence of rituximab (top, right panel); concomitantly, apoptotic nuclei appear within the hypodiploid region (M1) of the DNA histogram (bottom, right panel). (B) Freshly isolated B-CLL cells from 7 patients were cultured for 24 hours in triplicate with the indicated concentrations of rituximab and secondary antibody in 5-fold excess. Following harvest and washing, the cells were subsequently analyzed for DNA content as above. Dots and squares indicate the mean percentages of apoptotic cells at the indicated concentrations; error bars indicate the estimated 95% confidence intervals of the means. (C) Freshly isolated B-CLL cells were cultured as described in panel A, for 24 or 48 hours. After harvest, cells were incubated with FITC-conjugated annexin V and counterstained with PI in order to detect early apoptotic and late apoptotic/necrotic cells by flow cytometry. The lower left quadrants of each panel show the viable cells, which exclude PI and are negative for annexin V–FITC binding. The lower right quadrants represent the early apoptotic cells, annexin V–FITC+ and PI−, demonstrating cytoplasmic membrane integrity. The upper right quadrants contain the nonviable, late apoptotic/necrotic cells, positive for annexin V–FITC binding and for PI uptake.
Annexin V binds the membrane phospholipid phosphatidylserine, which is externalized from the inner to the outer leaflet of the plasma membrane in the early stage of apoptosis. When membrane integrity is lost, as seen in the later stage of cell death resulting from either the apoptotic or the necrotic processes, PI staining becomes positive. According to the results of our time-course study, annexin-positive apoptotic cells, after 24 hours of culture, were almost equally distributed between PI− early apoptotic (mean, 43%; range, 26%-71%; n = 7) and PI+ late apoptotic (mean, 35%; range, 22%-59%; n = 7) cells (Figure 2C). In some, but not all cases, we did observe some induction of apoptosis in the presence of the secondary antibody alone, or in the presence of the cross-linked IgG1 control, but cross-linked rituximab was always more potent in induction of apoptosis (Figure 2B). To address the role of the secondary antibody itself, we performed experiments in which the culture plates were coated with immobilized IgG1 or rituximab, and even under these conditions rituximab induced apoptosis, whereas IgG1 did not (data not shown). Furthermore, antibodies against CD19, alone or cross-linked, failed to induce apoptosis in B-CLL cells, and the rituximab preparation did not induce apoptosis in primary human T lymphocytes cultured under identical conditions (data not shown). These results demonstrate that cross-linking of rituximab bound to CD20 on the surface of freshly isolated B-CLL cells induces apoptosis.
Rituximab-induced apoptosis in B-CLL cells is related to MAP-kinase phosphorylation and dependent on p38 activity
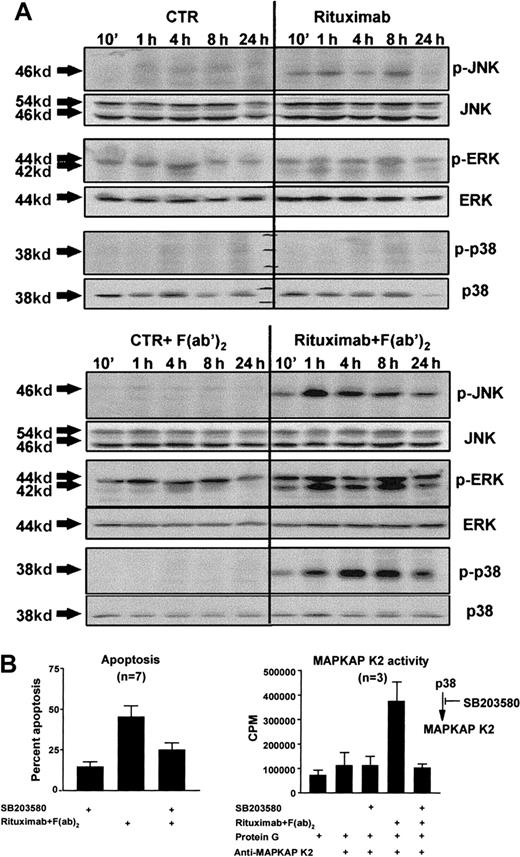
Binding of anti-CD20 antibodies to CD20 induces phosphorylation of protein tyrosine kinases and calcium mobilization in B-cell lines.15-17 These proximal membrane signals could theoretically lead to activation of MAP kinases in a CD20-mediated signaling pathway.22 We analyzed whether this was the case in the CD20-mediated apoptosis signaling pathway. MAP-kinase activation was assessed by Western blot analysis on whole cell lysates with anti–phospho kinase (MAPK) antibodies, which specifically recognize an activated form of MAPK (ie, MAPK phosphorylated at tyrosine and serine residues). We cultured freshly isolated B-CLL cells in the presence or absence of rituximab and the cross-linking F(ab)2 fragment. Rituximab, when added alone, induced weak phosphorylation of JNK and ERK and had little or no impact on the phosphorylation of p38 (Figure 3A, top). However, cross-linking of rituximab resulted in strong and sustained phosphorylation of all 3 MAP kinases (observed in 3 out of 3 independent experiments) (Figure 3A, bottom). To determine if this rituximab-induced MAP-kinase activation was related to the induction of apoptosis, we next analyzed the effect of inhibitors of the MAP-kinase pathways. The MEK-inhibitor UO126 had no inhibitory effect on CD20-mediated apoptosis, even though it completely inhibited rituximab-induced ERK activation (data not shown). In contrast, the p38 inhibitor SB203580 completely abrogated rituximab-induced MAPKAP K2 activity downstream of p38 (observed in 3 out of 3 independent experiments) (Figure 3B, bottom), and decreased rituximab-induced apoptosis by a mean of 41% (range, 16%-67%) (Figure 3B, top). In additional dose-response experiments, SB203580 significantly inhibited CD20-mediated apoptosis, even at doses from 2.5 μM (data not shown).
Rituximab-induced apoptosis and p38 MAP-kinase activity.
(A) Freshly isolated B-CLL cells (2 × 106/mL) were cultured for the indicated time periods, in the presence or absence of 4 μg/mL rituximab with or without the cross-linking mouse anti–human IgG1F(ab)2 fragment (20 μg/mL). Cells were harvested, and whole cell lysates were quantified and loaded on 10% polyacrylamide gels. Following electrophoresis and blotting, the membranes were developed by means of antibodies specific for the phosphorylated forms of JNK, ERK, and p38 and by chemiluminiscence. Equal loading of the gels was confirmed by reprobing of the membranes with antibodies specific for total JNK, ERK, and p38. Shown is 1 representative experiment out of 3. (B) Freshly isolated B-CLL cells from 7 B-CLL patients were cultured (2 × 106/mL) in duplicates and preincubated for 1 hour with 10μM SB203580, an inhibitor of the p38 MAP-kinase pathway. Then, the B-CLL cells were cultured in the presence or absence of 4 μg/mL rituximab and 20 μg/mL mouse anti–human IgG1F(ab)2 fragment for 24 hours. For quantification of the degree of apoptosis, isolated nuclei from 5 × 105 were lysed, stained with PI, and analyzed for DNA content on a FACSort flow cytometer (left). To demonstrate inhibition of the p38 MAP-kinase pathway under these conditions, MAPKAP K2 in vitro kinase assays were carried out. B-CLL cells were preincubated with 10 μM SB203580 for 1 hour and stimulated as described above for 8 hours. The B-CLL cells were lysed and immunoprecipitated with specific MAPKAP K2 antibodies, and kinase assays were performed with the use of a specific MAPKAP K2 substrate (right; mean and SEM).
Rituximab-induced apoptosis and p38 MAP-kinase activity.
(A) Freshly isolated B-CLL cells (2 × 106/mL) were cultured for the indicated time periods, in the presence or absence of 4 μg/mL rituximab with or without the cross-linking mouse anti–human IgG1F(ab)2 fragment (20 μg/mL). Cells were harvested, and whole cell lysates were quantified and loaded on 10% polyacrylamide gels. Following electrophoresis and blotting, the membranes were developed by means of antibodies specific for the phosphorylated forms of JNK, ERK, and p38 and by chemiluminiscence. Equal loading of the gels was confirmed by reprobing of the membranes with antibodies specific for total JNK, ERK, and p38. Shown is 1 representative experiment out of 3. (B) Freshly isolated B-CLL cells from 7 B-CLL patients were cultured (2 × 106/mL) in duplicates and preincubated for 1 hour with 10μM SB203580, an inhibitor of the p38 MAP-kinase pathway. Then, the B-CLL cells were cultured in the presence or absence of 4 μg/mL rituximab and 20 μg/mL mouse anti–human IgG1F(ab)2 fragment for 24 hours. For quantification of the degree of apoptosis, isolated nuclei from 5 × 105 were lysed, stained with PI, and analyzed for DNA content on a FACSort flow cytometer (left). To demonstrate inhibition of the p38 MAP-kinase pathway under these conditions, MAPKAP K2 in vitro kinase assays were carried out. B-CLL cells were preincubated with 10 μM SB203580 for 1 hour and stimulated as described above for 8 hours. The B-CLL cells were lysed and immunoprecipitated with specific MAPKAP K2 antibodies, and kinase assays were performed with the use of a specific MAPKAP K2 substrate (right; mean and SEM).
Discussion
Anti-CD20–based immunotherapy of B-cell malignancies has been developed in an attempt to activate immune-effector mechanisms, such as ADCC and complement-mediated lysis, and thereby induce cell death in the malignant clone.10 Activation of both effector mechanisms have been demonstrated in vitro and in vivo, but these observations do not preclude the possibility that the clinical efficacy of rituximab may involve additional effector mechanisms.13,14 23
Because CD5+ B-CLL cells have been reported to express less CD20 than other B cells,24 we first examined the phenotype of B-CLL cells in terms of CD5 and CD20 coexpression. We confirmed that CD20 expression is weaker on B-CLL cells compared with normal CD5+ and CD5− B lymphocytes and malignant CD5− B cells from samples of low-grade NHLs. In healthy donors, we identified a small, but very consistent, population of cells that have a CD5brightCD20dim phenotype, indistinguishable from that of B-CLL cells. Interestingly, as previously reported by others,25 this population is in fact a small population of CD20dim T lymphocytes. Studies are underway to characterize this population further. Given that the dim expression of CD20 has been noted on some T-cell malignancies,26 including both leukemias and lymphomas, it is important to assess the exact frequency of CD20 expression on T-cell malignancies and, in particular, to investigate the functional consequences of binding of rituximab to such cells.
We next demonstrated that, despite the dim CD20 expression, rituximab induced apoptosis in freshly isolated B-CLL cells under conditions in which the contributions from the above-mentioned effector mechanisms are negligible. First, complement is not active in heat-inactivated serum. Second, our samples contained a median of 96.1% B-CLL cells, and the vast majority of the residual cells are CD5+T-cells. Therefore, the ratio between B-CLL cells and cells capable of mediating the cytotoxic response, ie, NK cells and monocytes, is at best 1:100 or lower, making it unlikely that cytotoxicity has any significant impact on the apoptotic response. The induction of apoptosis requires cross-linking or immobilization of the antibody and appears to be specific for rituximab. First, although the cross-linking antibody by itself, or together with an IgG1 control preparation, can induce a small degree of apoptosis, this response is not observed in all cases in which rituximab induces apoptosis. The mechanism for this unspecific induction of apoptosis is not clear, but may be attributed to interaction through Fc receptors or immunoglobulin on the surface of the B-CLL cell. Second, antibodies against CD19, both alone and cross-linked, failed to induce apoptosis in B-CLL cells. Third, the rituximab preparation did not induce a measurable apoptotic response in normal T lymphocytes. Therefore, we conclude that the major mechanism by which rituximab works in our system is induction of apoptosis.
In cell lines, rituximab-induced apoptosis has been reported to involve calcium mobilization and protein tyrosine kinase phosphorylation.16 In many instances, such signals result in activation of serine/threonine MAP kinases. The dynamic balance of activation of the MAP-kinase pathways is thought to be important in determining whether a cell survives or undergoes apoptosis.27 An important finding in our study is the association between activation of MAP kinases and rituximab-induced apoptosis. Specifically, our results indicate that CD20-mediated apoptosis is dependent on the activity of the p38 MAP-kinase in B-CLL cells. The data presented in this study demonstrate that only cross-linking of rituximab induces a strong, sustained activation of p38 and a significant degree of apoptosis. When the activity of p38 was completely blocked, as evidenced by the absence of MAPKAP K2 activity in SB203580-treated cultures, the response to rituximab in terms of apoptosis induction was significantly decreased in 7 out of 7 experiments. The ERK inhibitor UO126 did not prevent rituximab-induced apoptosis. Although the unavailability of a specific JNK inhibitor precludes any conclusions about the role of JNK activation in rituximab-induced apoptosis, our results strongly indicate that rituximab-induced apoptosis is dependent on p38 activation in B-CLL cells. The specificity of the p38 inhibitor we applied has recently been questioned, but the data on this issue are not equivocal.28-30 Furthermore, we observed the inhibitory effect even at low doses at which the specificity of the inhibitor is not questioned (data not shown).
The p38 MAP kinase has been found to be important in many cellular apoptosis systems. A dependence of p38 MAPK activity in B-cell–mediated apoptosis has, for example, been demonstrated in B-cell receptor (BCR)–mediated apoptosis in the B104 B-cell line.31 Recently, both rituximab and anti-BCR antibodies were shown to induce apoptosis and lead to ERK activation in B-cell lines at various maturational stages.32 In contrast, analysis of Fas-mediated apoptosis in the BJAB B-cell line showed no dependence on p38 activity.31 These data suggests that the p38 MAPK pathway plays a complex role in different apoptosis pathways in B cells. Although our results demonstrate dependence on p38 MAP-kinase activitation for the induction of CD20-mediated apoptosis, complete inhibition of p38 activity resulted in only partial inhibition of apoptosis. This observation is consistent with previous reports, in which CD20-mediated apoptosis was shown to be partially dependent on calcium mobilization as well as on protein tyrosine kinase activation.16 These studies, along with the data presented here, suggest that the ability of rituximab to induce apoptosis involves a specific receptor-ligand–like interaction between the antibody and CD20. Specific components of the resulting CD20-mediated signaling cascade appear to be important for the ability of the antibody to induce apoptosis in B lymphocytes, and our results indicate that such molecules are to be defined downstream of p38. Identification of these molecules, and dissection of the pathway(s) that link CD20 to the apoptotic machinery, may provide new therapeutic targets in B-CLL and possibly other B-cell neoplasms.
Supported in part by Danish Cancer Society grants 96-00-009 (J.J.) and 99-100-39 (A.M.B.), the A. L. Vengs Foundation, and The John and Birthe Meyer Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jesper Jurlander, The Leukemia Laboratory, Department of Hematology L4041, The Finsen Centre, Rigshospitalet, Blegdamsvej 9, DK-2100 Copenhagen, Denmark; e-mail: jjurland@rh.dk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal