Oxygen-sensing chemoreceptors contribute significantly to the regulation of the respiratory drive and arterial PO2levels. The hypoxic ventilatory response (HVR) decreases strongly with age and is modulated by prolonged hypoxia and physical exercise. Several earlier studies indicated that the regulation of the ventilatory response and erythropoietin (EPO) production by the respective oxygen sensors involves redox-sensitive signaling pathways, which are triggered by the O2-dependent production of reactive oxygen species. The hypothesis that HVR and EPO production are modulated by thiol compounds or changes in the plasma thiol–disulfide redox state (REDST) was investigated. It was demonstrated that both responses are enhanced by oral treatment with N-acetyl-cysteine (NAC) and that HVR is correlated with plasma thiol level and REDST. Results suggest the possibility that age-related changes in plasma REDST may account for the age-related changes in HVR.
Introduction
Oxygen tension regulates a series of important physiological responses in practically all cells and organisms.1,2 This is exemplified by the O2-sensing arterial chemoreceptors that contribute significantly to regulation of the efferent respiratory drive and arterial PO2.3,4 The hypoxic ventilatory response (HVR) is triggered by acute hypoxia and precedes a series of other hypoxic cellular responses that include changes in smooth muscle tone and the increased expression of hypoxia-inducible proteins such as erythropoietin (EPO). Earlier studies showed that the HVR decreases strongly with age5,6 and is modulated by prolonged exposure to hypoxia7 and by physical exercise.8 The O2 sensitivity of the chemoreceptors may become a determining factor in the pathogenesis of diseases associated with hypoxemia, including cardiorespiratory diseases.1,2 9-12
The 2 types of O2 sensors that regulate ventilation and EPO production in response to changes in arterial oxygen concentration are not completely understood and, to some extent, are still the subject of controversy.1 Although the oxygen sensors for the HVR in the carotid body and in certain other chemoreceptors have been identified as NADPH oxidase isoforms13-17 (reviewed in Dröge18), the regulation of EPO production was shown to involve activation of the hypoxia-inducible transcription factor (HIF), which depends on redox-sensitive stabilization of its α subunit.19,20 Oxygen-sensing involves in this case the oxygen-dependent hydroxylation of a proline residue (HIF-1α P564) and the subsequent ubiquitination and degradation of HIF-1α.21,22 A more recent study in mice suggested that S-nitrosothiols may play a decisive role in the ventilatory effect of hypoxia at the level of the nucleus tractus solitarius.23In spite of the conspicuous differences in the mechanisms involved, O2 sensing involves typically redox-sensitive signaling processes that are likely to be altered by thiols or by changes in the thiol–disulfide redox state (REDST). We are, therefore, investigating the hypothesis that the reactivity of the various O2sensors in healthy human subjects may be modulated by thiols or by changes in the plasma REDST. As a first test of this hypothesis, we performed a randomized, placebo-controlled study of the effect of the thiol compound NAC on HVR and plasma EPO concentration.
Patients, materials, and methods
Double-blind clinical trial on the effect of NAC on HVR and on plasma EPO level
Eligible subjects were healthy normotonic male nonsmokers who had not consumed alcohol, caffeine, or any drugs for at least 20 hours and had not been engaged in intense physical exercise for at least 12 hours. Twenty-eight subjects were recruited and randomized by the Pharmacology Department of the University of Heidelberg. The sample size was estimated on the basis of an unblinded pilot study to achieve a significance of P < .05. The study was approved by the ethics committee and was conducted according to the principles of the Declaration of Helsinki. After randomization, one subject (placebo) had to be excluded because of an infection. Another subject (NAC) had to be excluded because of a blood donation. Anthropometric data are shown in Table 1. NAC (200-mg capsules; HEXAL AG, Holzkirchen, Germany) or placebo was administered in 3 doses of 600 mg/d at 8 am, 4pm, and 11 pm for 5 days (days 2-6).
Anthropometric data
| . | Placebo (n = 13) . | NAC (n = 13) . | P . |
|---|---|---|---|
| Age (y) | 24.1 ± 1.3 | 25.2 ± 1.0 | .49 |
| Body weight (kg) | 73.4 ± 3.3 | 77.9 ± 4.1 | .13 |
| Body height (cm) | 177.3 ± 2.7 | 177.7 ± 1.1 | .90 |
| Body mass index (kg m−2) | 22.4 ± 0.4 | 24.5 ± 1.2 | .12 |
| . | Placebo (n = 13) . | NAC (n = 13) . | P . |
|---|---|---|---|
| Age (y) | 24.1 ± 1.3 | 25.2 ± 1.0 | .49 |
| Body weight (kg) | 73.4 ± 3.3 | 77.9 ± 4.1 | .13 |
| Body height (cm) | 177.3 ± 2.7 | 177.7 ± 1.1 | .90 |
| Body mass index (kg m−2) | 22.4 ± 0.4 | 24.5 ± 1.2 | .12 |
Values are mean ± SEM.
Blood, plasma, and serum
Blood samples were drawn from a cubital vein and immediately placed in ice water. Within 10 minutes, the samples were subjected to centrifugation at 2000g and 4°C for 10 minutes. Aliquots of plasma were stored at −70°C.
Plasma amino acid concentrations were determined with an amino acid analyzer, and the concentration of acid-soluble thiol in the plasma was determined photometrically within 1 hour of sampling, as described.24 Typically, 0.93 mL plasma samples from heparinized blood were incubated with 0.07 mL sulfosalicylic acid (50%) for 10 minutes at 4°C and subsequent centrifugation (4°C, 15 minutes, 7000 rpm, 110g). Acid-soluble thiols in the supernatant (acid-soluble fraction) were determined by mixing 0.4 mL supernatant with 0.4 mL pH 8.0 buffer (0.2 M phosphate plus 0.01 M EDTA). The increase of the absorbance at 412 nm was then determined photometrically before and after the addition of 0.02 mL 10 mM 5.5-dithiobis-2-nitrobenzoate. Cysteine was used as a standard. The term [thiol]2 [cystine]−1 (μM) was defined as the plasma REDST. Serum levels of EPO were analyzed by the EPO enzyme-linked immunosorbent assay (IBL, Hamburg, Germany). Plasma total peroxide concentrations were determined photometrically in EDTA plasma by the plasma peroxide concentration assay (Immundiagnostik, Bensheim, Germany), which is based on the reaction of horseradish peroxidase with plasma peroxides using tetramethylbenzidine as a chromogen substrate (450-nm wavelength).
Ventilation, respiratory gas analysis, and pulse oximetry
Ventilation (VE) and inspiratory and end-tidal CO2 were measured breath-by-breath by the respiratory monitoring system Oxyconbeta (Mijnhardt, Bunnik, The Netherlands) using the software version 3.12 with elimination of gliding averages. Each subject wore a nose clip and a mouthpiece connected to a flowmeter (Triple V) with an integrated gas-sampling capillary. The flowmeter was attached to a low-resistance T-shape valve system (Haward, Edenridge, United Kingdom) with a dead space of 95 mL. The inspiratory side was connected to a 110-cm tube (inner diameter, 4.5 cm) through which room air and hyperoxic or hypoxic mixtures were inhaled. Oxygen saturation (SaO2) was measured continuously by a pulse oximeter (3740 Biox Pulse Oximeter; Ohmeda Biox, Louisville, KY) using the finger probe placed at heart level.
Protocol of the study under normoxia
Resting minute ventilation, end-tidal PCO2, and isocapnic HVR were determined on day 1 and day 5. Subjects ingested 400 mL water at 7:00 am and arrived at the laboratory in a fasting state at 7:45 am. Blood samples were drawn after 15 minutes of rest. For ventilatory measurements between 8:30am and 12:00 pm, subjects equilibrated to the semi-reclined test for at least 20 minutes. Breath-by-breath values of VE and end-tidal PCO2 (PetCO2) were monitored for several minutes. When stable conditions were reached, values were recorded for 5 minutes, and HVR measurements were performed under isocapnic conditions. This procedure was repeated between 4:30 pm and 8:00 pm. Additional plasma thiol determinations were made at 0:00, 4:00, 6:00, and 8:00pm. HVR in isocapnia was determined as described.25Nitrogen was admixed to the inspiratory air reservoir to lower the FiO2 level (initially 35%) in such a way that SaO2 fell within 6 to 10 minutes in a linear fashion to 80%. The slope of the ventilatory response (ΔVE/ΔSaO2,) was calculated by linear regression of breath-by-breath values. For isocapnic HVR measurements, CO2 was added to the inspiratory air to maintain PetCO2 at the initial individual level during normoxic baseline before the induction of progressive hypoxia. All HVR data were determined in duplicate and are presented as mean of both values. Whenever the lower HVR measurement was less than 50% of the higher value, the measurement was repeated.
Study under prolonged hypoxia after 5 days of medication
On day 6, blood samples were drawn at 8:00 am from fasted subjects in normoxia. At 10:00 am, after a small breakfast, subjects entered a 14-m2 normobaric hypoxia chamber that provided a constant FiO2 level of 12% by admixture of N2-enriched air through a feedback O2-sensor control of an air inlet valve (AGA, Hamburg, Germany). FiCO2 levels were kept below 0.1% by admixture of fresh air to the chamber. Subjects stayed in the chamber for 6 hours in a comfortable sitting position and were occupied with reading or TV watching. O2 saturation and heart rate were monitored continuously. Resting VE and PetCO2 were recorded after 30 minutes, 2 hours, and 6 hours in hypoxia, and the hypoxic ventilatory response under these poikilocapnic conditions (HVR poikilo) was calculated as Δ VE/Δ SaO2() for each time point. Blood samples were drawn after 15 minutes and 5.5 hours in hypoxia, and 15 minutes, 1 hour, 2 hours, and 4 hours after hypoxia.
Statistics
Statistical procedures were performed by SPSS for Windows (version 6.1). Results are presented as mean ± SEM. The 2 treatment groups (Table 1) were compared by the Student ttest for unpaired samples. Statistical differences within either group were assessed by one-factorial analysis of variance for repeated measures and posthoc test (paired Student t test). Linear regression analysis was used to determine correlations. A difference was considered significant if P < .05.
Results
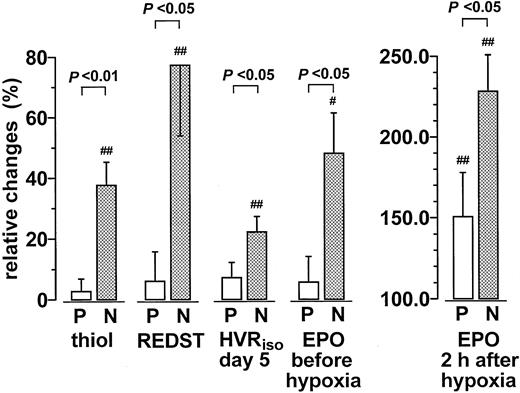
The randomized double-blind trial showed that relatively moderate doses of NAC caused a significant increase in the plasma thiol concentration and a shift in the plasma REDST (thiol2cystine−1) to more reducing conditions (Figures 1 and2). NAC treatment also caused a significant increase in the isocapnic hypoxic ventilatory response (HVRiso) and the plasma EPO level (Figures 1 and 2). Control experiments showed that the addition of cysteine or NAC to EPO standard or plasma samples did not cause an increase in EPO measurements. Measurements of EPO standard mixed with 15 μM or 50 μM cysteine, or 15 μM and 50 μM NAC, were 83.4% ± 5.5%, 73.2% ± 2.3%, 79.2% ± 2.5%, and 72.1% ± 6.6%, respectively, of the measurements without thiols. Corresponding mean values of 5 human plasma samples were 89.4% ± 5.3%, 93.2% ± 13.1%, 93.1% ± 7.6%, and 123.5% ± 24.3% of the corresponding measurements without thiols. Prolonged hypoxia (day 6) was found to cause a conspicuous increase in the total plasma peroxide concentration that was not seen in the NAC-treated group (Figure2).
Effect of NAC treatment on the plasma thiol, REDST, HVR, and EPO levels.
Relative changes during medication between baseline and terminal examination expressed as percentage of baseline values. With EPO, baseline examination took place on day 1 at 8 am, and the terminal examination was on day 6 at 8 am (before hypoxia) or at 6 pm (2 hours after hypoxia), as indicated. In all other cases, the relative changes were computed from the means of all measured values on day 1 and day 5, respectively. Similar data (P < .05) were obtained if the HVR data were normalized according to the individual body weight (not shown). Values are mean ± SEM (P = placebo, n = 13; N = NAC, n = 13). Significant differences between baseline and terminal values are indicated (#P < .05; ##P < .01).
Effect of NAC treatment on the plasma thiol, REDST, HVR, and EPO levels.
Relative changes during medication between baseline and terminal examination expressed as percentage of baseline values. With EPO, baseline examination took place on day 1 at 8 am, and the terminal examination was on day 6 at 8 am (before hypoxia) or at 6 pm (2 hours after hypoxia), as indicated. In all other cases, the relative changes were computed from the means of all measured values on day 1 and day 5, respectively. Similar data (P < .05) were obtained if the HVR data were normalized according to the individual body weight (not shown). Values are mean ± SEM (P = placebo, n = 13; N = NAC, n = 13). Significant differences between baseline and terminal values are indicated (#P < .05; ##P < .01).
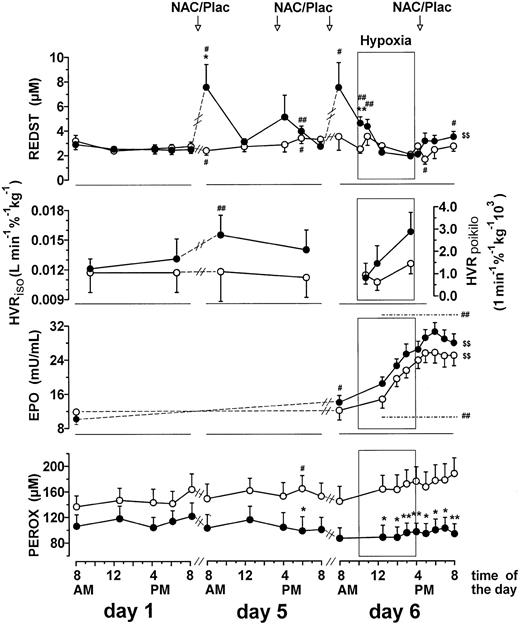
Longitudinal changes in plasma thiol, REDST, HVR, EPO level, and plasma peroxide concentration.
Longitudinal changes during NAC treatment and prolonged poikilocapnic hypoxia. Values are means ± SEM of the data before (day 1) and after 4 days of treatment (day 5) and the data on day 6 before, during, and after a 6-hour exposure to prolonged hypoxia indicated by the windows. The 6-hour exposition to normobaric hypoxia on day 6 started at 10 am. Three subjects (all placebo) of the total group (n = 26) refused to participate in the hypoxic exposition. Significant differences between the NAC group (●) and the placebo group (○) (*P < .05; **P < .01), significant changes compared with corresponding time points on day 1 (#P < .05; ##P < .01), and significant posthypoxic changes compared with the last value in hypoxia (day 6, 3:30 pm) ($P < .05; $$ P < .01) are indicated.
Longitudinal changes in plasma thiol, REDST, HVR, EPO level, and plasma peroxide concentration.
Longitudinal changes during NAC treatment and prolonged poikilocapnic hypoxia. Values are means ± SEM of the data before (day 1) and after 4 days of treatment (day 5) and the data on day 6 before, during, and after a 6-hour exposure to prolonged hypoxia indicated by the windows. The 6-hour exposition to normobaric hypoxia on day 6 started at 10 am. Three subjects (all placebo) of the total group (n = 26) refused to participate in the hypoxic exposition. Significant differences between the NAC group (●) and the placebo group (○) (*P < .05; **P < .01), significant changes compared with corresponding time points on day 1 (#P < .05; ##P < .01), and significant posthypoxic changes compared with the last value in hypoxia (day 6, 3:30 pm) ($P < .05; $$ P < .01) are indicated.
The simplest interpretation of these results is that the plasma thiol concentration or REDST might have had a direct effect on the reactivity of the oxygen sensors. As an additional test for this hypothesis, we determined the corresponding quantitative correlations. Relative changes in the HVRiso (percentage baseline) were indeed found to be correlated with changes in the plasma thiol concentration (μM) in 2 different settings—before NAC treatment during the 12-hour period from 8 am to 8 pm on day 1 (r = 0.48, P < .02, detailed data not shown) and during NAC treatment between day 1 and day 5 as determined by the differences between the means of all measurements on day 1 and day 5, respectively (r = 0.54, P < .01, detailed data not shown). A stronger correlation (r = 0.66,P < .001, detailed data not shown) was seen between the relative changes in HVRiso (percentage baseline) and the changes in the REDST (μM). In addition, the absolute HVRiso per body weight (l minute−1%−1 kg−1) was correlated with the mean REDST (μM) of day 5 (r = 0.53, P < .01, detailed data not shown).
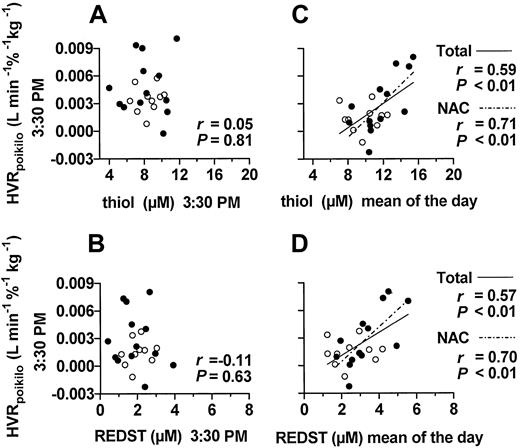
Longitudinal analysis on day 5 illustrated that the mean plasma thiol level (not shown) and REDST (Figure 2) of the NAC group decreased within a few hours of ingestion of NAC. This decrease was even more pronounced on day 6 during prolonged hypoxia under poikilocapnic conditions (Figure 2). At 3.30 pm on day 6, after 5.5 hours of hypoxia, the plasma thiol level and REDST showed essentially pretreatment values, little interindividual variation (Figure3A-B), and, accordingly, no significant correlation with the poikilocapnic HVR (HVRpoikilo) (Figure 3A-B). However, the HVRpoikilo values from this time point were significantly correlated with the mean plasma thiol levels and mean REDST values of the day (Figure 3C-D), implying that persons who happened to have a relatively highmean thiol level and REDST during the day also maintained a relatively high HVR value during hypoxia, even several hours after the temporary decline in thiol level and REDST. A significant correlation between HVRpoikilo and mean plasma thiol level and mean REDST of the day was also seen within the NAC-treated group alone (Figure 3C-D), indicating that the correlation was not merely based on differences between treatment groups. Collectively, these data support the paradigm that the HVR is indeed modulated by the REDST and that a temporary increase in plasma REDST may cause a long-lasting effect. There was no significant correlation between EPO level and thiol concentration or REDST (not shown).
Correlations between HVR and REDST.
Each point represents one subject (●, NAC; ○, placebo group). Shown are regression functions when P < .05 and the coefficients of correlation and P values for the total group and the NAC group. Correlations of the poikilocapnic HVR at 3:30pm on day 6 with the corresponding plasma thiol level and REDST at 3:30 pm are shown in panels A and B, respectively. Panels C and D show the corresponding correlations of the poikilocapnic HVR at 3:30 pm with the mean thiol level or the mean REDST of day 6, respectively (n = 6).
Correlations between HVR and REDST.
Each point represents one subject (●, NAC; ○, placebo group). Shown are regression functions when P < .05 and the coefficients of correlation and P values for the total group and the NAC group. Correlations of the poikilocapnic HVR at 3:30pm on day 6 with the corresponding plasma thiol level and REDST at 3:30 pm are shown in panels A and B, respectively. Panels C and D show the corresponding correlations of the poikilocapnic HVR at 3:30 pm with the mean thiol level or the mean REDST of day 6, respectively (n = 6).
Discussion
Our study has shown that the HVR and the EPO concentration are enhanced by oral treatment with NAC. Given that HVR and EPO production are 2 distinct physiological functions rigorously controlled by oxygen sensors, the results suggest strongly that the response of the respective redox sensors is modulated by changes in the plasma thiol level or the plasma REDST. In view of the fact that the regulation of ventilation in the carotid body in response to changing O2concentrations involves the intermediate production of superoxide radicals,13-15 it is reasonable to assume that NAC or its biochemical derivatives, cysteine and glutathione, may act by scavenging the O2-derived radicals or by direct reaction with redox-reactive components of the signaling cascade. Plasma REDST was previously shown to be correlated with the intracellular glutathione redox state, at least in some tissues,26 and NAC treatment was previously shown to cause a decrease in the production of superoxide anion by stimulated neutrophils.27-30 In view of recent evidence for a role of nitrosothiols in regulating the ventilatory response at the level of the nucleus tractus solitarius,23 there is also the possibility that oral NAC application or the endogenous concentration of thiols or REDST may modulate HVR by altering nitric oxide production.
Because the γ-glutamyl-cysteine synthetase reaction, the first and generally rate-limiting step in glutathione biosynthesis, is largely determined by the concentrations of its substrates glutamate and cysteine,31 it makes sense that the HVR was correlated not only with the REDST but also with the plasma glutamate and cystine concentrations. These correlations were most pronounced under the conditions of prolonged hypoxia at 3:30 pm on day 6 (ventilation VE vs glutamate, r = 0.76,P < .001; VE vs cystine,r = 0.51, P < .02; detailed data not shown). Weaker but still significant correlations were seen between the HVRiso and plasma glutamate and cystine levels under normoxic conditions on day 5 (not shown). Collectively, these correlations support the hypothesis that the oxygen sensor may be modulated by plasma REDST and, at least to some extent, by intracellular glutathione levels.
The production of EPO may be modulated by direct interference of NAC or endogenous thiols with the hydroxylation of the proline residues of HIF-1α, an obviously redox-sensitive process that controls the activity of this transcription factor.21,22 Several authors suggested that oxygen-dependent reactive oxygen species production may be involved in the down-regulation of HIF-1 activity.32-34 If this is confirmed, the increase in EPO levels by NAC treatment may also involve the scavenging of reactive oxygen species and may be associated with an increased expression of other proteins under control of HIF-1 such as the vascular endothelial growth factor. These points require further investigation.
Earlier studies have shown that plasma thiol level decreases with age and that corresponding shifts in the plasma REDST may contribute to the process of age-related wasting and may be a suitable target for therapeutic intervention with NAC.35 Moreover, studies from 2 different laboratories have shown that the HVR of elderly subjects in the 7th and 8th decades of life is approximately 50% lower than that of healthy young subjects.5,6 The emerging paradigm that aging may result, at least in part, fromdysregulation resulting from an oxidative shift in REDST may be seen as an extension of the free radical theory of aging.36 Oral treatment with NAC has served as a useful investigative tool and may be an effective pharmacologic option to increase the plasma thiol level, REDST, HVR, and plasma EPO concentration. This treatment may be useful for elderly subjects and for patients who have other conditions with an oxidative shift in plasma REDST, such as coronary heart disease and malignant diseases.35
We thank Ingrid Fryson for assistance in the preparation of this manuscript. We also thank Martina Haselmaier, Ute Winter, and Helge Lips for expert technical assistance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Wulf Dröge, Department of Immunochemistry, Deutsches Krebsforschungszentrum, Im Neuenheimer Feld 280, D-69120 Heidelberg, Germany; e-mail: w.droege@dkfz.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal