Abstract
Similar to solid tumors, growth of leukemias may also be angiogenesis dependent. Furthermore, tyrosine kinase receptors specific to endothelial cells are expressed on certain subsets of leukemias. We have previously demonstrated the existence of a VEGF/VEGFR-2 autocrine loop on leukemic cells that supports their growth and migration. Here, we demonstrate that in response to leukemia-derived proangiogenic and proinflammatory cytokines such as basic fibroblast growth factor and IL-1, endothelial cells release increasing amounts of another vascular endothelial growth factor (VEGF) family member, VEGF-C. In turn, interaction of VEGF-C with its receptor VEGFR-3 (FLT-4) promotes leukemia survival and proliferation. We demonstrate in 2 cell lines and 5 FLT-4+ leukemias that VEGF-C and a mutant form of the molecule that lacks the KDR-binding motif induce receptor phosphorylation, leukemia proliferation, and increased survival, as determined by increased Bcl-2/Bax ratios. Moreover, VEGF-C protected leukemic cells from the apoptotic effects of 3 chemotherapeutic agents. Because most leukemic cells release proangiogenic as well as proinflammatory cytokines, our data suggest that the generation of a novel paracrine angiogenic loop involving VEGF-C and FLT-4 may promote the survival of a subset of leukemias and protect them from chemotherapy-induced apoptosis. These results identify the VEGF-C/FLT-4 pathway as a novel therapeutic target for the treatment of subsets of acute leukemia.
Introduction
It has been suggested that, similar to solid tumors, neovasculature in the bone marrow of leukemia patients may regulate the growth of leukemia in a paracrine fashion.1-3We and others have shown that bone marrow endothelium releases leukemic growth factors such as granulocyte-macrophage colony-stimulating factor, interleukin (IL)-6, and IL-10.4-6 In turn, tumor cells such as leukemias release angiogenic growth factors—namely, vascular endothelial growth factor (VEGF)—which promote endothelial proliferation and expansion of the bone marrow vasculature.7 Furthermore, we have recently shown that certain leukemic cells not only release VEGF but also express its receptors, resulting in the establishment of an autocrine loop that supports their migration and survival.8 The physiologic significance of such angiogenic paracrine and autocrine loops in regulating leukemia growth and dissemination remains to be determined, but they have facilitated the identification of novel targets for possible therapeutic intervention.
In the present report, we demonstrate that another member of the VEGF family, VEGF-C, promotes survival and proliferation of a subset of acute leukemias. VEGF-C has been characterized as a lymphangiogenic and angiogenic growth factor and shown to signal through the receptors VEGFR-3 (FLT-4) and VEGFR-2 (KDR).9-12In addition, recent studies in solid tumor murine models have correlated increased tumor-derived VEGF-C levels with lymphangiogenesis and lymphatic metastasis,13 suggesting a role of VEGF-C in tumor progression by acting on lymphatic endothelium.
Endothelial cells express the VEGF-C receptor, FLT-4, and produce VEGF-C, particularly in response to proinflammatory cytokines such as IL-1 and tumor necrosis factor.14,15 Subsets of leukemic cells have also been shown to express FLT-4.16 However, it has not been demonstrated whether FLT-4 is functional on these malignant cells and conveys signals similar to those seen on endothelial cells. Given that leukemic cells release proangiogenic as well as proinflammatory cytokines like basic fibroblast growth factor (bFGF), IL-1, and tumor necrosis factor, respectively,17-19 we hypothesize that VEGF-C production by endothelial cells may be up-regulated in response to such cytokines. In turn, as indicated by our results, VEGF-C may then support leukemic survival and proliferation and protect FLT-4+ leukemias from chemotherapy-induced apoptosis. Taken together, our results suggest the existence of a novel angiogenic paracrine loop involving VEGF-C and its receptor FLT-4, which may contribute to the progression of a subset of acute leukemias and may function to protect the leukemic cells from the proapoptotic effects of chemotherapy. We identify the VEGFC/FLT-4 system as a potential target for therapeutic intervention in acute leukemia.
Materials and methods
All materials were obtained from Sigma Chemicals unless otherwise stated.
Cytokines/reagents
VEGF-C, mutant VEGF-C, and anti–FLT-4 monoclonal antibody were provided by K.A. Recombinant human bFGF, human VEGF165, and human IL-1α were obtained from R&D Systems (Minneapolis, MN). The tyrosine kinase inhibitor AG1433 was obtained from Calbiochem (San Diego, CA). Anti-KDR and secondary horseradish peroxidase–conjugated antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti–Bcl-2 antibody was purchased from Oncogene Research Products (Boston, MA), and anti-Bax antibody was purchased from Santa Cruz Biotechnology.
Primary cells and cell lines
Human umbilical vein endothelial cells (HUVECs) were isolated as previously described and cultured in complete endothelial culture medium.20 Primary leukemic cells (a total of 6) were isolated from the peripheral blood of patients and cultured as previously described.8 Primary leukemias and leukemic cell lines representing different types of acute myeloid leukemias, including HL-60, HEL, and THP-1, were cultured in 10% fetal calf serum, RPMI 1640 supplemented with l-glutamine, 25 mM HEPES, 1 μg/mL streptomycin, and 1 μg/mL penicillin.
RNA extraction, complementary DNA synthesis, and RT-PCR
Endothelial and leukemic cells were analyzed for KDR, VEGF-C, and FLT-4 expression by reverse transcriptase–polymerase chain reaction (RT-PCR) and Western analysis. For PCR, total RNA was extracted using TRI reagent following the manufacturer's instructions (Life Technologies, Rockville, MD). Complementary DNA was subsequently synthesized from total cellular RNA using First Strand Synthesis Kit (Life Technologies), and PCR was performed using a PCR thermal cycler (MWG Biotech, High Point, NC). The PCR program used to amplify VEGF-C, FLT-4, and β-actin consisted of a precycle of 5 minutes at 94°C, 45 seconds at 60°C, and 45 seconds at 72°C. Following this initial cycle, the reaction was continued for 35 cycles of 1 minute at 94°C, 45 seconds at 65°C, and 2 minutes at 72°C and concluded with 7 minutes at 72°C. The primer sequences were as follows: β-actin forward primer: TCATGTTTGAGACCTTCAA; β-actin reverse primer: GTCTTTGCGGATGTCCACG (β-actin PCR product: 513 base pairs [bp]); VEGF-C forward primer: GTGACCAACATGGAGTCGTG; VEGF-C reverse primer: CCAGAGATTCCATGCCACTT (VEGF-C PCR product: 660 bp); FLT-4 forward primer: ATTTGTGATTTTGGCCTTGC; FLT-4 reverse primer: CAGGCTCATGAACTTGAAAGC (FLT-4 PCR product: 550 bp); KDR forward primer: GTGACCAACATGGAGTCGTG; and KDR reverse primer: CCAGAGATTCCATGCCACTT (KDR PCR product: 660 bp).
Protein extraction and Western blotting
Phosphorylated VEGFR-3/FLT-4 was detected by Western blotting. Cells were serum-starved overnight, and specified wells were pretreated 1 hour with the tyrosine kinase inhibitor AG1433 (2 μg/mL). Cells were then incubated with 100 ng/mL VEGF-C or 200 ng/mL mutant VEGF-C for 10 minutes at 37°C. After this brief stimulation, total protein extracts from leukemic cell lines and HUVECs were obtained by lysing the cells in cold radioimmunoprecipitation assay (RIPA) buffer (50 mM/L Tris, 5 mM/L ethylenediaminetetraacetic acid, 1% Triton X-114, 0.4% sodium cacodylate, and 150 mM NaCl) in the presence of protease inhibitors (1 mg/mL aprotinin, 10 mg/mL leupeptin, 1 mmol/L β-glycerophosphate, 1 mM sodium orthovanadate, and 1 mM/L phenylmethylsulfonyl fluoride). After centrifugation to remove cell debris, supernatants (a total protein minimum of 500 ng) were immunoprecipitated overnight at 4°C with protein G–agarose beads and an antiphosphotyrosine antibody (Santa Cruz, Biotechnology) to precipitate phosphorylated proteins (in the case of leukemia cell lines) or with a mouse antihuman FLT-4 monoclonal antibody (provided by K.A.) (in the case of the primary leukemias). Precipitated proteins/antibody/beads were washed twice, resuspended in loading buffer, and then subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (7.5% gels) under reducing conditions (in the presence of β-mercaptoethanol). Proteins were subsequently blotted onto a nitrocellulose membrane following conventional protocols. Finally, blots were blocked in 1% bovine serum albumin/phosphate-buffered saline–0.1% Tween 20 for 1 hour at room temperature followed by incubation with primary and secondary antibodies. Antibodies used included the mouse antihuman FLT-4 and a secondary peroxidase-labeled goat antimouse antibody. For coimmunoprecipitation experiments, cell extracts were incubated with anti–FLT-4 antibody and blotted with an anti-KDR antibody. The ECL chemiluminescence detection system and ECL film (Amersham Pharmacia Biotech, Piscataway, NJ) were used to visualize the presence of proteins on the nitrocellulose blots.
Cell proliferation/survival assays
For proliferation studies, cells (1 × 105/mL) were cultured in serum-free RPMI medium for 6 hours before addition of the cytokines. Cells were collected, spun down, and counted after 24 and 48 hours. Cell number and viability were determined by trypan blue exclusion. Each experiment was done in triplicate, and experiments with the leukemic cell lines were performed twice. To investigate in greater detail the prosurvival mechanisms of VEGF-C, HEL and THP-1 cells were treated 24 hours in serum-free conditions in the absence or presence of VEGF-C (100 ng/mL) or mutVEGF-C (200 ng/mL), and levels of proapoptotic and antiapoptotic molecules, Bcl-2 and Bax, were measured by Western analysis.
Chemotherapy and leukemia survival
All 6 primary leukemias and the cell lines HEL and THP-1 (1 × 106 cells per condition) were left untreated or pretreated with VEGF-C (100 ng/mL) for 24 hours in serum-free conditions. In some conditions, VEGF-C was readded to the culture medium together with chemotherapeutic agents daily. After 48 hours, cells were collected and stained with the annexin V apoptosis kit following the manufacturer's instructions. Results are shown as the total number of annexin+ cells after 48 hours, as determined by flow cytometry analysis. Appropriate treatment concentrations of chemotherapeutic agents were assessed by preliminary titration experiments treating serum-starved HEL cells over a 48- to 72-hour period. Treatment concentrations used for subsequent experiments were as follows: cytarabine (Ara-C 200 ng/mL), daunorubicin (200 ng/mL), and etoposide (1 μM).
Statistical analysis
Statistical analysis was performed with data expressed as mean (SEM of 3 independent experiments, each experimental condition done in triplicate). To detect differences between data sets, the Studentt test (2-tailed) was applied in which P < .05 was considered statistically significant.
Results
Production of VEGF-C by HUVECs and up-regulation by leukemia-derived cytokines
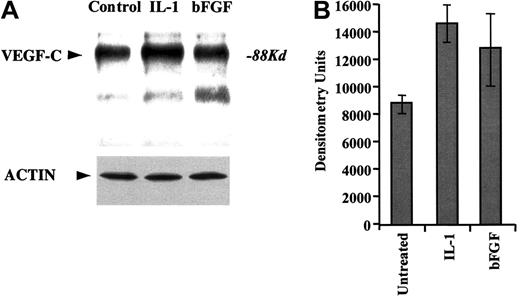
We demonstrate that HUVECs transcribe VEGF-C, as determined by RT-PCR (data not shown). Moreover, HUVECs also release VEGF-C into the cell culture medium, as determined by immunoprecipitation of cell culture supernatants (in serum-free conditions) with VEGF-C–specific antibodies (Figure 1A). Treatment of HUVECs with the proinflammatory cytokine IL-1β or proangiogenic factor bFGF increased the production of VEGF-C over a 24-hour period (Figure 1). Importantly, this increase is not due to an overall increase in protein synthesis, as shown with the Western blot against actin (Figure 1A).
HUVECs produce VEGF-C.
(A) Primary HUVECs were cultured serum-free for 24 hours in the absence or presence of IL-1α (5 ng/mL) or FGF-2 (10 ng/mL). Supernatants were collected, immunoprecipitated for VEGF-C, and immunoblotted with antibodies against VEGF-C. Actin Western blot is also shown as a control for even protein concentration. (B) Densitometry measurements of total band intensities of the VEGF-C levels in HUVEC supernatants were measured and graphed. Error bars (SEM) were calculated from 3 independent experiments and represent the mean variation in signal intensity.
HUVECs produce VEGF-C.
(A) Primary HUVECs were cultured serum-free for 24 hours in the absence or presence of IL-1α (5 ng/mL) or FGF-2 (10 ng/mL). Supernatants were collected, immunoprecipitated for VEGF-C, and immunoblotted with antibodies against VEGF-C. Actin Western blot is also shown as a control for even protein concentration. (B) Densitometry measurements of total band intensities of the VEGF-C levels in HUVEC supernatants were measured and graphed. Error bars (SEM) were calculated from 3 independent experiments and represent the mean variation in signal intensity.
FLT-4 is expressed by subsets of leukemia and activated upon VEGF-C stimulation
We examined FLT-4 and KDR expression, by RT-PCR, in leukemic cell lines representing different leukemic subsets, including HEL (megakaryocytic), THP-1 (monocytic), and HL-60 (promyelocytic). As previously shown, the 3 leukemic cell lines expressed KDR, whereas only HEL and THP-1 were FLT-4+ (data not shown).
We also analyzed 19 freshly isolated peripheral blood leukemic samples for FLT-4 and KDR expression by RT-PCR. Of the 19 samples, 6 acute myeloid leukemias, 2 acute lymphocytic leukemias, and 1 chronic lymphocytic leukemia expressed FLT-4 at the messenger RNA level (data not shown). Six samples were subsequently chosen for further analysis, including 1 acute lymphocytic leukemia (referred to as sample 1) and 5 acute myeloid leukemias (samples 2-6). Of note, samples 1, 3, 4, 5, and 6 expressed FLT-4 and KDR, as determined by RT-PCR and flow cytometry, whereas sample 2 expressed KDR but not FLT-4 (data not shown).
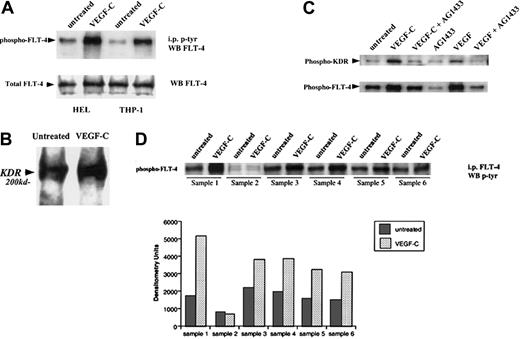
We next investigated whether FLT-4, present on primary leukemias and the cell lines HEL and THP-1, was functional by analyzing ligand-induced receptor autophosphorylation. VEGF-C induced FLT-4 phosphorylation on HEL, THP-1 (Figure2A), and the 5 FLT-4+ primary leukemias (Figure 2D), similar to its effects on HUVECs. In addition, we demonstrate in coimmunoprecipitation experiments that VEGF-C may induce FLT-4/KDR heterodimerization (data for HEL shown in Figure 2B). In a subsequent immunoprecipitation study in HEL cells (Figure 2C), stimulation with either VEGF or VEGF-C led to the phosphorylation of both FLT-4 and KDR, an effect abrogated by a general tyrosine kinase inhibitor (AG1433). Similar results were seen in THP-1 cells and the FLT-4+ leukemias (data not shown). This suggests that binding of VEGF family members to their receptor(s) on leukemias may lead to the recruitment and activation of other signaling molecules such as other VEGF receptor tyrosine kinases.
VEGF-C induces FLT-4 phosphorylation on HEL and THP-1 cells.
(A) Serum-starved leukemic cells were left untreated or stimulated with VEGF-C (100 ng/mL) for 10 minutes and then immunoprecipitated for tyrosine-phosphorylated proteins. Subsequent blots were probed with antibody against FLT-4. As a control, total FLT-4 levels are also shown (FLT-4 Western blot). (B) HEL cells placed in serum-free conditions were treated in the absence or presence of VEGF-C (100 ng/mL) for 10 minutes and then lysed with RIPA buffer. Lysates were immunoprecipitated for FLT-4, and blots were probed with anti-KDR antibodies. (C) HEL cells placed in serum-free conditions were treated in the absence or presence of AG1433 (2 μg/mL) for 1 hour and then treated with VEGF-C (100 ng/mL) or VEGF (20 ng/mL) for 10 minutes. Cells were then lysed with RIPA buffer and lysates immunoprecipitated for tyrosine-phosphorylated proteins. Subsequent blots were probed with anti-KDR or anti–FLT-4 antibodies. (D) Six primary leukemias were left untreated or stimulated with VEGF-C (100 ng/mL) for 10 minutes, immunoprecipitated for FLT-4, and probed with an antibody for phosphorylated proteins. As shown, FLT-4 induced tyrosine phosphorylation in FLT-4+ samples (nos. 1, 3, 4, 5, and 6). Bar graph represents the densitometry quantification of phosphorylated FLT-4 and is representative of 3 independent experiments.
VEGF-C induces FLT-4 phosphorylation on HEL and THP-1 cells.
(A) Serum-starved leukemic cells were left untreated or stimulated with VEGF-C (100 ng/mL) for 10 minutes and then immunoprecipitated for tyrosine-phosphorylated proteins. Subsequent blots were probed with antibody against FLT-4. As a control, total FLT-4 levels are also shown (FLT-4 Western blot). (B) HEL cells placed in serum-free conditions were treated in the absence or presence of VEGF-C (100 ng/mL) for 10 minutes and then lysed with RIPA buffer. Lysates were immunoprecipitated for FLT-4, and blots were probed with anti-KDR antibodies. (C) HEL cells placed in serum-free conditions were treated in the absence or presence of AG1433 (2 μg/mL) for 1 hour and then treated with VEGF-C (100 ng/mL) or VEGF (20 ng/mL) for 10 minutes. Cells were then lysed with RIPA buffer and lysates immunoprecipitated for tyrosine-phosphorylated proteins. Subsequent blots were probed with anti-KDR or anti–FLT-4 antibodies. (D) Six primary leukemias were left untreated or stimulated with VEGF-C (100 ng/mL) for 10 minutes, immunoprecipitated for FLT-4, and probed with an antibody for phosphorylated proteins. As shown, FLT-4 induced tyrosine phosphorylation in FLT-4+ samples (nos. 1, 3, 4, 5, and 6). Bar graph represents the densitometry quantification of phosphorylated FLT-4 and is representative of 3 independent experiments.
VEGF-C induces leukemia proliferation
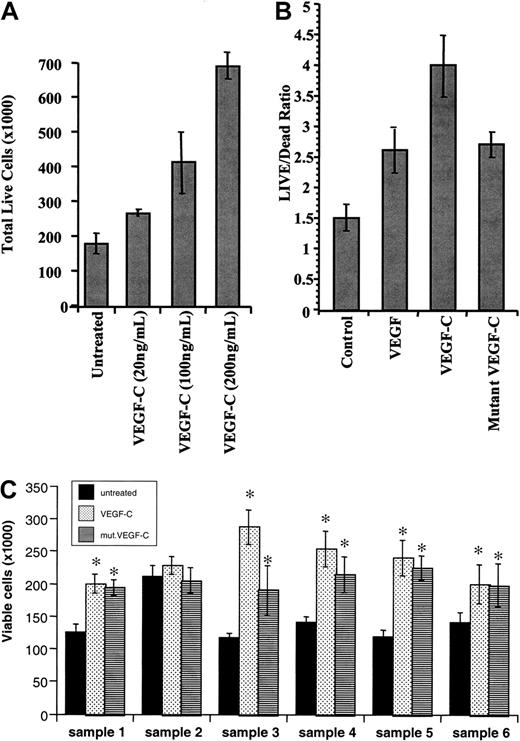
We next investigated whether VEGF-C binding to its receptor on leukemic cells resulted in any phenotypic changes. VEGF-C increased leukemic cell proliferation in a dose-dependent manner (data shown for THP-1 cells, Figure 3A). Treatment at a concentration of 200 ng/mL led to an approximately 2-fold increase in viable cells compared with the untreated population. Notably, a mutant-form of VEGF-C (mutVEGF-C), capable of binding and signaling only through FLT-4 and not KDR, also induced leukemic cell proliferation, although to a lesser extent than that seen for VEGF-C (shown for HEL cells, Figure 3B). The proliferative effects of VEGF-C and mutVEGF-C were observed in both HEL and THP-1 cell lines as well as the 5 FLT-4+ primary samples analyzed (*P < .05 compared with untreated cells, Figure 3C). Importantly, sample 2 (KDR+ but FLT-4−) did not respond to the proliferative effects of VEGF-C (Figure 3C). This result demonstrates that on subsets of leukemias VEGF-C induces leukemic proliferation by interacting through FLT-4 and KDR.
VEGF-C and mutant VEGF-C promote leukemic cell proliferation.
(A) THP-1 leukemic cells were serum-starved for 48 hours and then left untreated or incubated in the presence of increasing concentrations of VEGF-C (20, 100, and 200 ng/mL), and total live cells were counted by trypan blue exclusion. (B) HEL leukemic cells were serum-starved for 48 hours, same as above, in the absence or presence of VEGF (20 ng/mL), VEGF-C (100 ng/mL), or mutVEGF-C (200 ng/mL), and total live cells were counted by trypan blue exclusion. (C) Primary leukemias (a total of 6, as described in “Materials and methods”) were cultured in serum-free conditions for 48 hours, in the presence or absence of VEGF-C (100 ng/mL) or mutVEGF-C (200 ng/mL). The total number of viable cells was determined by trypan blue exclusion. *VEGF-C and mutVEGF-C induced a significant increase in viable cells (P < .05 compared with untreated cells).
VEGF-C and mutant VEGF-C promote leukemic cell proliferation.
(A) THP-1 leukemic cells were serum-starved for 48 hours and then left untreated or incubated in the presence of increasing concentrations of VEGF-C (20, 100, and 200 ng/mL), and total live cells were counted by trypan blue exclusion. (B) HEL leukemic cells were serum-starved for 48 hours, same as above, in the absence or presence of VEGF (20 ng/mL), VEGF-C (100 ng/mL), or mutVEGF-C (200 ng/mL), and total live cells were counted by trypan blue exclusion. (C) Primary leukemias (a total of 6, as described in “Materials and methods”) were cultured in serum-free conditions for 48 hours, in the presence or absence of VEGF-C (100 ng/mL) or mutVEGF-C (200 ng/mL). The total number of viable cells was determined by trypan blue exclusion. *VEGF-C and mutVEGF-C induced a significant increase in viable cells (P < .05 compared with untreated cells).
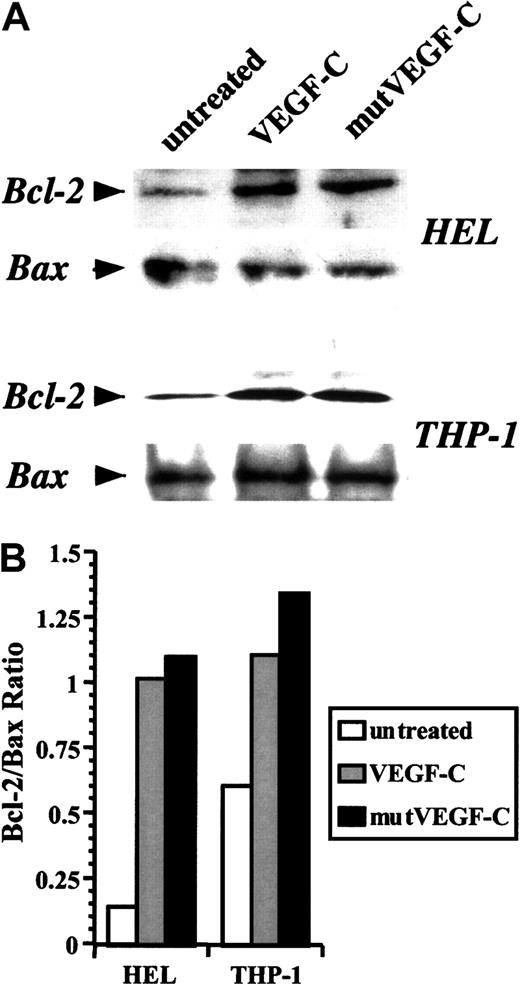
VEGF-C and mutVEGF-C also induce prosurvival effects
We used the 2 leukemic cell lines to investigate further the mechanisms by which VEGF-C promoted cell survival. Treatment of serum-starved THP-1 and HEL cells with VEGF-C or mutVEGF-C induced an up-regulation in Bcl-2 expression within 24 hours (Figure4A). The levels of Bax remained fairly constant (Figure 4A), resulting in increased Bcl-2/Bax ratios (Figure4B). This suggests that one mechanism by which VEGF-C may promote leukemic cell survival is through inhibition of apoptosis. Because mutVEGF-C produces similar effects on leukemia survival and Bcl-2 induction, these results also suggest that VEGF-C promotes leukemic cell survival by signaling through FLT-4.
Induction of Bcl-2 levels by VEGF-C and mutVEGF-C.
(A) Serum-starved HEL and THP-1 cells were treated in the absence or presence of VEGF-C (100 ng/mL) or mutVEGF-C (200 ng/mL) for 24 hours. Cells were lysed in RIPA buffer, proteins resolved by acrylamide gel electrophoresis, and immunoblotted for Bcl-2 and Bax. (B) Bcl-2 and Bax band densities were measured, and Bcl-2/Bax ratios were graphed for the different conditions.
Induction of Bcl-2 levels by VEGF-C and mutVEGF-C.
(A) Serum-starved HEL and THP-1 cells were treated in the absence or presence of VEGF-C (100 ng/mL) or mutVEGF-C (200 ng/mL) for 24 hours. Cells were lysed in RIPA buffer, proteins resolved by acrylamide gel electrophoresis, and immunoblotted for Bcl-2 and Bax. (B) Bcl-2 and Bax band densities were measured, and Bcl-2/Bax ratios were graphed for the different conditions.
VEGF-C and mutVEGF-C protect leukemia from chemotherapy-induced apoptosis
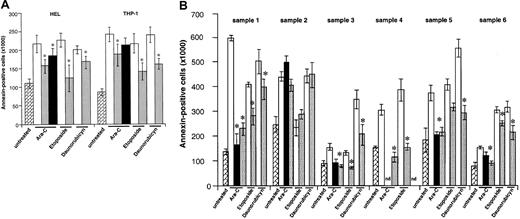
As seen in Figure 5A, VEGF-C protected HEL and THP-1 leukemic cells from chemotherapy-induced cell death. This effect was seen with daunorubicin, etoposide, and Ara-C, with variable results for the 3 agents but achieving significance (P < .005, Figure 5A). As an example, VEGF-C increased the average total viable Ara-C–treated population by approximately 230% in THP-1 cells and 60% in HEL cells (after 48 hours, Figure 5,P < .05). Similar results, and confirming also the mitogenic effects of VEGF-C, were seen in the 5 FLT-4+primary leukemias. VEGF-C protected the primary leukemic cells from chemotherapy-induced cell death, an effect that was significant (P < .05) for all 3 agents used but achieving its most impressive effects against Ara-C (Figure 5B). Importantly, VEGF-C or its mutant failed to protect leukemic cells from sample 2 from chemotherapy-induced apoptosis (Figure 5B). Finally, VEGF had only a marginal effect at protecting the 2 cell lines HEL and THP-1 from chemotherapy-induced apoptosis (results shown for Ara-C, significant for HEL cells, Figure 5A). These results suggest that VEGF-C signaling through FLT-4 induces leukemic cell survival in serum-free conditions and protects leukemic cells from the proapoptotic effects of chemotherapy.
Partial rescue of leukemic cells from chemotherapy-induced death.
(A) HEL and THP-1 cells (1 × 106 per well) were exposed to different chemotherapy agents (Ara-C, 200 ng/mL; etoposide, 1 μM; daunorubicin, 200 ng/mL) for 48 hours, and the number of apoptotic cells was determined by annexin V staining, as described in “Materials and methods.” In some conditions, cells were pretreated with 100 ng/mL VEGF-C, mutVEGF-C, or VEGF (100 ng/mL) for 24 hours, before addition of the chemotherapeutic agents. The cytokines, as with the chemotherapeutic agent, were readded to the cultures daily. As shown, VEGF-C and mutVEGF-C exerted a significant protective effect against the 3 agents used in this study. VEGF also protected HEL cells from Ara-C–induced apoptosis. Results shown were obtained from 3 independent experiments, and each condition was assayed in triplicate. *P < .05 (significant difference compared with chemotherapeutic agent alone). , indicates untreated; □, chemotherapy;
, indicates untreated; □, chemotherapy; , chemotherapy + VEGF-C; ■, Arg-C+VEGF. (B) Similar to the cell lines, 6 primary leukemia samples were cultured in serum-free conditions for 48 hours in the presence of different chemotherapeutic agents (Ara-C, etoposide, daunorubicin). In some conditions the cells were pretreated with VEGF-C (100 ng/mL) or mutVEGF-C (200 ng/mL) for 24 hours, prior to the addition of the chemotherapeutic agent. In these conditions, the cells received cytokines plus chemotherapeutic agent daily. Apoptotic cells were determined by annexin V staining, as described in “Materials and methods.” As shown, VEGF-C and mutVEGF-C protected FLT-4+ primary cells from chemotherapy-induced apoptosis (samples1, 3, 4, 5, and 6). Importantly, neither VEGF-C nor mutVEGF-C protected a FLT-4− sample (number 2) from chemotherapy-induced apoptosis. Results shown were obtained from 3 independent experiments, and each condition was assayed in triplicate. *P < .05 (significant difference compared with chemotherapeutic agent alone). nd: due to sample constraints, some conditions could not be assayed in triplicate and were therefore removed from the final graph.
, chemotherapy + VEGF-C; ■, Arg-C+VEGF. (B) Similar to the cell lines, 6 primary leukemia samples were cultured in serum-free conditions for 48 hours in the presence of different chemotherapeutic agents (Ara-C, etoposide, daunorubicin). In some conditions the cells were pretreated with VEGF-C (100 ng/mL) or mutVEGF-C (200 ng/mL) for 24 hours, prior to the addition of the chemotherapeutic agent. In these conditions, the cells received cytokines plus chemotherapeutic agent daily. Apoptotic cells were determined by annexin V staining, as described in “Materials and methods.” As shown, VEGF-C and mutVEGF-C protected FLT-4+ primary cells from chemotherapy-induced apoptosis (samples1, 3, 4, 5, and 6). Importantly, neither VEGF-C nor mutVEGF-C protected a FLT-4− sample (number 2) from chemotherapy-induced apoptosis. Results shown were obtained from 3 independent experiments, and each condition was assayed in triplicate. *P < .05 (significant difference compared with chemotherapeutic agent alone). nd: due to sample constraints, some conditions could not be assayed in triplicate and were therefore removed from the final graph. , indicates untreated; □, chemotherapy;
, indicates untreated; □, chemotherapy; , chemotherapy + VEGF-C; ■, Arg-C+mutVEGF-C.
, chemotherapy + VEGF-C; ■, Arg-C+mutVEGF-C.
Partial rescue of leukemic cells from chemotherapy-induced death.
(A) HEL and THP-1 cells (1 × 106 per well) were exposed to different chemotherapy agents (Ara-C, 200 ng/mL; etoposide, 1 μM; daunorubicin, 200 ng/mL) for 48 hours, and the number of apoptotic cells was determined by annexin V staining, as described in “Materials and methods.” In some conditions, cells were pretreated with 100 ng/mL VEGF-C, mutVEGF-C, or VEGF (100 ng/mL) for 24 hours, before addition of the chemotherapeutic agents. The cytokines, as with the chemotherapeutic agent, were readded to the cultures daily. As shown, VEGF-C and mutVEGF-C exerted a significant protective effect against the 3 agents used in this study. VEGF also protected HEL cells from Ara-C–induced apoptosis. Results shown were obtained from 3 independent experiments, and each condition was assayed in triplicate. *P < .05 (significant difference compared with chemotherapeutic agent alone). , indicates untreated; □, chemotherapy;
, indicates untreated; □, chemotherapy; , chemotherapy + VEGF-C; ■, Arg-C+VEGF. (B) Similar to the cell lines, 6 primary leukemia samples were cultured in serum-free conditions for 48 hours in the presence of different chemotherapeutic agents (Ara-C, etoposide, daunorubicin). In some conditions the cells were pretreated with VEGF-C (100 ng/mL) or mutVEGF-C (200 ng/mL) for 24 hours, prior to the addition of the chemotherapeutic agent. In these conditions, the cells received cytokines plus chemotherapeutic agent daily. Apoptotic cells were determined by annexin V staining, as described in “Materials and methods.” As shown, VEGF-C and mutVEGF-C protected FLT-4+ primary cells from chemotherapy-induced apoptosis (samples1, 3, 4, 5, and 6). Importantly, neither VEGF-C nor mutVEGF-C protected a FLT-4− sample (number 2) from chemotherapy-induced apoptosis. Results shown were obtained from 3 independent experiments, and each condition was assayed in triplicate. *P < .05 (significant difference compared with chemotherapeutic agent alone). nd: due to sample constraints, some conditions could not be assayed in triplicate and were therefore removed from the final graph.
, chemotherapy + VEGF-C; ■, Arg-C+VEGF. (B) Similar to the cell lines, 6 primary leukemia samples were cultured in serum-free conditions for 48 hours in the presence of different chemotherapeutic agents (Ara-C, etoposide, daunorubicin). In some conditions the cells were pretreated with VEGF-C (100 ng/mL) or mutVEGF-C (200 ng/mL) for 24 hours, prior to the addition of the chemotherapeutic agent. In these conditions, the cells received cytokines plus chemotherapeutic agent daily. Apoptotic cells were determined by annexin V staining, as described in “Materials and methods.” As shown, VEGF-C and mutVEGF-C protected FLT-4+ primary cells from chemotherapy-induced apoptosis (samples1, 3, 4, 5, and 6). Importantly, neither VEGF-C nor mutVEGF-C protected a FLT-4− sample (number 2) from chemotherapy-induced apoptosis. Results shown were obtained from 3 independent experiments, and each condition was assayed in triplicate. *P < .05 (significant difference compared with chemotherapeutic agent alone). nd: due to sample constraints, some conditions could not be assayed in triplicate and were therefore removed from the final graph. , indicates untreated; □, chemotherapy;
, indicates untreated; □, chemotherapy; , chemotherapy + VEGF-C; ■, Arg-C+mutVEGF-C.
, chemotherapy + VEGF-C; ■, Arg-C+mutVEGF-C.
Discussion
There is a great deal of interest in determining whether growth of liquid tumors such as leukemia also involves an increase in angiogenesis. In this regard, emerging data suggest that there is an increase in bone marrow angiogenesis of patients with different hematologic malignancies such as leukemia.2,3,21-23Moreover, disease progression in leukemic patients has been associated with increased plasma levels of proangiogenic cytokines, including VEGF and bFGF,24 secreted by leukemic cells in vitro and in vivo.1,8,25 As a result of an increase in circulating angiogenic growth factors, the expanding bone marrow endothelial mass may support leukemic growth in a paracrine fashion by releasing factors such as granulocyte-macrophage colony-stimulating factor and IL-6.5
In the present study, we demonstrate that VEGF-C may support leukemic growth by acting in a paracrine fashion. VEGF-C is a member of the VEGF growth factor family that has been previously shown to induce proliferation of lymphatic as well as normal endothelium.26,27 It is capable of signaling through both VEGFR2 (KDR) and VEGFR3 (FLT-4) and induces endothelial proliferation and migration.9,27 Recent studies in a murine breast cancer model have shown that overexpression of VEGF-C in tumors leads to increased lymphangiogenesis and lymphatic metastasis.13While those studies examined the role of VEGF-C signaling on the vasculature, our studies suggest an important role for vasculature-derived VEGF-C signaling on subsets of leukemias. We show that endothelial cells release VEGF-C into the cell culture supernatants, a phenomenon augmented in response to leukemia-derived proinflammatory and angiogenic cytokines such as IL-1 and bFGF. In turn, VEGF-C induced proliferation and promoted cell survival of FLT-4+ leukemias cultured in serum-free conditions. More importantly, VEGF-C protected FLT-4/KDR+ leukemic cells from chemotherapy-induced apoptosis in response to 3 different agents: Ara-C, etoposide, and daunorubicin. The antiapoptotic effects of VEGF-C involved induction of Bcl-2 and increased Bcl-2/Bax ratios. These effects of VEGF-C were not observed in the FLT-4−/KDR+ leukemic cells, such as the HL-60 cell line and one primary leukemia sample (results not shown). Furthermore, a mutant-form of VEGF-C that binds and signals only through VEGFR-3 (FLT-4) produced similar effects to those seen with VEGF-C. The results suggest that on subsets of leukemias, VEGF-C exerts most of its effects through FLT-4. However, we also demonstrate that VEGF-C treatment leads to FLT-4/KDR heterodimerization as well as both FLT-4 and KDR autophosphorylation, suggesting that upon VEGF-C stimulation these receptors may interact and perhaps use common signaling cascades.
During leukemia progression, mobilization of leukemic blasts into the peripheral circulation will result in increased circulating levels of angiogenic or proinflammatory cytokines, which in turn may increase VEGF-C production by bone marrow endothelium. An increase in VEGF-C production may result in augmented leukemia proliferation and resistance to chemotherapy. In this paradigm, it remains to be demonstrated whether VEGF-C production by bone marrow endothelial cells in leukemic patients is higher than in their normal counterparts or whether antiangiogenic therapies down-regulate VEGF-C production. Additionally, because endothelial cells have also been shown to express FLT-4 and certain subsets of leukemias release VEGF-C, it is unclear whether additional paracrine or autocrine FLT-4/VEGF-C signaling loops play an important role in disease progression. Although further investigation is needed to address these questions, the present results suggest that circulating VEGF-C levels may correlate with disease prognosis.
To our knowledge, the present report is the first demonstration that VEGF-C, by acting in a paracrine fashion, may actually contribute to the development of leukemia. A previous study documented the expression of FLT-4 in over one third of human primary acute leukemias screened.16 Therefore, agents that target the VEGF-C/FLT-4 signaling pathway may have therapeutic potential for these subsets of leukemias. Our results also emphasize the role of the microenvironment—namely, the vascular component—in the growth of liquid tumors such as leukemias. Furthermore, in light of the recent findings that VEGF-C may promote lymphangiogenesis and lymphatic metastasis in a solid tumor model,13 therapies blocking the FLT-4/VEGF-C signaling pathway may prove to be effective for the treatment of leukemia by targeting not only leukemic cells but also the neovasculature.
Finally, the specific contribution of the different VEGF family members to the process of solid or liquid tumor angiogenesis has not been properly defined. We recently reported that besides stimulating endothelial growth, VEGF by interaction with its VEGFR-2 may support leukemic cell growth and migration in an autocrine manner.8 The results shown here suggest that antiangiogenic strategies may require targeting autocrine and paracrine signaling of several VEGF family members. Considering the effects of VEGF-C in chemotherapy-treated leukemias, a combination of chemotherapy and anti-VEGF–C/FLT-4 therapy may prove to be the most effective in treating certain subsets of leukemias.
Supported by a Translational Research Award from the Leukemia and Lymphoma Society; National Heart, Lung, and Blood Institute (NHLBI) grants R01s HL-58707, HL-61849, HL-66592, HL-67839; Research Scholar Grant from American Cancer Society (RSG-01-091-01); and the Lupin Foundation; (S.R.). S.D. was supported by Portuguese Science and Technology Foundation.
S.D. and M.C. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shahin Rafii, Weill Medical College of Cornell University, Division of Hematology/Oncology, 1300 York Ave, Rm C-606, New York, NY 10021; e-mail: srafii@mail.med.cornell.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal