Hypogammaglobulinemia is the hallmark of common variable immunodeficiency (CVID) syndrome, a heterogeneous disorder predisposing patients to recurrent bacterial infections. In this study, we investigated the peripheral B-cell compartment of 30 well-characterized CVID patients in comparison to 22 healthy controls. Flow cytometric analysis of peripheral blood lymphocytes revealed a reduction of class-switched CD27+IgM−IgD− memory B cells below 0.4% in 77% of our patients (group I), while this B-cell subpopulation exceeded 0.5% in all healthy donors and in 23% of CVID patients (group II). These results correlate well with the capacity of peripheral blood lymphocytes to produce immunoglobulins in vitro upon stimulation with Staphylococcus aureus Cowan I (SAC) plus interleukin-2 because the production of immunoglobulin G in vitro is entirely dependent on the presence of switched memory B cells. The subdivision of group I into patients with an increased proportion of CD21− peripheral B cells (> 20%; group Ia) and patients with normal percentages of CD21− B cells (< 20%; group Ib) revealed a significant clustering of patients with splenomegaly and autoimmune cytopenias in group Ia. Based on these observations, we propose a fast and reliable new classification for CVID patients by flow cytometric quantification of class-switched memory and immature B cells in the peripheral blood of patients. Our results point toward defects at various stages of B-cell differentiation in CVID subgroups and support the value of a B-cell–oriented classification principle. A consensus on this new classification system will hopefully provide a tool for rapidly defining homogeneous subgroups of CVID for functional studies and genetic linkage analysis.
Introduction
Common variable immunodeficiency (CVID) comprises a heterogeneous group of humoral immunodeficiencies of unknown etiology, with a prevalence of about 1 in 50 000. It is characterized by reduced serum levels of all switched immunoglobulin (Ig) isotypes (IgG, IgA, IgE), predisposing patients to frequent infections of the respiratory tract with encapsulated bacteria. Splenomegaly as well as malignancies and autoimmune phenomena may develop in 20% to 30% of the patients.1 Multiple etiologies are likely to result in this common clinical phenotype. Depending on the patients analyzed, primary T-cell defects,2-4 an exaggerated T-cell suppression,5 and primary B-cell defects,6-8all leading to a complex failure of T- and B-cell cooperation, have been reported.
Therefore, a classification of the patients is of primary importance to acknowledge the heterogeneity of the disease. About 80% of all CVID cases display normal T- and B-cell numbers in their peripheral blood. They have been functionally classified by Bryant et al9according to their capacity to produce IgM, IgA, and IgG in vitro upon stimulation with Staphylococcus aureus Cowan I(SAC) plus interleukin-2 (IL-2) or anti-IgM plus IL-2 (see “Patients, materials, and methods”). Peripheral blood lymphocytes (PBLs) of patients in group A fail to produce any Ig isotype in vitro, while group B patients produce IgM only and group C patients are indistinguishable from healthy controls in producing normal amounts of all isotypes in vitro despite low serum Ig levels in vivo. A minority of CVID patients (5%-10%) have strikingly low peripheral B-cell counts (< 1% of PBLs), suggesting defects at the early B-cell differentiation stages in bone marrow.10Another subtype (5%-10%) exhibits noncaseating, sarcoidlike granulomas in different organs and tends to additionally develop a progressive T-cell deficiency.11 12 In our studies we included only CVID patients with normal B-cell counts (> 1% of PBLs) and without evidence of granulomatous disease.
The recent observation13 that X-linked hyper-IgM syndrome patients are lacking CD27+IgD−IgM− memory B cells led us to examine this compartment in CVID. Mature class-switched CD27+IgD−IgM− memory B cells were profoundly diminished or absent in all CVID patients of group A and almost all of group B. In contrast, CVID patients of group C were almost indistinguishable from healthy controls. Based on this finding we suggest a new, fast, and reliable fluorescence-activated cell sorting (FACS)–based classification defining 2 groups: CVID group I comprises patients with switched memory B cells below 0.4% of total PBLs, and CVID group II includes all patients with normal numbers of switched memory B cells (> 0.4%). Group I can be subdivided according to increased (Ia) or normal (Ib) numbers of CD19+CD21− immature B cells. This classification not only shows a good correlation with the previous, more laborious functional classification of Bryant et al,9but it also associates clinical features like splenomegaly and autoimmune cytopenia preferentially with group Ia.
Patients, materials, and methods
Patients and controls
All patients were diagnosed as having CVID based on typical medical history of recurrent bacterial infections associated with hypogammaglobulinemia (serum IgG < 3.0 g/L, IgA < 0.5 g/L). Other diseases causing hypogammaglobulinemia such as myeloma, non-Hodgkin lymphoma, exudative gastroenteropathy, nephrotic syndrome, chronic immunosuppression, or catabolic states due to malnutrition were excluded. Our group of 38 CVID patients included 2 with sarcoidlike granulomas and 6 with low peripheral B-cell counts (< 1% CD19+ cells of PBLs) who were not subjected to detailed B-cell phenotyping. All patients were on regular intravenous IgG substitution (15-20 g IgG) every 4 to 7 weeks. Most patients were hyporeactive (usually one positive reaction to tetanus toxoid) or anergic in delayed-type skin tests (Multitest Immignost, Biosyn, Fellbach, Germany) but responded normally to mitogens (phytohemagglutinin, concanavalin A, pokeweed) and antigens (tetanus toxoid, purified protein derivative) in lymphoproliferative tests (results not shown). Table 1 summarizes patients' age, onset of disease, sex, percentage of peripheral B cells, and their classification according to Bryant et al.9
Characterization of CVID patients and healthy donors
| Group . | No. of patients . | Age, y . | Age at onset, y . | Sex M/F . | % peripheral B cells . | IgM in vitro, ng/mL . | IgG in vitro, ng/mL . |
|---|---|---|---|---|---|---|---|
| A | 6 | 39.7 ± 18.9 | 35.0 ± 20.7 | 4:2 | 6.1 ± 3.8 | 110 ± 130 | 0 |
| B | 19 | 42.7 ± 16.2 | 32.4 ± 12.9 | 11:8 | 7.8 ± 4.6 | > 4000* | 160 ± 200 |
| C | 5 | 44.4 ± 8.7 | 34.5 ± 8.6 | 2:3 | 10.8 ± 3.9 | > 7500 | > 1500 |
| Low B cells | 6 | 39.7 ± 8.5 | 28.1 ± 5.1 | 5:1 | 0.4 ± 0.4 | 20 ± 25 | 35 ± 42 |
| Sarcoidlike | 2 | 28 ± 4 | 24 ± 4 | 2:0 | 12.5 ± 10.5 | > 7500 | 190 ± 100 |
| HD | 22 | 32.4 ± 7.6 | NA | 12:10 | 7.7 ± 2.7 | > 7500 | > 1200† |
| Group . | No. of patients . | Age, y . | Age at onset, y . | Sex M/F . | % peripheral B cells . | IgM in vitro, ng/mL . | IgG in vitro, ng/mL . |
|---|---|---|---|---|---|---|---|
| A | 6 | 39.7 ± 18.9 | 35.0 ± 20.7 | 4:2 | 6.1 ± 3.8 | 110 ± 130 | 0 |
| B | 19 | 42.7 ± 16.2 | 32.4 ± 12.9 | 11:8 | 7.8 ± 4.6 | > 4000* | 160 ± 200 |
| C | 5 | 44.4 ± 8.7 | 34.5 ± 8.6 | 2:3 | 10.8 ± 3.9 | > 7500 | > 1500 |
| Low B cells | 6 | 39.7 ± 8.5 | 28.1 ± 5.1 | 5:1 | 0.4 ± 0.4 | 20 ± 25 | 35 ± 42 |
| Sarcoidlike | 2 | 28 ± 4 | 24 ± 4 | 2:0 | 12.5 ± 10.5 | > 7500 | 190 ± 100 |
| HD | 22 | 32.4 ± 7.6 | NA | 12:10 | 7.7 ± 2.7 | > 7500 | > 1200† |
Patients are characterized according to Bryant et al9 into subgroups A, B, and C and are compared with healthy donors (HD). Age, onset of disease, sex, percentage of peripheral B-cell count (as percentage of PBLs), and IgM and IgG in vitro production after SAC plus IL-2 stimulation are given. There was no statistically significant difference in age, age of onset, sex distribution, and percentage of peripheral B cells between the different subgroups of CVID patients.
CVID patients with low B-cell counts (< 1%) and sarcoidlike lesions were not included in further studies.
NA indicates not applicable.
Twelve of 19 produced more than 7500 ng/mL IgM.
Most healthy donors produced more than 1500 ng/mL IgG.
Cell preparation
After informed consent was obtained, 20 to 30 mL of blood anticoagulated with ethylenediaminetetraacetic acid (EDTA) was drawn from CVID patients and age-matched healthy controls. Blood samples from CVID patients were always taken prior to intravenous Ig substitution. The fraction of peripheral blood mononuclear cells (PBMCs) was isolated by Ficoll-Hypaque density gradient centrifugation (Biochrom, Berlin, Germany) and washed twice with phosphate-buffered saline. PBMCs were centrifuged through a layer of 100% heat-inactivated fetal calf serum (PANBiotech, Aidenbach, Germany) to reduce cell-bound IgG and resuspended in RPMI 1640 (Biochrom) supplemented with 10% fetal calf serum.
Antibodies and flow cytometry
PBMCs (2.5 × 105/50 μL RPMI 1640 plus 10% fetal calf serum) were stained for 20′ at 4°C with 10 μL of a mixture of the following antibodies at optimal concentrations: CD27–fluorescein isothiocyanate (FITC) (Dako, Glostrup, Denmark) or CD21-FITC (Coulter-Immunotech, Hamburg, Germany), anti-IgD–phycoerythrin (Southern Biotechnology Associates, Birmingham, AL), CD19-PC5 (Coulter-Immunotech), and anti-IgM–Cy5 (Dianova, Hamburg, Germany). Four-color data acquisition was performed with a FACSCalibur (Becton Dickinson, Mountain View, CA). Data analysis (CellQuest, Becton Dickinson) was performed by forward versus side-scatter gating on viable lymphocytes (PBLs) in combination with gating on CD19+ cells.
Ig synthesis in vitro
For in vitro Ig synthesis, 5 × 105 PBMCs were stimulated for 8 days with the T-cell–independent stimulus SAC (Calbiochem, La Jolla, CA) diluted to 1:10 000 plus 20 U/mL IL-2. Cultures were set up in 500 μL RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, penicillin, streptomycin, andl-glutamine (Biochrom). Control cultures were kept in medium without B-cell stimulants. Supernatants were collected after 8 days and stored at −20°C until assayed for IgG, IgA, and IgM content by enzyme-linked immunosorbent assay.6 In this study we only report IgG and IgM concentrations and omitted IgA because it paralleled IgG and provided no additional information. Results are expressed as nanograms per milliliter of Ig isotype produced in stimulated cultures minus control cultures. Based on the results of the Ig synthesis following SAC plus IL-2 stimulation in vitro,CVID patients were assigned into one of the 3 subgroups defined by Bryant et al9 (Table 1): group A, no significant synthesis of IgM (< 500 ng/mL) or IgG (< 500 ng/mL); group B, significant synthesis of IgM (> 4000 ng/mL) but not of IgG (< 500 ng/mL); and group C, normal synthesis of IgM (> 4000 ng/mL) and IgG (> 500 ng/mL).
Sorting of CD19+CD27−IgG−naive B cells
After isolation of CD19+ B cells (purity > 95%) by magnetic beads (DETACHaBEADs, Dynal, Hamburg, Germany), cells were stained for IgG (goat-F(ab′)2 anti-IgG–phycoerythrin, Southern Biotechnology Associates) and CD27 (CD27-FITC, Dako). Then 5 to 10 × 106 CD19+ B cells were sorted on a FACStar Plus (Becton Dickinson) into CD27−IgG− naive B cells and CD27+IgG−IgM+ memory B cells. Purified B cells or B-cell subpopulations were cultured at 1 × 105/200 μL in U-shaped microtiter plates and stimulated with SAC plus IL-2 (20 U/mL). Parallel 0.5 × 105 B cells were cocultured with 2 × 105 CD4+ T cells in flat-bottomed microtiter plates. CD4+ T cells had been positively selected by DETACHaBEADs (Dynal) and depleted of contaminating monocytes by incubation (20′, 4°C) with anti-CD14 and anti-CD16 monoclonal antibodies (Coulter-Immunotech) and subsequent treatment with immunomagnetic beads coated with antimouse IgG (Dynal). The purity of CD4+ T cells was more than 99%. Supernatants of B-cell cultures were collected after 8 days and stored at −20°C until assayed for Ig isotypes by enzyme-linked immunosorbent assay.
Statistical analysis
Statistical comparisons of numeric data were made using an unpaired Student t test. Classified data were evaluated by the χ2 or Fisher exact test. Differences between groups were considered significant at P < .05.
Results
Decrease of class-switched CD27+IgM−IgD− B cells in CVID group A and B patients
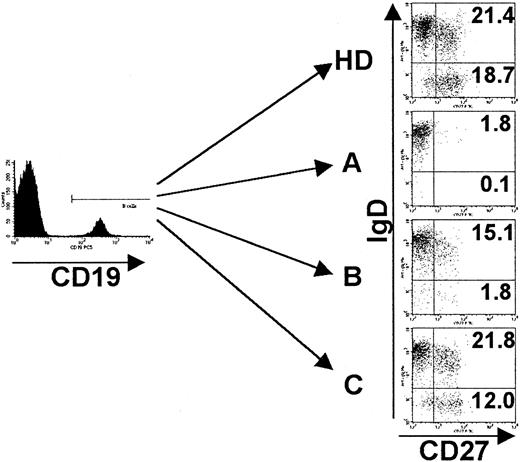
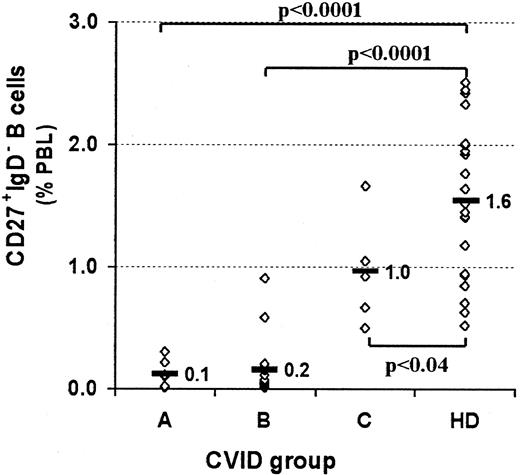
We examined B-cell subpopulations in the blood of 22 healthy donors and 30 CVID patients previously classified into groups A (n = 6), B (n = 19), and C (n = 5) (Table 1). The analysis revealed a severe decrease of CD27+ memory B cells in 77% of those CVID patients who had normal B-cell counts and no sarcoidlike lesions. When analyzed separately according to the classification of Bryant et al,9 CD27+ B cells were severely depleted in group A and B samples (1.1% ± 0.7%,P < .001, and 1.7% ± 1.7%, P = .006, respectively) while group C samples (4.1% ± 1.6%,P = .3) showed no significant differences to healthy controls (3.2% ± 1.5%).
A recent report of Rajewski's group14 demonstrated a substantial number of non–class-switched CD27+IgD+IgM+ memory B cells in the peripheral blood of humans. When dissecting the B-cell compartment according to surface Ig and CD27 expression, PBLs of healthy donors comprised 4.3% ± 1.6% naive B cells (CD27−IgM+IgD+), 1.6% ± 1.1% non–class-switched memory B cells (CD27+IgM+IgD+), less than 0.2% “IgM only” memory B cells (CD27+IgM+IgD−), and 1.6% ± 0.6% class-switched memory B cells (CD27+IgM−IgD−). We could not detect a significant decrease in the non–class-switched compartment of group A or B patients (1.0% ± 0.7%, P = .11, and 1.5% ± 1.5%, P = .79, data not shown), but both groups lack significant numbers of class-switched memory B cells (0.1% ± 0.1%, P < .0001, and 0.2% ± 0.2%,P < .0001, respectively) (Figures1 and2). The fraction of IgM+IgD− memory B cells was equally low and not significantly different in CVID and healthy controls (< 0.2% of PBLs). The analysis of group C samples showed a slight decrease of class-switched memory B cells (1.0% ± 0.4%, P < .04) (Figures 1 and 2) and an insignificant increase in non–class-switched memory B cells (3.2% ± 1.8%, P = .15, data not shown).
Phenotypes of peripheral B cells of CVID patients.
PBMCs were stained for the expression of CD19, CD27, IgM, and IgD and analyzed by FACS. Analysis was performed by gating on CD19+cells, and dead cells were excluded by forward/side scatter gating. Representative examples of each CVID subgroup (A-C) according to the classification by Bryant et al9 as well as one healthy control (HD) are given. Indicated values represent percentages of gated CD19+ B cells. See “Patients, materials, and methods.”
Phenotypes of peripheral B cells of CVID patients.
PBMCs were stained for the expression of CD19, CD27, IgM, and IgD and analyzed by FACS. Analysis was performed by gating on CD19+cells, and dead cells were excluded by forward/side scatter gating. Representative examples of each CVID subgroup (A-C) according to the classification by Bryant et al9 as well as one healthy control (HD) are given. Indicated values represent percentages of gated CD19+ B cells. See “Patients, materials, and methods.”
Profound decrease of switched memory B cells in CVID patients of subgroups A and B.
PBMCs were stained for the expression of CD19, CD27, IgM, and IgD, as shown in Figure 1. CVID patients are assigned to groups A-C according to the classification by Bryant et al9 and are compared with healthy controls (HD). Rhombus symbols represent the percentage of switched memory B cells (CD27+IgM−IgD−) for each patient or healthy donor. Statistical analysis was performed by the Student t test, and P values are given.
Profound decrease of switched memory B cells in CVID patients of subgroups A and B.
PBMCs were stained for the expression of CD19, CD27, IgM, and IgD, as shown in Figure 1. CVID patients are assigned to groups A-C according to the classification by Bryant et al9 and are compared with healthy controls (HD). Rhombus symbols represent the percentage of switched memory B cells (CD27+IgM−IgD−) for each patient or healthy donor. Statistical analysis was performed by the Student t test, and P values are given.
Good correlation between the frequency of CD27+IgM−IgD− switched memory B cells in peripheral blood and IgG synthesis in vitro
The reduced size of memory B-cell populations in the subgroups of CVID patients producing little or no Ig in vitro suggests a correlation between the production of Ig isotypes in vitro and the size of memory B-cell subpopulations in vivo. While we were unable to determine a clear-cut correlation between IgM production in vitro and the IgM memory B-cell pool (see below), we found a significant dependence of IgG production in vitro on the frequency of switched memory B cells (CD27+IgM−IgD−) in the peripheral blood (r = 0.81, Figure 3). This correlation was independent of whether the cells were derived from CVID patients or healthy donors, suggesting a normal intrinsic function of class-switched memory B cells in CVID patients of group C.
Good correlation between percentage of switched memory B cells in peripheral blood and IgG synthesis in vitro.
The percentage of switched memory B cells (CD27+IgM−IgD−) in the peripheral blood and IgG production after SAC and IL-2 stimulation in vitro show a significant correlation (r = 0.81) when analyzed by linear regression. Healthy controls as well as CVID patients are included in this plot.
Good correlation between percentage of switched memory B cells in peripheral blood and IgG synthesis in vitro.
The percentage of switched memory B cells (CD27+IgM−IgD−) in the peripheral blood and IgG production after SAC and IL-2 stimulation in vitro show a significant correlation (r = 0.81) when analyzed by linear regression. Healthy controls as well as CVID patients are included in this plot.
Isolated naive B cells of healthy donors do not produce IgM or IgG in vitro after SAC plus IL-2 stimulation
Because more than 90% of B cells from group A CVID patients express a naive CD27− phenotype, we analyzed for more adequate comparison the capacity of naive B cells of healthy donors to produce Ig in vitro after stimulation with SAC plus IL-2. Highly purified CD19+CD27−IgG− B cells were stimulated for 8 days with SAC plus IL-2 in the presence or absence of autologous CD4+ T cells. In supernatants of T-cell–free cultures, no traces of Igs were detectable, indicating that SAC plus IL-2 is unable to induce Ig synthesis in isolated CD27−IgM+IgD+ naive B cells (Table2). However, in the presence of autologous CD4+ T cells, naive, mature B cells produced large amounts of IgM, but no class switch to IgG was observed (Table2). Therefore, the Ig synthesis pattern in vitro of mature CD27−IgM+IgD+ normal B cells closely resembles that of PBLs of CVID group B patients. Cultures set up with highly purified CD27+IgM+IgD+ memory B cells of healthy donors produced large amounts of IgM even in the absence of T cells (Table 2). They, however, failed also to produce significant levels of IgG, confirming the previous finding that SAC plus IL-2 is not sufficient to induce class switch in vitro.15 The data presented in Table 2 also explain why in cultures of PBLs stimulated with SAC plus IL-2 it is impossible to correlate IgM production in vitro to the IgM memory B-cell pool: In the presence of CD4+ T cells, both CD27−IgM+ naive B cells and CD27+IgM+ memory B cells are induced to IgM production.
IgM production in vitro by naive (CD27−) and IgM memory (CD27+) B cells of healthy donors
| Stimulus . | CD4+ T cells* . | CD19+ B cells . | CD27−IgG− B cells . | CD27+IgG− B cells . | |||
|---|---|---|---|---|---|---|---|
| IgM, ng/mL . | IgG, ng/mL . | IgM, ng/mL . | IgG, ng/mL . | IgM, ng/mL . | IgG, ng/mL . | ||
| IL-2 | None | 0 | 145 | 0 | 0 | 0 | 0 |
| 0 | 62 | 0 | 0 | 953 | 0 | ||
| 0 | 0 | 0 | 0 | 251 | 0 | ||
| SAC plus IL-2 | None | 628 | > 1500 | 0 | 0 | > 7500 | 69 |
| > 7500 | 1461 | 0 | 0 | > 7500 | 43 | ||
| 3392 | 1624 | 0 | 0 | > 7500 | 0 | ||
| IL-2 | + | 652 | 963 | 0 | 0 | 1005 | 0 |
| 823 | 331 | 0 | 0 | > 7500 | 146 | ||
| 3193 | 1691 | 25 | 55 | > 7500 | 183 | ||
| SAC plus IL-2 | + | > 7500 | > 1500 | > 7500 | 0 | > 7500 | 127 |
| > 7500 | > 1500 | 2684 | 0 | > 7500 | 228 | ||
| > 7500 | > 1500 | 3441 | 0 | > 7500 | 99 | ||
| Stimulus . | CD4+ T cells* . | CD19+ B cells . | CD27−IgG− B cells . | CD27+IgG− B cells . | |||
|---|---|---|---|---|---|---|---|
| IgM, ng/mL . | IgG, ng/mL . | IgM, ng/mL . | IgG, ng/mL . | IgM, ng/mL . | IgG, ng/mL . | ||
| IL-2 | None | 0 | 145 | 0 | 0 | 0 | 0 |
| 0 | 62 | 0 | 0 | 953 | 0 | ||
| 0 | 0 | 0 | 0 | 251 | 0 | ||
| SAC plus IL-2 | None | 628 | > 1500 | 0 | 0 | > 7500 | 69 |
| > 7500 | 1461 | 0 | 0 | > 7500 | 43 | ||
| 3392 | 1624 | 0 | 0 | > 7500 | 0 | ||
| IL-2 | + | 652 | 963 | 0 | 0 | 1005 | 0 |
| 823 | 331 | 0 | 0 | > 7500 | 146 | ||
| 3193 | 1691 | 25 | 55 | > 7500 | 183 | ||
| SAC plus IL-2 | + | > 7500 | > 1500 | > 7500 | 0 | > 7500 | 127 |
| > 7500 | > 1500 | 2684 | 0 | > 7500 | 228 | ||
| > 7500 | > 1500 | 3441 | 0 | > 7500 | 99 | ||
After isolation of CD19+ B cells of 3 healthy donors, naive (CD27−IgG−) as well as IgM memory (CD27+IgG−) cells were sorted by FACS. Purified B cells (CD19+ B cells) or B-cell subpopulations (CD27− or CD27+IgG− B cells) were then stimulated with IL-2 or SAC plus IL-2. IgG and IgM concentrations in 8-day culture supernatants are given in nanograms per milliliter. While naive B cells produce IgM only in the presence of SAC plus IL-2 and CD4+ T cells, IgM secretion by IgM memory B cells is sufficiently activated by either stimulus alone. There was no class switch in vitro detectable in either subpopulation under the applied conditions.
In the presence (+) or absence (None) of CD4+ T cells according to the protocol. For details, see “Patients, materials, and methods.”
IgM production of CVID group A patients in vitro is dependent on culture conditions
We have applied the CVID classification of Bryant et al9 over the last 10 years in our clinic and found that patients usually stayed in the same group when tested on repeated occasions. However, in 2 patients repeatedly classified as type A, a reversion to type B could be observed. Interestingly, we have never observed the opposite—that is, progression from type B to A. To clarify the validity of a negative IgM production in vitro we studied in a more systematic approach the influence of culture conditions on the outcome of Ig synthesis in vitro of CVID group A patients. We therefore coincubated 0.5 × 105/200 μL purified B cells and 2 × 105/200 μL autologous CD4+ T cells. In one patient (Table 3, patient no. 2), this increase of B-cell numbers in the culture normalized the IgM production, suggesting that a low B-cell frequency of 1.5% (7500 B cells/200 μL of culture) in the original PBL preparation might have been a cause for the “defective” IgM production in vitro. Another type A patient with 10% B cells in the original PBL fraction (Table 3, patient no. 3) showed normal IgM production in vitro when 0.5 × 105/200 μL isolated B cells were cocultured with 2 × 105/200 μL autologous CD4+ T cells, but when 2 × 105 PBLs were added to the isolated B cells the IgM production subsided again. This suggests suppression by a population other than B cells and CD4+ T cells. Similar observations have previously been reported.16Moreover, in a recent study of our group using the “Zubler system” to maximally stimulate B cells17 and the enzyme-linked immunospot assay technique as the read-out system, all type A patients produced IgM spots but no or very few IgG spots.18 In accordance with these and previous data,19 we found a normal, spontaneous IgM synthesis by 23 of 23 CVID-derived Epstein-Barr virus lines (data not shown). Taken together, all these findings indicate that failing IgM production in vitro is not an absolute and irreversible defect in CVID patients; it may have various causes and does not seem to be a reliable marker for a specific subgroup of CVID patients.
Dependence of IgM production on culture condition
| Cell population . | No. of incubated B cells per well . | IgM, ng/mL . | IgG, ng/mL . | IgA, ng/mL . |
|---|---|---|---|---|
| Patient no. 1 (CVID group B) | ||||
| PBMCs | 30 000 | > 7 500 | 170 | 0 |
| CD19+ B cells alone | 100 000 | 0 | 0 | 0 |
| CD19+ B cells plus CD4+ T cells | 50 000 | > 7 500 | 40 | 0 |
| Patient no. 2 (CVID group A) | ||||
| PBMCs | 7 500 | 0 | 0 | 0 |
| CD19+ B cells alone | 100 000 | 0 | 0 | 0 |
| CD19+ B cells plus CD4+ T cells | 50 000 | > 7 500 | 110 | 0 |
| Patient no. 3 (CVID group A) | ||||
| PBMCs | 60 000 | 0 | 0 | 0 |
| CD19+ B cells alone | 100 000 | 0 | 0 | 0 |
| CD19+ B cells plus CD4+ T cells | 50 000 | > 7 500 | 290 | 80 |
| CD19+ B cells plus CD19− PBMCs | 50 000 | 0 | 0 | 0 |
| Cell population . | No. of incubated B cells per well . | IgM, ng/mL . | IgG, ng/mL . | IgA, ng/mL . |
|---|---|---|---|---|
| Patient no. 1 (CVID group B) | ||||
| PBMCs | 30 000 | > 7 500 | 170 | 0 |
| CD19+ B cells alone | 100 000 | 0 | 0 | 0 |
| CD19+ B cells plus CD4+ T cells | 50 000 | > 7 500 | 40 | 0 |
| Patient no. 2 (CVID group A) | ||||
| PBMCs | 7 500 | 0 | 0 | 0 |
| CD19+ B cells alone | 100 000 | 0 | 0 | 0 |
| CD19+ B cells plus CD4+ T cells | 50 000 | > 7 500 | 110 | 0 |
| Patient no. 3 (CVID group A) | ||||
| PBMCs | 60 000 | 0 | 0 | 0 |
| CD19+ B cells alone | 100 000 | 0 | 0 | 0 |
| CD19+ B cells plus CD4+ T cells | 50 000 | > 7 500 | 290 | 80 |
| CD19+ B cells plus CD19− PBMCs | 50 000 | 0 | 0 | 0 |
Ig production was measured either after incubation of PBMCs (5 × 105 cells/500 μL), isolated CD19+B cells alone (0.5 × 105 cells/200 μL), or in coculture with 2 × 105 CD4+ T cells or 2 × 105 CD19-depleted PBMCs (CD19−PBMCs) for 8 days. The results are presented for patient no. 1 (CVID group B according to Bryant et al9) and patient no. 2 and patient no. 3 (both CVID group A). See Table 2 for comparison with healthy donors and “Patients, materials, and methods” for further details. The numbers of B cells incubated per test are recorded in the second column. In patient no. 2, the low peripheral B-cell number is likely to be the main reason for failure of a detectable IgM production. It normalized after increasing the number of incubated B cells. PBMCs of patient no. 3, however, contained a sufficient number of B cells, but IgM production was only detectable after cocultivation of purified CD4+ T cells and CD19+ B cells. The missing IgM production after adding CD19-depleted PBMCs to isolated B cells suggests a suppressive effect of the remaining populations.
A new, easy FACS-based classification of CVID patients with normal B-cell numbers
The percentage of class-switched CD27+IgM−IgD− memory B cells of the PBL fraction was a highly reliable marker for a classification of CVID patients. In all healthy donors more than 0.5% (1.6% ± 0.6%) of PBLs belong to the CD27+IgM−IgD− population. In contrast, PBLs of 77% of our CVID patients with normal B-cell counts contained less than 0.35% of class-switched memory B cells (0.1% ± 0.1%, P < .0001). We designated this group of patients as CVID group I (Table 4). A total of 23% of our patients showed a less significant reduction of the peripheral switched memory B-cell compartment (0.9% ± 0.4%,P = .005); they were classified as CVID group II. The difference to group I was also highly significant (P < .002) (Table 4). At the same time this group showed a significant increase of total B cells (12.6% ± 4.7%) compared with group I (6.5% ± 3.5%, P = .019) and healthy donors (7.7% ± 2.7%, P = .046) (Table 4). Our new CVID group I comprises only former group A and B patients of the Bryant classification, while group C patients segregate with the new group II.
New classification of CVID
| CVID . | Subgroup . | No. of patients . | CD19+ B cells, % of PBLs . | CD27− IgM/D+, % of PBLs . | CD27+IgM/D+, % of PBLs . | CD27+IgM/D−, % of PBLs . | CD21−, % of B cells . | Bryant classification . | Splenomegaly . | Autoimmunity4-150 . | Vaccination4-151 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group I | a | 10 | 4.9 ± 2.6‡ | 3.5 ± 1.8 | 1.2 ± 0.9 | 0.1 ± 0.14-153 | 44.7 ± 11.04-153 | A/B | 10/10 (100%) | 6/10 | Neg. |
| b | 13 | 7.8 ± 3.6 | 6.5 ± 3.24-155 | 0.9 ± 0.4‡ | 0.1 ± 0.14-153 | 9.9 ± 5.7 | A/B | 5/12 (42%) | 6/13 | Interm. | |
| Group II | 7 | 12.6 ± 4.74-155 | 7.6 ± 4.3 | 3.8 ± 1.94-155 | 0.9 ± 0.44-154 | 12.6 ± 9.04-159 | C | 1/7 (14%)4-159 | 3/7 | Pos. | |
| HD | 22 | 7.7 ± 2.7 | 4.3 ± 1.6 | 1.6 ± 1.1 | 1.6 ± 0.6 | 7.0 ± 2.7 | NA | NA | NA | NA |
| CVID . | Subgroup . | No. of patients . | CD19+ B cells, % of PBLs . | CD27− IgM/D+, % of PBLs . | CD27+IgM/D+, % of PBLs . | CD27+IgM/D−, % of PBLs . | CD21−, % of B cells . | Bryant classification . | Splenomegaly . | Autoimmunity4-150 . | Vaccination4-151 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group I | a | 10 | 4.9 ± 2.6‡ | 3.5 ± 1.8 | 1.2 ± 0.9 | 0.1 ± 0.14-153 | 44.7 ± 11.04-153 | A/B | 10/10 (100%) | 6/10 | Neg. |
| b | 13 | 7.8 ± 3.6 | 6.5 ± 3.24-155 | 0.9 ± 0.4‡ | 0.1 ± 0.14-153 | 9.9 ± 5.7 | A/B | 5/12 (42%) | 6/13 | Interm. | |
| Group II | 7 | 12.6 ± 4.74-155 | 7.6 ± 4.3 | 3.8 ± 1.94-155 | 0.9 ± 0.44-154 | 12.6 ± 9.04-159 | C | 1/7 (14%)4-159 | 3/7 | Pos. | |
| HD | 22 | 7.7 ± 2.7 | 4.3 ± 1.6 | 1.6 ± 1.1 | 1.6 ± 0.6 | 7.0 ± 2.7 | NA | NA | NA | NA |
The new classification includes only CVID patients with peripheral B-cell numbers above 1% of PBLs. PBLs of group I CVID patients comprise less than 0.4% of class-switched memory B cells (CD27+IgM−IgD−), while in group II, like healthy donors (HD), this population accounts for more than 0.4%. Group I is further subdivided by the percentage of immature CD21− B cells into subgroups Ia (more than 20%) and Ib (less than 20%). All former group A and B patients according to the classification by Bryant et al9 fall into group I, except 2 former group B patients who fall into group II. All values are given as mean ± SE.
NA indicates not applicable.
There is a significant clustering of splenomegaly and autoimmune cytopenia in group Ia. Autoimmune phenomena in group Ia patients included 5 patients with thrombocytopenia and 1 with autoimmune hemolytic anemia; group Ib included 2 patients with pernicious anemia, 2 with vitiligo, 1 with autoimmune hemolytic anemia, and 1 with CREST syndrome; group II included 2 patients with pernicious anemia and 1 with vitiligo.
The new classification may allow a prediction of the response to vaccinations. Neg. indicates no response (n = 1); Interm., intermediate (n = 3); and Pos., normal response (n = 3) to vaccination with phage φ X174.
P < .02.
P < .0001 compared with HD.
P < .05.
P < .005 compared with HD.
One patient of group II had more than 20% immature B cells in the peripheral blood, and he was the only one with splenomegaly in this group.
As can be seen in Figure 4, CD21− immature B cells normally amount to less than 20% of the CD19+ B-cell compartment in healthy donors. In contrast, patients of CVID group I can be divided into a subgroup Ia, with an increased proportion of CD21− B cells, and a subgroup Ib, with normal numbers of this cell population (Table 4). CVID patients of group Ib showed a significant decrease of IgM memory B cells (CD27+IgM+IgD+, 0.9% ± 0.4%, P < .02) compared with healthy donors (1.6% ± 1.1%) (Table 4). Interestingly, all 10 patients of subgroup Ia were clinically characterized by splenomegaly, and 6 of 10 had recurrent autoimmune cytopenia. Some patients of CVID subgroup Ib and II, rather, exhibited other autoimmune phenomena than cytopenias, eg, pernicious anemia or vitiligo (Table 4). The clustering of splenomegaly (10 of 10) and autoimmune cytopenia (6 of 10) was significant in comparison to group Ib (5 of 12,P = .005, and 1 of 13, P = .019, respectively) and group II (1 of 7, P = .0006, and 0 of 7,P = .034, respectively). There was only one patient with increased counts of CD21− B cells (and splenomegaly) in group II, suggesting a more complex defect in this patient.
Increase of CD21− B cells in CVID patients of group Ia.
PBMCs were stained for the expression of CD19, CD21, IgM, and IgD and analyzed by FACS. Analysis was performed by gating on CD19+B cells, and dead cells were excluded by forward/side scatter gating. Representative FACS dot blots of the new CVID subgroups Ia, Ib, II, and one healthy control (HD) are shown. Indicated values represent percentages of gated CD19+ B cells. See “Patients, materials, and methods.”
Increase of CD21− B cells in CVID patients of group Ia.
PBMCs were stained for the expression of CD19, CD21, IgM, and IgD and analyzed by FACS. Analysis was performed by gating on CD19+B cells, and dead cells were excluded by forward/side scatter gating. Representative FACS dot blots of the new CVID subgroups Ia, Ib, II, and one healthy control (HD) are shown. Indicated values represent percentages of gated CD19+ B cells. See “Patients, materials, and methods.”
Our recent results of an in vivo vaccination study (A. Rump, manuscript in preparation) with the neoantigen phage φ X174 20showed a surprising correlation with the new classification. The only tested patient of group Ia produced no detectable specific antibody response, while all 3 patients of group Ib had transiently detectable titers. In contrast, normal antiphage antibody responses were seen in 3 patients of group II. This suggests a predictive value of the new classification for potential vaccination studies.
Discussion
After antigen-independent development in the bone marrow,21 immature B cells leave the bone marrow and gather in the longer-lived mature, naive IgD+IgM+CD27− B-cell pool.22 When these cells are stimulated by antigen in the presence of the appropriate costimulation they will engage in a germinal center (GC) reaction and develop into plasma cells or memory B cells.23 In most patients with CVID the development of both cells types is greatly disturbed while mature B cells are present in normal numbers, indicating defects in the late B-cell differentiation. The functional classification of Bryant et al9 has certainly helped to identify disease subtypes but is not generally accepted. Reasons for its rare application are the laborious and expensive technique of Ig synthesis in vitro, which still awaits an international standardization, and the lack of a correlation to lymphocyte phenotypes and/or clinical features (eg, splenomegaly, autoantibodies). Based on current concepts of B-cell differentiation, we suggest 2 checkpoint markers for a new, rapid, and reliable FACS-based classification of CVID: first, CD27 as a marker for memory B cells that have undergone a GC reaction and, second, CD21 as a marker for the progression of immature via transitional into mature B cells. We want to emphasize that our proposed classification is only applicable in patients with firmly established diagnosis of CVID according to the World Health Organization definition.24We purposely excluded CVID patients with very low B-cell numbers (< 1%; CVID group III) because of the difficulty of analyzing their peripheral B-cell compartment. We also excluded patients with hypogammaglobulinemia and histologically proven sarcoidlike lesion because this subgroup tends to develop T-cell deficiency.
In the last 2 years CD27 as well as CD148 25 have been established as reliable markers for human memory B cells.26,27 In all healthy donors more than 0.5% of PBLs belong to the CD27+IgM−IgD−switched memory B-cell population; in contrast, PBLs of 77% of our CVID patients contained less than 0.35% of this cell type. We therefore set as a cutoff 0.4% of class-switched memory B cells and assigned all patients who fell below that percentage to CVID group I. The remaining 23% of our patients maintained switched memory B cells in the lower normal range. They were assigned to CVID group II. Two recent papers by Brouet et al28 and Jacquot et al29 also describe subsets of CVID patients with normal and decreased percentages of CD27+ B cells. In the latter group, Jacquot et al29 identified severe forms of CVID and suggested that defects of CD27 expression or function contribute in some patients to the pathogenesis of CVID. The progression of immature via transitional to mature B cells can be monitored by the expression of CD21.22,30 Interestingly, patients of CVID group I could be subdivided according to the percentage of CD21− B cells in peripheral blood. PBLs of healthy donors never exceeded 20% of CD21− immature B cells (7.0% ± 2.7%), whereas 10 of 23 patients of CVID group I exhibited a remarkable expansion of this population (44.9% ± 11.6%, P < .0001). This accumulation of immature B cells in the blood points toward a disturbed maturation or premature exodus of immature B cells from the bone marrow. The necessary signals for further differentiation seem to derive from the splenic microenvironment but are still elusive. Most interestingly, 10 of 10 patients in this subgroup Ia showed splenomegaly, and 6 of 10 (60%) suffered from recurrent autoimmune cytopenia (IgM-mediated idiopathic thrombocytopenia and/or autoimmune hemolytic anemia), while only 1 of 13 patients in subgroup Ib (P < .02) and none in group II (P = .03) presented with autoimmune cytopenia. Other autoimmune phenomena, such as vitiligo and pernicious anemia, were more common in these groups. One potential mechanism leading to autoimmune cytopenia may be the decrease of competing mature B cells for the entry into secondary follicles.31 Failure of appropriate up-regulation of CD21 or its signaling complex may by itself interfere with appropriate antibody production.32 33
When we compared our results with the classification procedure based on Ig synthesis in vitro,9 we found that, with the exception of 2 group B patients, all A and B patients belong to the new group I. We could show a significant correlation between IgG production in vitro and the percentage of switched memory B cells in vivo and demonstrated a variability of IgM production in vitro depending on the protocol. Thus, low to absent IgM production in vitro may be due to low B-cell numbers (1%-2%) in some patients and may normalize with a proportionate increase of B cells in the cell culture system (Table 3). In other patients of group A, the removal of suppressive cell populations may result in significant IgM production.5 16Therefore, IgM production is not a reliable marker for a CVID classification. Neither was the percentage of IgM memory B cells, because the size of this population in healthy donors varied considerably.
Because the development of memory B cells is essentially linked to the formation of GCs in secondary lymphoid organs,23 the finding of a significantly reduced switched memory B-cell compartment in CVID type I strongly supports the hypothesis that GC reactions are disturbed in this disease.34 Many possible causes for disturbed GC reactions have been described. Mutations in the CD40 ligand gene,35,36 defects of the tumor necrosis factor-α family37 and its receptors,38 and impaired expression of costimulatory molecules like CD86 39 and chemokines like B-lymphocyte chemoattractant40 all interfere strongly with the formation and function of GCs. Well-coordinated interactions of T cells and B cells within the histoarchitectural network of follicular dendritic cells are essential for the normal development of a primary into a secondary follicle.
In contrast to mice, up to 25% of human peripheral B cells express hypermutated IgM and CD27 on their surface and were therefore classified as IgM memory B cells.14 The origin of the IgM memory population is discussed controversially. Patients of group Ib exhibit an expansion of mature B cells and a concomitant reduction of switched and IgM memory B cells (Table 4), suggesting defects in the formation of both memory compartments, while the IgD−(“IgM only”) memory B-cell compartment was only very small and indistinguishable from healthy donors and group II patients. If the IgM memory population is dependent on GC formation as thought by some authors,26 steps within the GC leading to isotype class switch41 ought to be disturbed in patients with normal numbers of IgM memory but decreased numbers of switched memory B cells (group Ia). Alternatively, the presence of IgM memory B cells in X-linked hyper-IgM syndrome (CD40L deficiency)42 as well as findings in sheep43 point toward a GC-independent origination of this population. The recent observation that the development of IgM memory (but not switched memory) is crucially dependent on the presence of the spleen and that splenectomy leads to a permanent abolition of more than 90% of this population (Wardermann H, Weber H, et al, manuscript in revision) adds an intriguing new facet to the origin of IgM memory in man.
A different pathogenesis seems to apply for the CVID group II patients. This group comprises all former C patients and 2 additional patients of group B. On average, PBLs of these patients contained a higher percentage of total B cells compared with patients in group I and healthy donors, suggesting an increased proliferation, an augmented lifespan, or decreased apoptosis of B cells. Their IgM memory B cells especially seem to be expanded compared with healthy donors. The switched memory B cells were only slightly decreased, and quantitative flow cytometry of memory B-cell subsets as well as the synthesis of Ig in vitro could not reliably distinguish them from healthy controls. CVID group II patients probably have a normal GC reaction but somehow fail to produce substantial amounts of antibodies in vivo or have an increased catabolism of Ig. The presence of functional GC reactions in some CVID patients with switched memory B cells has additionally been supported by the finding of normal hypermutation rates in 6 of 8 tested CVID patients by Levy et al.44 The hypogammaglobulinemia in group II patients may be due to impaired terminal plasma cell differentiation, a disturbed homing of plasma cell precursors,45 or a shortened lifespan of plasma cells in vivo.46 Many interactions like CD27/CD70,47,48 CD134/CD134L,49,50 and IL-6 with its receptor51,52 promote the terminal differentiation of B cells into plasma cells. Interestingly, one report indicated an increase in IL-6 in CVID-derived PBLs.53
In 2 recent papers, Brouet et al28 and Jacquot et al29 analyzed the role of CD27 expression and function in patients with CVID. Both found a substantial decrease in CD27+ B cells in a subgroup of CVID patients, as similarly observed for our patients. Brouet et al28 demonstrated for 3 of 5 of these patients a defective up-regulation of CD27 on isolated B cells after in vitro activation, suggesting a defect in CD27 expression. This finding was confirmed by the report of Jacquot et al29 describing 2 of 6 patients with defective up-regulation of CD27 after in vitro activation. However, it does not distinguish between a primary defect in CD27 up-regulation and a defect secondary to a disturbed activation signal following SAC plus IL-2 stimulation. Interestingly, both authors describe another subgroup with normal CD27 expression but impaired in vitro function despite costimulation with CD70 transfectants. Thus, Brouet et al28reported that isolated B cells from 2 of these patients failed to produce Ig when costimulated with CD70 transfectants. Unfortunately, this finding was not correlated to Ig production in the absence of CD70/CD27 costimulation, thus rendering it impossible to distinguish defects in CD27 function from defects in SAC plus IL-2/CD40 stimulation. The group of Jacquot et al,29 however, clearly shows for one patient a disturbed costimulatory signaling via CD27. Unfortunately, neither group correlated their data to extended clinical phenotypes and the existing classification of Bryant et al.9 These findings point at an important role of CD27/CD70 costimulation in the pathogenesis of CVID and deserve further investigation. Recent results from our group show that naive, pre–germinal center B cells (CD27−) from CVID group I show an impaired up-regulation of CD70 and CD86 upon stimulation with anti-IgM plus IL-2, while CD27 regulation was not altered (C.G., R.D., K.W., et al, manuscript submitted). Functional investigations on the interaction between CD27 on B cells and its CD70 ligand on T cells are underway.
In conclusion, this study proposes a new CVID classification that is based on flow cytometric analysis of different B-cell subsets in peripheral blood. It accommodates the previous functional classification of Bryant et al9 and confirms and extends reports by Brouet et al28 and Jacquot et al29on low CD27+ B-cell counts in a subset of severely immunocompromised CVID patients. It shows a correlation with clinical findings and may permit the prediction of successful vaccination in some CVID patients. A consensus on this classification system will provide a powerful tool to rapidly define and compare homogeneous subgroups of CVID for functional studies and genetic linkage analysis.
Supported by the Landesstiftung Baden Wuerttemberg. C.G. is a recipient of an award of the Hans-Hench Stiftung.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
H. H. Peter, Div of Rheumatology and Clinical Immunology, Hugstetter-Strasse 55, 79106 Freiburg, University Hospital of Freiburg, Germany; e-mail: peter@mm61.ukl.uni-freiburg.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal