Abstract
We have studied the impact of B-cell receptor (BCR) or CD40 ligation on the in vitro chemotactic response of tonsillar B cells to 4 chemokines: stromal cell–derived factor (SDF)–1α, macrophage inflammatory protein (MIP)–3α, MIP-3β, and B-cell–attracting chemokine (BCA)–1. In the tonsil, SDF-1 and MIP-3α are both expressed in the crypt epithelium, while MIP-3β is found in the T zone and BCA-1 in the follicles. Resting virgin and memory B cells display a similar chemotaxis pattern, and they both have the potential to migrate in vitro to all 4 chemokines studied. This pattern of responsiveness is strongly modified by a surrogate antigen (Ag) but is not altered by CD40 ligand. We report here that surrogate Ag induces a profound and sustained suppression of the response to the crypt chemokines SDF-1α and MIP-3α, while it exacerbates the migratory response to MIP-3β. The effect of surrogate Ag on the response to BCA-1 is biphasic: After an initial phase of suppression, chemotaxis toward BCA-1 is strongly up-regulated. Our results suggest that Ag is primarily responsible for reprogramming the B-cell chemotaxis responsiveness during the humoral response. We propose that it initiates an ordered change of the chemotaxis machinery allowing Ag-activated B cells to relocate in the T zone and B-cell follicles sequentially.
Introduction
Development of the humoral response to T-cell–dependent antigens (Ag) requires a series of orchestrated movements of T cells, B cells, and antigen-presenting cells within the lymphoid tissues.1 These movements allow the encounter of the different cell partners involved in the response. The B-cell response is initiated in the T zone, where B cells that have taken up Ag present it to Ag-specific primed T cells. As a result of this T-cell–B-cell cognate interaction, B cells enter 2 developmental pathways.2 The first one takes place in the outer part of the T zone and leads to the production of unmutated plasma cells. It is also referred to as the extrafollicular reaction. The second one takes place in the follicle, where activated B lymphocytes, most likely originating from the extrafollicular foci, give rise to the germinal center (GC) reaction. During this reaction, B cells undergo a severe process of positive selection that results in the differentiation of the best-fit B cells into either memory B cells or mutated plasma cells. These 2 B-cell subsets then exit the follicle to migrate to other sites, either to take part into the effector phase of the response (plasma cells) or to reach the microanatomic niche able to support their long-term survival (memory B cells and plasma cells). Two main signals drive the development of B cells during T-dependent antibody (Ab) responses. The first one is Ag and its signaling through the B-cell receptor (BCR). The second one is CD40 ligand (CD40L) induced on T cells during cognate interactions with professional Ag-presenting cells or Ag-activated B cells. The CD40/CD40L couple is required for the initiation and maintenance of the GC reaction but not for the extrafollicular reaction.3-5 Several lines of evidence from both in vivo and in vitro experiments suggest that the cellular traffic into and within secondary lymphoid tissues is controlled by both lymphoid chemokines and cell-cell adhesion processes. Two important parameters contribute to guide the movements of B and T cells within the lymphoid tissue during the immune response. The first one is the restricted location of the sources of lymphoid chemokines to certain microanatomic sites. The second one is the pattern of chemokine receptor expression, which is subjected to regulation depending on the activation or differentiation status of the cells.
Here we have explored the regulation of the responsiveness of human tonsil B cells to 4 lymphoid chemokines: stromal cell–derived factor-1 (SDF-1/CXCL12), macrophage inflammatory protein-3α (MIP-3α/CCL20), MIP-3β (CCL19), and B-cell–attracting chemokine-1 (BCA-1/CXCL13). In the tonsil, SDF-1 and MIP-3α are both exclusively produced in the crypt epithelium, which is the main site for Ag entry in this tissue.6,7 While SDF-1 is produced throughout the spongy epithelium of the crypt, expression of MIP-3α is restricted to the epithelial cells lining the lumen of the crypt. It has been postulated that MIP-3α could play a role in recruiting immature dendritic cells (DCs) with high Ag capture capacity to the sites of Ag penetration.8,9 On the other hand, the close interactions between SDF-1–producing cells and memory B cells in tonsils suggest that SDF-1 may contribute to attract and retain memory B cells in the supportive epithelial microenvironment of the crypt.7
MIP-3β is expressed in the T zone of secondary lymphoid tissues, where it is produced by local DCs.10 MIP-3β shares its receptor with another chemokine, secondary lymphoid chemokine (SLC/CCL21),11,12 which is produced by endothelial cells of the high endothelial venules. SLC plays a key role in the entry of both T cells and DCs into secondary lymphoid organs.13
BCA-1 is classically described as a follicle chemokine. This assertion is based on 2 types of experimental evidence. First, expression of the BCA-1 protein is prominent in the follicle,14 and recent results have documented its production by follicular DCs and GC DCs.15 Second, mice rendered deficient for CXCR5, the receptor for BCA-1, exhibit an altered follicular architecture and a default in the recruitment of both B and T cells into these structures during the humoral response.16
Our results show that virgin and memory B cells have a similar pattern of in vitro chemotaxis and migrate to the 4 lymphoid chemokines analyzed here: SDF-1, MIP-3α, MIP-3β, and BCA-1. Nevertheless, their responsiveness to these chemokines is strongly modified after ligation of the BCR. Surrogate Ag inhibits their migration to SDF-1α, MIP-3α, and BCA-1, while it exacerbates their response to MIP-3β. In contrast, CD40 ligation does not alter the pattern of B-cell migration but affects the amplitude of the response to MIP-3β and BCA-1. The inhibition of the B-cell responsiveness to MIP-3α and SDF-1α by the surrogate Ag is stable along time. By contrast, the suppressive effect exerted by the surrogate Ag on the B-cell chemotaxis toward BCA-1 is only transitory. When B cells undergo a short exposure to surrogate Ag, the initial phase of suppression of their responsiveness to BCA-1 is followed by a strong up-regulation of their migratory response to this chemokine. The profound alterations in both the pattern and the amplitude of the chemotactic responses in activated B cells are not correlated with a dramatic modulation of expression of the chemokine receptors. This suggests that the regulatory function of the BCR on the B-cell chemotaxis is mainly exerted downstream of the chemokine receptors. Altogether our findings suggest that BCR ligation reprograms B cells to migrate toward MIP-3β and BCA-1 sequentially.
Materials and methods
Antibodies and reagents
The following Abs and conjugates were used in the experiments described herein. The uncoupled anti-CD38 monoclonal Ab (mAb, OKT10) was purchased from the American Type Culture Collection (Montluçon, France). The fluorescein isothiocyanate (FITC)–coupled anti-CD38 (T16) and anti-CD44 (IOL44) mAbs were from Immunotech (Marseille, France). The polyclonal sheep anti-immunoglobulin D (IgD) Ab was from The Binding Site (Birmingham, United Kingdom). The biotinylated polyclonal goat anti-IgD Ab and phycoerythrin (PE)–coupled streptavidin were from Sigma Chemical (St Louis, MO). The polyclonal goat anti-IgD Ab coupled to PE was from Southern Biotechnology Associates (Birmingham, AL). The PE-coupled anti-CCR6, biotinylated anti-CXCR4, and biotinylated anti-CXCR5 mAbs were all from R&D Systems (Minneapolis, MN). The uncoupled anti-CCR7 mAb was from Pharmingen (NJ) and was revealed using a biotinylated goat anti-mouse IgM Ab from Caltag (Burlingame, CA). The R-PE–cyanine 5 (RPE-Cy5)–coupled streptavidin was from Dako (Glostrup, Denmark). F(ab′)2 fragments of rabbit anti-human IgG, IgA, and IgM Ab were from Jackson ImmunoResearch Laboratories (West Grove, PA). The trimeric CD40L/leucine zipper fusion protein was kindly provided by Dr R. Armitage (Immunex, Seattle, WA) and was used at 500 ng/mL throughout the study. The 4 chemokines used for the chemotaxis assays, MIP-3α, MIP-3β, SDF-1α, and BCA-1, were all purchased from R&D Systems.
Isolation of tonsil B cells
Tonsil B cells were isolated as described elsewhere.17 Briefly, tonsils were gently teased, and the cell suspension was subjected to T-cell depletion by incubation with sheep red blood cells (SRBCs) followed by a density gradient centrifugation. The B-cell–enriched fraction was then resuspended in RPMI 1640 supplemented with 10 IU penicillin, 10 μg streptomycin, 10 mL HEPES, 2 mM l-glutamine, and 10% fetal calf serum, referred to as “complete medium” herein. All reagents were purchased from Gibco BRL Laboratories (Grand Island, NY). GC B cells were obtained after removal of virgin and memory B cells by rosetting with SRBCs coated with anti-IgD and anti-CD44 Abs as described elsewhere.18 Memory B cells were isolated after removal of virgin and GC B cells using SRBCs coated with anti-IgD and anti-CD38 Abs. For enrichment of virgin B cells, GC and memory B cells were depleted after rosetting with SRBCs coupled to anti-CD38 and anti-CD80 Abs. The purity of the negatively selected cell populations ranged between 95% to 99% for the GC and memory B cells and 70% to 80% for virgin B cells. For the reverse transcriptase–polymerase chain reaction (RT-PCR) studies, both virgin and memory B cells were obtained by cell sorting after labeling the cells with anti-IgD and anti-CD44 Abs. The purity of the sorted cells was always higher than 99%. Most chemotaxis assays were performed with CD38− B cells, a population that includes both virgin and memory B cells and is devoid of GC B cells. CD38− B cells were isolated after depletion of GC B cells with SRBCs coupled to CD38 mAbs.
Chemotaxis assay
Migration assays were performed essentially as described by Bleul et al.19 We chose to preincubate B cells for 6 hours in complete medium before the migration assays because pilot experiments had shown that this treatment enhanced the number of cells migrating to chemokines without significantly affecting the levels of spontaneous migration. A shorter incubation of the cells (2 hours) resulted consistently in a lower number of cells migrating to the 4 chemokines but did not alter the pattern of B-cell migration. The quantitative difference is probably due to in vivo desensitization20 21 of these cells, which is completely overcome by the 6-hour preculture but not by 2 hours. A total of 5 × 105 cells of the appropriate tonsillar B-cell population were added in the upper 5 μm pore polycarbonate Transwell culture insert of 24-well plates (Costar, Cambridge, MA) in 100 μL HEPES-buffered RPMI 1640 supplemented with 7.5% fetal calf serum. Chemokines were added as 600 μL in the lower chamber. As described in “Results,” the concentrations of SDF-1α, MIP-3α, MIP-3β, and BCA-1 required for optimal B-cell migration were 100 ng/mL, 500 ng/mL, 500 ng/mL, and 1 μg/mL, respectively. Cells were left to migrate for 3 hours, and each migration was performed in duplicates. To estimate the numbers of B cells migrating to the tested chemokines, cells in the lower chamber were counted by flow cytometry for 50 seconds. Percentage of input cells migrating was calculated after a standard curve obtained using different known concentrations of B cells that were treated identically to the cells used for migration. In each experiment the number of cells migrating to medium containing no chemokines was considered as the spontaneous migration and therefore was subtracted from the number of cells migrating to the chemokines investigated to calculate the percentage of input cells specifically migrating to each chemokine. The spontaneous migration never exceeded 4% of the input cells. When the phenotype of migrating cells was required, the cells recovered in the lower chamber as well as the cells used to elaborate the standard curve were stained with a PE-conjugated anti-IgD Ab. Virgin and memory B cells were identified as IgD+ and IgD−, respectively.
The inhibition of the B-cell chemotactic response induced by BCR ligation was calculated as follows: % inhibition = 100 − (100 × % specific migration of anti-Ig–treated cells/% specific migration of untreated cells).
Cell cultures
All cell cultures were performed in complete medium at 37°C in a 5% CO2 atmosphere. Cells were seeded at a density of 3 × 106/mL for different periods of time as indicated in the text.
Reverse transcriptase–polymerase chain reaction
Isolation of total RNA was performed essentially as described by Chomczynski and Sacchi.22 For reverse transcription, 1 μg RNA was converted into single-stranded DNA by a standard 20 μL RT reaction using random primers P(dN)6(Boehringer Mannheim) and Superscript kit (RNAseH-MMLV RT; Gibco BRL, Gaithersburg, MD) according to the manufacturer's instructions. One tenth of the total complementary DNA product was amplified in a 50 μL reaction mixture using 1 μM each of sense and antisense primers and 1.25 U Taq polymerase (Perkin Elmer/Cetus, Norwalk, CT). Expression of the β-actin messenger RNA was used as a control for RNA integrity and equal gel loading. PCR products were run on a 1.5% agarose gel, stained with ethidium bromide, and visualized by ultraviolet illumination.
The amplification primers for CCR6, CCR7, CXCR4, CXCR5, and β-actin used are as follows: CCR6, 5′-CAGATGGTCATCACATTGGTG-3′ and 5′-CCATGTTATGAGGAATCTGAC-3′; CCR7, 5′-GACGATTACATCGGAGACAAC-3′ and 5′-TATGGGGAGAAGGTGGTGGTG-3′; CXCR4, 5′-CCACCGCATCTGGAGAACCAG-3′ and 5′-AGAAAGCTAGGGCCTCGGTGA-3′; CXCR5, 5′-TTCACCT-CCCGATTCCTCTAC-3′ and 5′-AGGAAAAGCAGCAATAAAAGG-3′; and β-actin, 5′-GGGTCAGAAGGATTCCTATG-3′ and 5′-GGTCTCAAACATGATCTGGG-3′.
Immunofluorescence staining
The expression of CCR6, CCR7, CXCR4, and CXCR5 by B cells as well as the phenotype of cells migrating to SDF-1α, MIP-3α, MIP-3β, and BCA-1 were analyzed by flow cytometry. The distribution of chemokine receptors was studied by triple staining of freshly isolated B cells. Cells were labeled with anti-CD38, anti-IgD mAbs, and the appropriate antichemokine receptor Ab. Double labeling for CD38 and IgD allows discrimination of the 3 main B-cell subsets found in the tonsil. Virgin B cells are CD38−/IgD+, GC B cells are CD38+/IgD−, and memory B cells are CD38−/IgD−. For staining, the cells were resuspended as 107/mL in cold phosphate-buffered saline supplemented with 10% normal human serum and 0.1% sodium azide. For the CCR6 staining, B cells were incubated with optimal dilutions of FITC-coupled anti-CD38, PE-coupled anti-CCR6, and biotinylated anti-IgD Abs for 15 minutes at 4°C. For the CXCR4 and CXCR5 stainings, cells were incubated with FITC-coupled anti-CD38, PE-coupled anti-IgD, and the biotinylated forms of anti-CXCR4 or CXCR5 mAbs. All cells were then washed twice and incubated with streptavidin coupled to RPE-Cy5 for 15 minutes before flow cytometry analysis. For CCR7 staining, cells were incubated with FITC-coupled anti-CD38, PE-coupled anti-IgD, and unconjugated CCR7 Abs. Cells were then washed twice and incubated with a biotinylated goat anti-mouse IgM Ab before being washed and incubated with streptavidin coupled to RPE-Cy5 for 15 minutes. For double staining with CCR6 and CCR7, the memory B cells were first incubated with PE-coupled anti-CCR6 and the unconjugated anti-CCR7 mAbs, then with biotinylated goat antimouse IgM, and finally with streptavidin coupled to RPE-Cy5. When intracytoplasmic staining was required, the cells were washed in cold phosphate-buffered saline plus 2% fetal calf serum and permeabilized with PermeaFix (Ortho Diagnostics Systems) according to the manufacturer's instructions. Once permeabilized, the cells were washed and treated as for surface staining. All cells were analyzed on a FACScan flow cytometer (Becton Dickinson, Heidelberg, Germany).
The relative mean fluorescence intensity (MFI) of the chemokine receptor stainings was calculated as follows: MFI of chemokine receptor staining/MFI of irrelevant isotype-matched Ab staining. The percentage of inhibition of the relative MFI of CCR6, CCR7, CXCR4, and CXCR5 stainings were calculated as follows: % inhibition = 100 − (100 × relative MFI of the chemokine receptor staining on anti-Ig–treated cells/relative MFI of the chemokine receptor staining on untreated cells).
Results
CCR6 and CCR7 are heterogeneously expressed by mature B cells
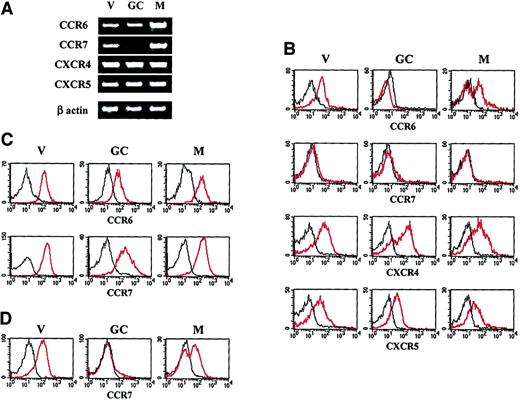
Expression of the CCR6, CCR7, CXCR4, and CXCR5 transcripts was analyzed in freshly isolated virgin, GC, and memory B cells by semiquantitative RT-PCR. As shown in Figure1A, while the messenger RNAs for CCR6, CXCR4, and CXCR5 are present in all 3 B-cell subsets, the CCR7 transcript is absent in GC B cells. To investigate the possible posttranscriptional regulation of chemokine receptor expression, surface expression of CCR6, CCR7, CXCR4, and CXCR5 on B-cell subsets was analyzed by triple immunofluorescence analysis of the unfractionated tonsil B-cell population (Figure 1B). The surface expression of the CXCR4 and CXCR5 proteins matches the results obtained by RT-PCR and confirms that these 2 chemokine receptors are ubiquitously distributed in the mature B-cell compartment. In contrast, CCR6 was subjected to regulation because its expression is lost in the entire GC B-cell population and on approximately half of the memory B-cell subset. Strikingly, the CCR7 protein is undetectable on the surface of any freshly isolated mature B-cell subset. Despite their low or undetectable surface expression on certain B-cell types, both CCR6 and CCR7 were clearly expressed in the cytoplasm of all 3 B-cell subsets considered (Figure 1C). The presence of an intracytoplasmic pool of CCR7 in GC B cells was somehow unexpected because the transcript for CCR7 is not detectable in this B-cell subset.
Chemokine receptor expression by tonsillar B-cell subsets.
(A) Expression of the CCR6, CCR7, CXCR4, and CXCR5 transcripts in virgin (V), GC, and memory (M) B cells was analyzed by semiquantitative RT-PCR. (B) Surface expression of CCR6, CCR7, CXCR4, and CXCR5 was performed by triple immunofluorescence analysis of unfractionated tonsillar B cells using a combination of anti-IgD, anti-CD38, and the corresponding antichemokine receptor Abs. (C) Intracytoplasmic expression of CCR6 and CCR7 in virgin, GC, and memory B cells. (D) Surface expression of CCR7 on B cells after a 6-hour culture in complete medium. The data shown are representative of 4 independent experiments. The red lines correspond to the staining with the antichemokine receptor Ab, the black lines to the staining with an irrelevant isotype-matched Ab.
Chemokine receptor expression by tonsillar B-cell subsets.
(A) Expression of the CCR6, CCR7, CXCR4, and CXCR5 transcripts in virgin (V), GC, and memory (M) B cells was analyzed by semiquantitative RT-PCR. (B) Surface expression of CCR6, CCR7, CXCR4, and CXCR5 was performed by triple immunofluorescence analysis of unfractionated tonsillar B cells using a combination of anti-IgD, anti-CD38, and the corresponding antichemokine receptor Abs. (C) Intracytoplasmic expression of CCR6 and CCR7 in virgin, GC, and memory B cells. (D) Surface expression of CCR7 on B cells after a 6-hour culture in complete medium. The data shown are representative of 4 independent experiments. The red lines correspond to the staining with the antichemokine receptor Ab, the black lines to the staining with an irrelevant isotype-matched Ab.
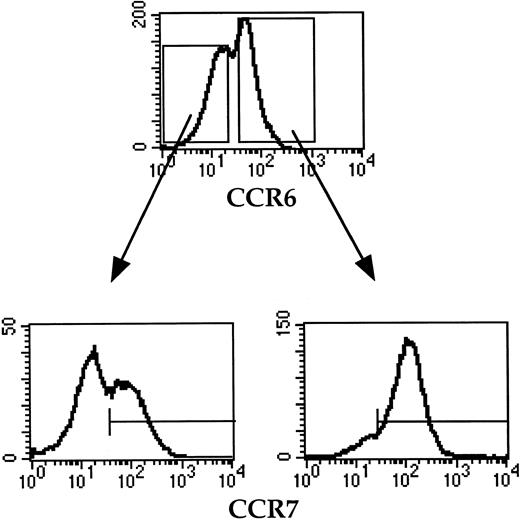
To investigate whether the lack of CCR7 expression by freshly isolated tonsil B cells is due to desensitization possibly induced by previous in vivo contact with either MIP-3β or SLC, B cells were precultured for 6 hours in complete medium before being assessed for CCR7 expression by triple immunofluorescence staining with anti-CD38, anti-IgD, and anti-CCR7 Abs. As shown in Figure 1D, while GC B cells remain CCR7−, all virgin B cells and about 60% memory B cells become CCR7+ upon 6-hour culture in complete medium. In contrast, neither the percentage of CCR6+ B cells nor the density of CCR6 expression is modified upon 6-hour culture (data not shown). Further analysis of memory B cells preincubated for 6 hours in complete medium shows that most CCR6+ memory B cells coexpress CCR7, while the CCR6− memory B-cell subset includes a CCR7+ and a CCR7− population (Figure 2).
CCR6 and CCR7 expression on memory B cells after 6-hour preculture in complete medium.
Memory B cells were double stained with anti-CCR6 and anti-CCR7 mAbs. The CCR7 expression by CCR6+ and CCR6− memory B cells is shown as histograms. The horizontal line indicates the negative threshold as determined by staining with an irrelevant isotype-matched Ab. The data shown are representative of 3 independent experiments.
CCR6 and CCR7 expression on memory B cells after 6-hour preculture in complete medium.
Memory B cells were double stained with anti-CCR6 and anti-CCR7 mAbs. The CCR7 expression by CCR6+ and CCR6− memory B cells is shown as histograms. The horizontal line indicates the negative threshold as determined by staining with an irrelevant isotype-matched Ab. The data shown are representative of 3 independent experiments.
Altogether, these findings indicate that (1) the expression of CCR6 in the mature B-cell compartment is regulated at the posttranslational level, while expression of CCR7 is regulated both at the transcriptional (GC B cells) and posttranslational (memory B cells) levels; (2) the surface expression of both CCR6 and CCR7 is completely repressed in GC B cells; and (3) the surface expression of CCR6 and CCR7 in the memory B cell is heterogeneous.
Both virgin and memory B cells migrate to MIP-3α, SDF-1α, MIP-3β, and BCA-1 in vitro
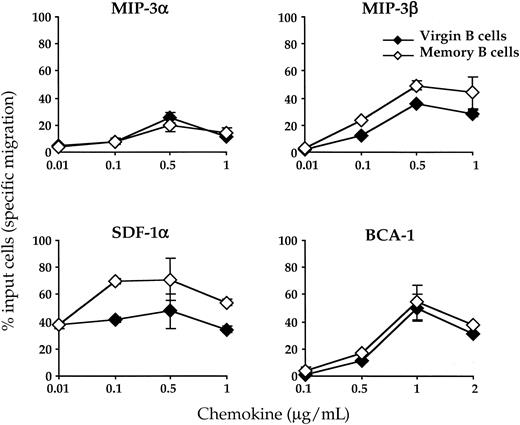
The sites of production of MIP-3α, MIP-3β, SDF-1α, and BCA-1 in the tonsillar tissue have been previously documented.6 7 As described above, GC B cells lack expression of CCR6, the receptor for MIP-3α, and CCR7, the receptor for MIP-3β. Furthermore, despite surface expression of CXCR4 and CXCR5, GC B cells fail to migrate in vitro to SDF-1 and BCA-1 (data not shown). This defective migration of GC B cells is not imputable to their apoptosis because their viability when cultured in the presence of virgin and memory B cells (unfractionated B-cell population) is only marginally impaired within the time course of the migration experiment. Thus, because the migratory capacity of GC B cells is strongly repressed, we decided to compare the in vitro chemotactic response of virgin and memory B cells to crypt chemokines (MIP-3α and SDF-1), the T-zone chemokine MIP-3β, and the B-cell follicle chemokine BCA-1. CD38− B cells isolated by negative selection procedures were thus exposed for 3 hours to graded concentrations of the 4 chemokines cited above. Their chemotactic response as well as the phenotype of the cells migrating to these chemokines were investigated as described in “Materials and methods.” As shown in Figure3, both virgin and memory B cells have the capacity to migrate in vitro to the 4 chemokines analyzed here. For all 4 chemokines, the optimal concentration—ie, that which attracts the higher number of cells—was identical for both B-cell subsets. In contrast for 2 of them (SDF-1α and MIP-3β), the amplitude of the response was higher in memory B cells than in virgin B cells. Whatever the subset considered, SDF-1α was the chemokine that attracted the highest number of cells, while MIP-3α was the chemokine with the lowest B-cell chemotactic activity. Therefore, our data are compatible with the notion that the chemotactic abilities of virgin and memory B cells differ quantitatively but not qualitatively.
Chemotactic response of virgin and memory B cells to lymphoid chemokines.
CD38− B cells were precultured for 6 hours in complete medium before being assessed for chemotaxis in response to MIP-3α, MIP-3β, SDF-1 α, and BCA-1 in the Transwell migration assay. Input and migrated B cells were stained with an anti-IgD Ab to calculate the percentage of virgin and memory B cells that migrated during the assay. The results are expressed as percent of input cells specifically migrating in response to the chemokines after subtraction of the percent of cells spontaneously migrating to complete medium containing no chemokine. The chemotactic response of virgin B cells and memory B cells is shown as open and solid diamonds, respectively. Results are expressed as mean ± SD of experimental duplicates. Representative of 3 independent experiments.
Chemotactic response of virgin and memory B cells to lymphoid chemokines.
CD38− B cells were precultured for 6 hours in complete medium before being assessed for chemotaxis in response to MIP-3α, MIP-3β, SDF-1 α, and BCA-1 in the Transwell migration assay. Input and migrated B cells were stained with an anti-IgD Ab to calculate the percentage of virgin and memory B cells that migrated during the assay. The results are expressed as percent of input cells specifically migrating in response to the chemokines after subtraction of the percent of cells spontaneously migrating to complete medium containing no chemokine. The chemotactic response of virgin B cells and memory B cells is shown as open and solid diamonds, respectively. Results are expressed as mean ± SD of experimental duplicates. Representative of 3 independent experiments.
BCR ligation dramatically alters the chemotaxis pattern of B cells
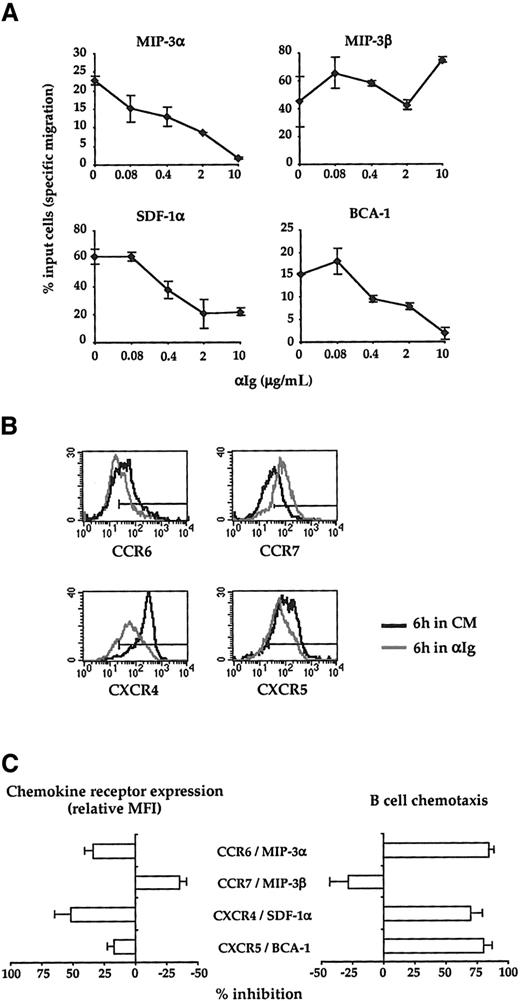
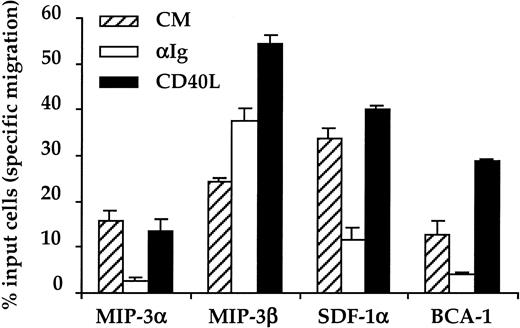
The data shown in Figure 3 indicate that, in the absence of exogenous stimuli, B cells have the potential to migrate to crypt, T-zone, as well as follicle chemokines. Nevertheless, during their response to an antigenic stimulus, B cells are expected to migrate sequentially to distinct microanatomic compartments. Therefore, we next examined whether a surrogate Ag would affect the chemotactic pattern of B cells to the 4 lymphoid chemokines analyzed here. Because both virgin and memory B cells display a similar pattern of responsiveness to MIP-3α, SDF-1, MIP-3β, and BCA-1, the effects of BCR ligation on the chemotactic response of B cells was analyzed on a mixed B-cell population that includes both virgin and memory B cells. CD38− B cells were first cultured for 6 hours with or without increasing concentrations of F(ab′)2 fragments of anti-human Ig Abs before being exposed to the optimal concentration of MIP-3α, SDF-1α, MIP-3β, and BCA-1 as defined in Figure 3. As shown in Figure 4A, the surrogate Ag induces a strong dose-dependent inhibition of the chemotactic response to both crypt chemokines, MIP-3α and SDF-1α, and to the follicle chemokine, BCA-1. In striking contrast, chemotaxis to the T-zone chemokine, MIP-3β, was consistently enhanced by BCR ligation. Figure4B,C illustrates that the changes of the B cell-chemotactic response to SDF-1α, MIP-3α, MIP-3β, and BCA-1 induced by the surrogate Ag are correlated with a modulation of expression of their corresponding receptors. Altogether, these data indicate that BCR ligation stimulates the migratory response of B cells to the T-zone chemokine, MIP-3β, while it inhibits their migration to the chemokines produced both in the crypt and the B-cell follicles.
Effect of BCR ligation on the B-cell chemotactic responses.
(A) CD38−B cells were preincubated for 6 hours with or without increasing doses of anti-Ig Abs (αIg) before being assessed for migration in Transwell plates. MIP-3α, MIP-3β, SDF-1α, and BCA-1 were used at 500 ng/mL, 500 ng/mL, 100 ng/mL, and 1 μg/mL, respectively. The data correspond to the percent of input cells that migrate specifically to each chemokine. Results are expressed as mean ± SD of experimental duplicates and are representative of 3 independent experiments. (B) Surface expression of CCR6, CCR7, CXCR4, and CXCR5 by CD38− B cells precultured for 6 hours with (grey line) or without (solid line) 10 μg/mL anti-Ig Abs. The horizontal line indicates the negative threshold as determined by staining with an irrelevant isotype-matched Ab. (C) The percent inhibition of both chemokine receptor expression and B-cell chemotaxis induced by anti-Ig Abs (10 μg/mL) was calculated as described in “Materials and methods.” The results correspond to the mean ± SD of the percent inhibition calculated on 3 independent experiments. CM indicates complete medium.
Effect of BCR ligation on the B-cell chemotactic responses.
(A) CD38−B cells were preincubated for 6 hours with or without increasing doses of anti-Ig Abs (αIg) before being assessed for migration in Transwell plates. MIP-3α, MIP-3β, SDF-1α, and BCA-1 were used at 500 ng/mL, 500 ng/mL, 100 ng/mL, and 1 μg/mL, respectively. The data correspond to the percent of input cells that migrate specifically to each chemokine. Results are expressed as mean ± SD of experimental duplicates and are representative of 3 independent experiments. (B) Surface expression of CCR6, CCR7, CXCR4, and CXCR5 by CD38− B cells precultured for 6 hours with (grey line) or without (solid line) 10 μg/mL anti-Ig Abs. The horizontal line indicates the negative threshold as determined by staining with an irrelevant isotype-matched Ab. (C) The percent inhibition of both chemokine receptor expression and B-cell chemotaxis induced by anti-Ig Abs (10 μg/mL) was calculated as described in “Materials and methods.” The results correspond to the mean ± SD of the percent inhibition calculated on 3 independent experiments. CM indicates complete medium.
Ligation of CD40 does not alter the chemotaxis pattern of B cells
The CD40/CD40L couple is instrumental in the development of T-dependent Ab responses. The induction and maintenance of the GC reaction are the most important contributions attributed to this pair of molecules during the humoral response. To analyze whether CD40 ligation affects the chemotactic response of B cells, CD38− B cells were preincubated with complete medium, anti-Ig Abs (10 μg/mL), or soluble trimeric CD40L prior to the chemotaxis assay. Data in Figure 5 show that, as opposed to surrogate Ag, CD40L does not modify the pattern of responsiveness of B cells to chemokines but enhances the “amplitude” of their response to MIP-3β and BCA-1. While BCR ligation modulates the B-cell chemotactic response both quantitatively and qualitatively, CD40 engagement only modulates the amplitude of this response.
Effect of CD40 ligation on the B-cell chemotactic response.
CD38− B cells were precultured for 6 hours in the absence (hatched bars) or in the presence of 10 μg/mL anti-Ig Abs (open bars) or CD40L (black bars) before performing the chemotaxis assay. The concentrations of chemokines used were the same as for Figure 4. Results correspond to the percent specific migration and are expressed as the mean ± SD of experimental duplicates. Representative of 3 separate experiments.
Effect of CD40 ligation on the B-cell chemotactic response.
CD38− B cells were precultured for 6 hours in the absence (hatched bars) or in the presence of 10 μg/mL anti-Ig Abs (open bars) or CD40L (black bars) before performing the chemotaxis assay. The concentrations of chemokines used were the same as for Figure 4. Results correspond to the percent specific migration and are expressed as the mean ± SD of experimental duplicates. Representative of 3 separate experiments.
We have also analyzed any possible modulation of the expression of CCR6, CCR7, CXCR4, and CXCR5 after CD40 cross-linking (data not shown). Despite the enhancement of B-cell migration to MIP-3β and BCA-1, the expression of both CCR7 and CXCR5 was not modified by CD40 ligation.
BCR ligation reprograms B cells to migrate to MIP-3β and BCA-1 sequentially
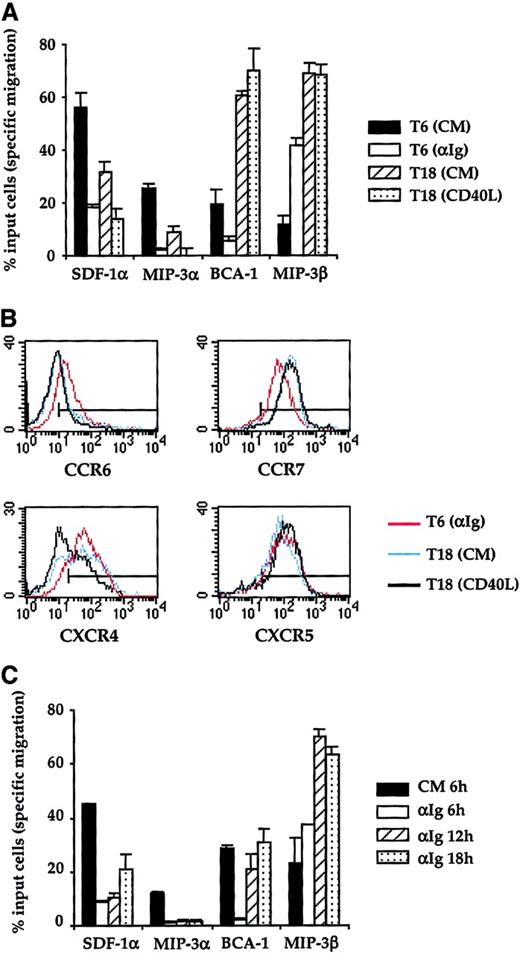
Ab responses to T-dependent Ag require that B cells take up Ag, process it, and present the peptide to Ag-specific T cells. CD40 ligation is likely to take place during this T-cell–B-cell cognate interaction, which will result in B-cell and T-cell migration to the follicle where they will give onset to the GC reaction. We made the assumption that B cells must receive the antigenic hit through their BCR before they establish a cognate interaction with T cells and receive a signal through CD40. Therefore, we designed an experimental protocol in which CD38− B cells were first exposed for 6 hours to the optimal dose of anti-Ig Abs (10 μg/mL). After the 6-hour primary culture, the surrogate Ag was washed off, and the cells were either tested for chemotaxis (T6) or were replaced in culture with medium containing CD40L for a further 12 hours. As a control, anti-Ig–pulsed B cells were also recultured for 12 hours in complete medium without CD40L. At the end of these secondary cultures, the cells were washed twice and their chemotactic responses assessed (T18). As expected, after BCR ligation B cells showed little chemotactism toward SDF-1α, MIP-3α, and BCA-1, while they migrated strongly toward MIP-3β (Figure 6A). The removal of surrogate Ag followed by a further 12-hour culture in complete medium did not significantly restore their ability to respond to SDF-1α or MIP-3α. Nevertheless, under this culture condition the chemotactic response to MIP-3β and BCA-1 was strikingly enhanced. The stimulation of the latter response was the most impressive because 60% of the input cells migrated to BCA-1 at T18 while only 5% of B cells responded to this chemokine at T6 in anti-Ig–stimulated cultures. This pattern of chemotactic response was not further modified when anti-Ig–activated B cells were exposed to CD40L in the secondary cultures. This suggests that the signal delivered through CD40 after the pulse with surrogate Ag has no impact on the migratory response of B cells. As shown in Figure 6B, the dramatic enhancement of the chemotactic response to BCA-1 observed in secondary cultures of anti-Ig–pulsed cultures was not accompanied by a modulation of the surface expression of CXCR5. Unlike for CXCR5, removal of surrogate Ag induces an up-regulation of CCR7.
A short pulse with surrogate Ag enhances responsiveness of B cells to MIP-3β and BCA-1.
(A) The chemotactic response of CD38− B cells was assessed at 2 time points: (1) at the end of a primary culture (T6) conducted with (white bars, T6 αIg) or without 10 μg/mL anti-Ig Abs (black bars, T6 CM) and (2) at the end of a secondary culture (T18) in which anti-Ig–pulsed B cells were cultured in the presence (gray bars, T18 CD40L) or absence of trimeric CD40L (hatched bars, T18 CM). The 4 chemokines were used at the same concentrations as for Figures 4 and 5. (B) Expression of CCR6, CCR7, CXCR4, and CXCR5 was analyzed after the primary culture with anti-Ig Abs (red line) and after the secondary cultures conducted with (black line) or without CD40L (blue line). The horizontal line indicates the negative threshold as determined by staining with an irrelevant isotype-matched Ab. (C) CD38−B cells were precultured for 6 hours in complete medium (white bars) or for 6 hours (black bars), 12 hours (hatched bars, αIg 12 hours), and 18 hours (gray bars, αIg 18 hours) with 10 μg/mL anti-Ig Abs before being analyzed for their chemotactic response to MIP-3α, MIP-3β, SDF-1α, and BCA-1. In panels A and C, the results are expressed as the mean ± SD of duplicate determinations. Representative of 1 experiment chosen among 3. CM indicates complete medium.
A short pulse with surrogate Ag enhances responsiveness of B cells to MIP-3β and BCA-1.
(A) The chemotactic response of CD38− B cells was assessed at 2 time points: (1) at the end of a primary culture (T6) conducted with (white bars, T6 αIg) or without 10 μg/mL anti-Ig Abs (black bars, T6 CM) and (2) at the end of a secondary culture (T18) in which anti-Ig–pulsed B cells were cultured in the presence (gray bars, T18 CD40L) or absence of trimeric CD40L (hatched bars, T18 CM). The 4 chemokines were used at the same concentrations as for Figures 4 and 5. (B) Expression of CCR6, CCR7, CXCR4, and CXCR5 was analyzed after the primary culture with anti-Ig Abs (red line) and after the secondary cultures conducted with (black line) or without CD40L (blue line). The horizontal line indicates the negative threshold as determined by staining with an irrelevant isotype-matched Ab. (C) CD38−B cells were precultured for 6 hours in complete medium (white bars) or for 6 hours (black bars), 12 hours (hatched bars, αIg 12 hours), and 18 hours (gray bars, αIg 18 hours) with 10 μg/mL anti-Ig Abs before being analyzed for their chemotactic response to MIP-3α, MIP-3β, SDF-1α, and BCA-1. In panels A and C, the results are expressed as the mean ± SD of duplicate determinations. Representative of 1 experiment chosen among 3. CM indicates complete medium.
Our experiments show that the anti-Ig–mediated modulation of the B-cell chemotactic response to BCA-1 is biphasic: suppression after 6 hours of primary culture; strong stimulation at the end of the secondary culture. We next addressed the question of whether the enhancement of the B-cell response to BCA-1 in the secondary cultures of anti-Ig–activated B cells was imputable to removal of the surrogate Ag or to the late time point at which the chemotaxis assay was performed. For this purpose, B cells were continuously cultured in the presence of anti-Ig Abs and were harvested after 6 hours, 12 hours, or 18 hours to be tested for chemotaxis (Figure 6C). The migratory response of B cells after a 6-hour preculture in complete medium was used as a control to illustrate the intrinsic migratory capacity of the B-cell population tested. As expected from our previous observations, a 6-hour stimulation with anti-Ig suppressed the response to SDF-1α, MIP-3α, and BCA-1 and enhanced the response to MIP-3β. The inhibition of the responsiveness of B cells to SDF-1α and MIP-3α was maintained after 12 hours and 18 hours of anti-Ig stimulation. The prolonged exposure of B cells to anti-Ig Abs confirmed that BCR ligation strongly stimulates their chemotactic response to MIP-3β because B-cell responsiveness to this chemokine was further increased after 12 hours of anti-Ig stimulation. After the initial suppression of their responsiveness to BCA-1 by 6 hours of anti-Ig stimulation, B cells regained the capacity to migrate in response to BCA-1. However, the amplitude of the migratory response of anti-Ig–stimulated B cells to BCA-1 at later time points (both 12 and 18 hours) did not exceed that of unstimulated B cells (CM 6 hours). The latter observation is in contrast with the fact that the migratory response of anti-Ig–pulsed B cells (Figure 6A) was significantly enhanced above the levels seen in untreated cultures. Altogether, these results suggest that removal of the surrogate Ag is necessary for B cells to fully develop their ability to migrate in response to BCA-1.
Discussion
In this report, we have studied the regulation of the chemotactic responses of tonsillar B cells to the lymphoid chemokines MIP-3α, MIP-3β, SDF-1α, and BCA-1. Our results show that there are at least 3 levels at which B-cell chemotaxis is regulated. First, the regulation can be exerted during transcription of the chemokine receptor. This is the case for CCR7, whose transcript is not detectable in GC B cells. Second, the regulation can be exerted at a posttranslational level. This is exemplified by our observation that, despite the presence of an intracytoplasmic pool of CCR6 protein in GC B cells, their receptor is not exported to the cell membrane. Finally, the third regulatory mechanism may operate downstream of the chemokine receptor, as suggested by our observation that in some cases the migratory response of B cells to chemokines can be profoundly modified without appreciable alterations of the density of expression of the corresponding receptors.
The developmental regulation of CCR7 expression on T cells has been documented previously.23-25 Expression of CCR7 on B cells has been inferred from the observation that both peripheral blood26 and tonsillar B cells27 weakly migrate in vitro to MIP-3β. However, the distribution of this receptor on mature B-cell subsets has remained elusive until now. Here we show that CCR7 is undetectable in freshly isolated tonsillar B cells but is expressed on almost all virgin B cells and about 60% of the memory B-cell population upon 6-hour culture in complete medium. This phenomenon is highly suggestive of desensitization of CCR7 expression on tonsillar B cells in vivo. One of the possibilities is that the down-regulation of CCR7 on virgin and memory B cells is consecutive to their interaction with any of the 2 CCR7 ligands (MIP-3β and SLC) during their recirculation circuit. Alternatively, the loss of CCR7 expression on tonsillar B cells could be due to heterologous desensitization promoted by their contact with other local chemokines.
In any case, surface CCR6 and CCR7 expression on GC B cells is likely to be repressed by a distinct mechanism because these receptors are not reacquired upon in vitro culture. Down-regulation of the chemokine receptor expression is likely to contribute to retain B cells in the GC until completion of the hypermutation and selection processes to avoid the exit of potentially harmful B cells not suitable for positive selection. It is not yet clear, though, why GC B cells retain an intracytoplasmic pool of CCR7 protein while the expression of the transcript is repressed.
In our hands, the expression of CCR6 and CCR7 delineates 2 memory B-cell subsets. This observation is reminiscent of the heterogeneous expression of these receptors on memory T cells, which has been postulated to distinguish 2 memory subsets with distinct homing capacities.23,28,29 In the tonsil, MIP-3α is exclusively produced by epithelial cells lining the lumen of the crypt, ie, the site of Ag penetration.6,7 Thus, CCR6+ memory B cells have the capacity to participate in the ongoing mucosal response that chronically takes place in the inflamed tonsils. Coexpression of CCR7 by these CCR6-expressing memory B cells could confer them the capacity to relocate in the T zone to initiate the humoral response. The CCR6− memory B-cell population is more heterogeneous than the CCR6+ one: It comprises both a CCR7+ and a CCR7− subset. It may represent cells that are not engaged in a humoral response and could include both newly generated memory B cells and cells responsible for the long-term memory. Our findings on CCR6 expression by memory B cells are in partial agreement with the findings of other studies conducted in human and murine B cells. Murine memory B cells lack CCR6 expression,30 while freshly isolated tonsil memory B cells have been reported to homogeneously express CCR6 and to strongly migrate to MIP-3α.31 The discrepancy between CCR6 expression on human versus murine B cells may relate to the origin of these B cells. The tonsil, unlike the spleen, is a lymphoid tissue continuously exposed to environmental Ag. Furthermore, MIP-3α is expressed in the tonsil but not in spleen.32 Splenic memory B cells are located mainly in the marginal zone and do not recirculate33; therefore, these B cells may not require expression of CCR6.
The distinct phases of the Ab responses to T-dependent Ag take place in different microanatomic compartments within the secondary lymphoid tissues. It is therefore expected that B cells exhibit different migratory behavior depending on their developmental stage. Our results are in apparent disagreement with this assumption because they show that virgin and memory B cells display a similar chemotactic pattern. This unexpected finding may relate to the fact that the in vitro migration assay is indicative of the chemotactic potential of the cells but does not necessarily reflect the actual movements of the cells in vivo. As demonstrated by Foxman et al,34 the trafficking of cells within a tissue is influenced by the interplay of parameters such as the concentration of the chemokines, the vicinity of their sources of production, and the effects of previous chemokine encounters (desensitization).20,21 Therefore, the in vitro migratory response of B cells to a set of chemoattractants applied separately cannot fully predict their orientation in the tissues. Nevertheless, our results indicate that the chemotactic pluripotentiality of resting B cells is lost upon BCR-mediated activation, because a surrogate Ag inhibits B-cell chemotaxis to SDF-1α, MIP-3α, and BCA-1, while it enhances their migration to MIP-3β. Our present findings are in agreement with the data of Bleul et al19 and Ngo et al10 showing, respectively, that BCR ligation suppresses chemotaxis of tonsil B cells to SDF-1α and enhances the response of murine splenic B cells to MIP-3β. The restricted migratory potential of Ag-activated B cells is likely to render them refractory to most chemokine gradients except that of MIP-3β in the T zone. The experiments performed in the 2-step culture model highlight that not all the modifications of the B-cell chemotactic potential promoted by BCR ligation occur simultaneously. This is exemplified by the observation that the BCR-induced enhancement of responsiveness to BCA-1 does not appear until 12 hours of removal of the surrogate Ag. This suggests that the capacity of B cells to migrate to the follicles is not an immediate consequence of BCR ligation. As we propose in the hypothetical model depicted in Figure 7, the delayed induction of BCA-1 responsiveness could be instrumental to allow Ag-activated B cells to relocate first in the T zone before migrating to the follicles. Interestingly, the outcome of BCR ligation on the B-cell responsiveness to BCA-1 seems to be subordinated to the duration of exposure to the surrogate Ag. When aggregation of the BCR is transient (ie, when surrogate Ag is withdrawn after 6 hours of contact), almost all activated B cells (75% on average) are induced to migrate to BCA-1 after the initial suppression phase. By contrast, when the surrogate Ag is not removed, activated B cells regain the ability to migrate to BCA-1 but the numbers of cells responding to this chemokine never exceed those seen in untreated cultures (20% responding cells on average). This observation is reminiscent of the strikingly different in vivo trafficking patterns between anergic self-reactive B cells and naive B cells. According to Cyster et al, self-reactive B cells that have been chronically exposed to a nondeleting form of auto-Ag are prevented from entering the follicles.35 They accumulate at the periphery of these follicles, where they will eventually undergo apoptosis. Thus, sustained inhibition of responsiveness to BCA-1 through persistent stimulation of the BCR might contribute to the censoring of self-reactive B lymphocytes in the periphery.
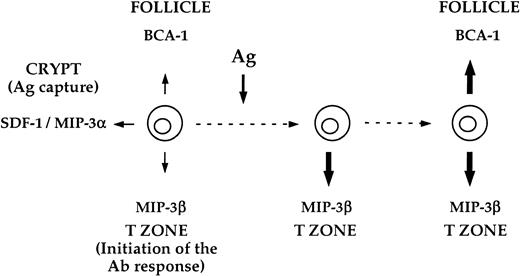
Hypothetical model for the reprogramming of the chemotactic responsiveness of B cells promoted by BCR ligation.
This model proposes that resting B cells have a broad chemokine responsiveness potential, which should equally allow for their migration to the crypt, the T zone, and the B-cell follicle. In contrast, Ag-activated B cells have a very restricted migratory potential, which should favor their relocation to defined microanatomic sites. Our data are compatible with the hypothesis that Ag is the main force that drives the reprogramming of B-cell chemotaxis responsiveness in the course of the immune response. Upon Ag binding, B cells initiate an ordered change of their chemotactic machinery that allows them to redistribute sequentially (1) in the T zone, where the B-cell response is initiated, and (2) in the follicle, where the B-cell response is amplified and improved.
Hypothetical model for the reprogramming of the chemotactic responsiveness of B cells promoted by BCR ligation.
This model proposes that resting B cells have a broad chemokine responsiveness potential, which should equally allow for their migration to the crypt, the T zone, and the B-cell follicle. In contrast, Ag-activated B cells have a very restricted migratory potential, which should favor their relocation to defined microanatomic sites. Our data are compatible with the hypothesis that Ag is the main force that drives the reprogramming of B-cell chemotaxis responsiveness in the course of the immune response. Upon Ag binding, B cells initiate an ordered change of their chemotactic machinery that allows them to redistribute sequentially (1) in the T zone, where the B-cell response is initiated, and (2) in the follicle, where the B-cell response is amplified and improved.
Unlike BCR ligation, cross-linking of CD40 does not induce dramatic changes in the pattern of in vitro migration of B cells. The response to MIP-3α and SDF-1 is not modified at all by CD40L, but a consistent increase in the chemotactic response to both MIP-3β and BCA-1 was observed. The weak impact of CD40 ligation on B-cell chemotaxis is somehow intriguing because in vivo CD40 ligation plays a key role in the fate of B cells during T-dependent Ab responses.3-5Our results would thus suggest that CD40 ligation may not be instrumental for B cells to migrate to the follicles to give rise to the GC reaction. This is in apparent contradiction to the classic notion that GC formation is a feature characteristic of T-dependent but not T-independent Ag responses. Nevertheless, recent data from the group of MacLennan show that T-independent type II Ag can indeed induce a GC reaction if given at a sufficient high dose.36Altogether, these findings support the notion that the BCR rather than CD40 is the driving force that initiates the relocation of B cells during the course of the humoral response.
We are grateful to Dr Christophe Caux for careful reading of this manuscript and for all of his helpful comments.
Supported by the Association pour la Recherche sur le Cancer grant 5641. M.C.-P. was originally funded by the Wellcome Trust Fundation and is now the recipient of a grant from La Fondation pour la Recherche Médicale.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Thierry Defrance, INSERM U404, 21 Ave Tony Garnier, 69365 Lyon, Cedex 07, France; e-mail:defrance@cervi-lyon.inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal