Abstract
Specific intracellular signals mediated by interleukin-6 (IL-6) receptor complexes, such as signal transducer and activator of transcription 3 (STAT 3) and extracellular signal–regulated kinase (ERK) 1/2, are considered to be responsible for inducing a variety of cellular responses. In multiple myeloma, IL-6 only enhanced the proliferation of CD45+ tumor cells that harbored the IL-6–independent activation of src family kinases even though STAT3 and ERK1/2 could be activated in response to IL-6 in both CD45+ and CD45− cells. Furthermore, the IL-6–induced proliferation of CD45+ U266 myeloma cells was significantly suppressed by Lyn-specific antisense oligodeoxynucleotides or a selective src kinase inhibitor. These results indicate that the activation of both STAT3 and ERK1/2 is not enough for IL-6–induced proliferation of myeloma cell lines that require src family kinase activation independent of IL-6 stimulation. Thus, the activation of the src family kinases associated with CD45 expression is a prerequisite for the proliferation of myeloma cell lines by IL-6. We propose a mechanism for IL-6–induced cell proliferation that is strictly dependent upon the cellular context in myelomas.
Introduction
Interleukin-6 (IL-6) has a variety of biologic functions in different cells1 and is a growth factor for human myeloma cells.2 IL-6 receptor complexes consist of the IL-6 receptor α chain (IL-6Rα) and gp130, the latter being commonly used as a signal-transducing molecule by the members of the IL-6 family of cytokines.3 Upon IL-6 binding to the IL-6Rα, phosphorylated tyrosine residues in gp130 using Janus kinases (JAKs), such as Jak1 and Tyk2, recruit signal transducer and activator of transcription 3 (STAT3), which is also phosphorylated by the JAKs. The phosphorylated STAT3 molecules form dimers, move to the nucleus, and regulate the target gene expression.4 IL-6 also induces the activation of Ras via Sos and Grb2 interacting with SHP-2, which is also recruited by the other phosphorylated tyrosine residue in gp130. Subsequently, the downstream signaling intermediates of Ras, such as Raf-1, mitogen-activated protein kinase (MAPK) or ERK kinase (MEK) 1/2, and extracellular signal–regulated kinase (ERK) 1/2, are activated.5
CD45 antigens are initially characterized as leukocyte common antigen expressed on all hematopoietic cells except for mature erythrocytes and platelets.6 CD45 molecules contain 2 domains harboring protein tyrosine phosphatase (PTP) activity in their cytoplasmic region and are crucial for both T- and B-cell activation through the corresponding antigen receptors.7 Engagement of B-cell antigen receptor by anti–immunoglobulin M antibodies induces the activation and recruitment of src family protein tyrosine kinases (PTKs), such as Lyn, Fyn, and Blk.8 The src family PTKs include Blk, Fgr, Fyn, Hck, Lck, Lyn, Src, and Yes, some of which are expressed exclusively in hematopoietic cells.9 They share src homology (SH) 2, SH3, catalytic domains of PTKs, and a conserved COOH-terminal tyrosine residue, which is selectively phosphorylated by a cytosolic PTK, Csk.10 Dephosphorylation of the COOH-terminal tyrosine residue of the src family PTKs by CD45 PTP has been implicated in a mechanism of src family PTK activation11-14 and results in the alteration of their intramolecular conformation that affects their kinase activities.9 The activated src family PTKs mediate downstream the signals of several extracellular stimuli, such as growth factors, cytokines, and antigen stimulation, leading to diversification and amplification of the initial signals.9
Multiple myeloma (MM) is a human plasma cell neoplasm usually found in bone marrow. IL-6 is a growth factor for myeloma cells in vitro2 and for murine plasmacytoma in vivo.15IL-6 plays a crucial role in the onset of plasma cell tumors in vivo16 and in vitro has an antiapoptotic effect on myeloma cells.17 Therefore, IL-6 supports the survival and expansion of myeloma cells by both stimulating cell division and by preventing apoptosis. However, only a portion of the tumor cells isolated from MM patients proliferates in response to IL-6. Despite the clonal origin of myeloma cells, defined by their immunoglobulin gene rearrangement, they include mixed subpopulations in accordance with the expressions of their surface antigens, such as CD45, CD49e, and MPC-1.18 Some populations of myeloma cells, such as MPC-1−CD49e− immature phenotype, show significant proliferative activity from IL-6, whereas others do not proliferate but secrete higher amounts of antibodies in response to IL-6.18 Thus, myeloma cells are heterogeneous in respect to their biologic characters as well as to their immunophenotypes.
Although the roles of CD45 have been extensively studied for antigen receptors in B and T cells,7 its physiologic consequences in other hematopoietic cells remain largely unknown. Along with others, we recently found that a few MPC-1−CD49e−immature myeloma cells expressing CD45 form a proliferating population in MM.19-21 Thus, MM seems a good example in which to address the biologic functions of CD45 molecules in cell proliferation promoted by the cytokine IL-6. In this study we show that in myeloma cell lines the activation of both STAT3 and ERK1/2 is not sufficient for IL-6–induced proliferation, which further requires IL-6–independent activation of the src family PTKs associated with CD45 PTP. We demonstrate that the cellular context, such as CD45 expression and src family PTK activation, is crucial for myeloma cells to proliferate in response to IL-6.
Materials and methods
Cell culture, flow cytometry
Human myeloma cell lines U266, ILKM2, ILKM3, ILKM8 (gifts from Dr S. Shimizu, Shimane, Japan), NOP2,22 and KMS5 23 were maintained as described.24CD45− or CD45+ U266 subpopulations were isolated via a cell sorter (Epics Elite ESP, Coulter, Hialeath, FL). Flow cytometry analysis using phyroerythrin-labeled anti–human CD45 or CD126 (IL-6Rα) monoclonal antibodies (Immunotech, Marseille, France) was carried out as described previously.24
Cell proliferation assay
Cells (1 × 104) depleted with IL-6 for 24 hours in 96-well plates were incubated with or without 2 ng/mL of IL-6 (a gift from Chugai Pharmaceutical, Shizuoka, Japan) in 100 μL medium for 48 to 60 hours, and cell proliferation was measured by 5-bromo-2′-deoxyuridine (BrdU) incorporation after 15 to 18 hours of culture with BrdU in each well. A peroxidase-labeled anti-BrdU antibody was used to detect the pyrimidine analog BrdU, incorporated instead of thymidine, in newly synthesized DNA. The immune complexes were detected by the Biotrak cell proliferation enzyme-linked immunosorbent assay system, version 2 (Amersham Pharmacia Biotech, Piscataway, NJ), according to the manufacturer's instructions.
Western blot analysis
Cells (1 × 106) depleted with IL-6 for 24 hours were stimulated with 2 ng/mL of IL-6 for 0, 10, 30, or 90 minutes. Cells after washing with phosphate-buffered saline were lysed in ice-cold cell lysis buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1 mM ethyleneglycotetraacetic acid, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM sodium orthovanadate, and 1 μg/mL leupeptin) containing 1 mM phenylmethylsulfonyl fluoride with sonication. Equivalent amounts of proteins (10 μg) were boiled in Laemmli sample buffers and fractionated on 8% or 10% polyacrylamide gel electrophoresis (PAGE) with sodium dodecyl sulfate (SDS). Separated proteins in gels were transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA) by an electroblot apparatus (Bio-Rad). Immunostaining of the membranes using specific antibodies, horseradish peroxidase–labeled antibodies, and a chemiluminescent substrate (KPL, Gaithersburg, MD) was performed according to manufacturers' protocols. Specific antibodies for STAT3, MEK1/2, stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK), p38 MAPK, phosphorylated STAT3 (Tyr705), phosphorylated MEK1/2 (Ser217/221), phosphorylated SAPK/JNK (Thr183/Tyr185), and phosphorylated p38 MAPK (Thr180/Tyr182) were purchased from New England Biolabs (Beverly, MA). Antibodies for Lyn, Fyn, Blk, SHP-1, SHP-2, CD45, and gp130 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Immunoprecipitation and Western blot analysis
Cell lysates cleared with either protein A–agarose or protein G–agarose (Santa Cruz Biotechnology) were incubated with specific antibodies, and immune complexes were then precipitated with either protein A– or G-agarose. After washing the immune complexes with the cell lysis buffer, bound proteins were disrupted by heating in Laemmli sample buffers, and supernatants were subjected to 8% or 10% PAGE with SDS followed by Western blot analysis.
Kinase assay
Cell lysates were immunoprecipitated with either anti–phosphorylated ERK1/2 (Thr202/Tyr204, New England Biolabs), anti-Lyn, or anti-Fyn antibodies, as described above. After washing the immunoprecipitates with the cell lysis buffer, the immune complexes were washed with kinase buffers composed of 25 mM Tris (pH 7.5), 5 mM β-glycerolphosphate, 2 mM dithiothreitol, 0.1 mM sodium orthovanadate, and 10 mM MgCl2. Then they were incubated at 30°C for 30 minutes in the kinase buffer supplemented with 200 μM adenosine triphosphate and a substrate followed by 8% or 10% PAGE with SDS and Western blot analysis. Either 2 μg of the recombinant Elk1 fusion protein (New England Biolabs) for ERK1/2 or 10 μg of enolase (Boehringer Mannheim, Mannheim, Germany) inactivated by acetic acid for Lyn or Fyn kinases were used as substrates for kinase assays. Either phosphorylated Elk1 antibodies (Ser383, New England Biolabs) for Elk1 fusion proteins, or phosphorylated tyrosine antibody 4G10 (Upstate Biotechnology, Lake Placid, NY) for enolase and src family PTKs, were used to detect phosphorylation of the substrates by Western blot analysis.
Antisense oligodeoxynucleotide treatment
CD45+ U266 cells were cultured in the presence of phosphorothioate oligodeoxynucleotides at a concentration of 10 μM. For proliferation assay, the oligodeoxynucleotides were added 24 hours prior to IL-6 stimulation and incubated for 72 hours. For Western blot analysis, cells were cultured with the oligodeoxynucleotides and IL-6 for 48 hours and then harvested. Nucleotide sequences of the oligodeoxynucleotides used have been described elsewhere.25 26
Pharmacologic experiments
CD45− or CD45+ U266 cells were cultured with either 300 ng/mL PP2 (Calbiochem-Novabiochem, La Jolla, CA) or 1 μg/mL herbimycin A (Sigma, St Louis, MO). For proliferation assay, these inhibitors were added 1 hour prior to IL-6 stimulation and then incubated for 72 hours. For Western blot analysis, these reagents were preincubated for 30 minutes followed by IL-6 stimulation for 10 minutes.
Results
Human myeloma cell lines expressing CD45 antigens proliferated in response to IL-6
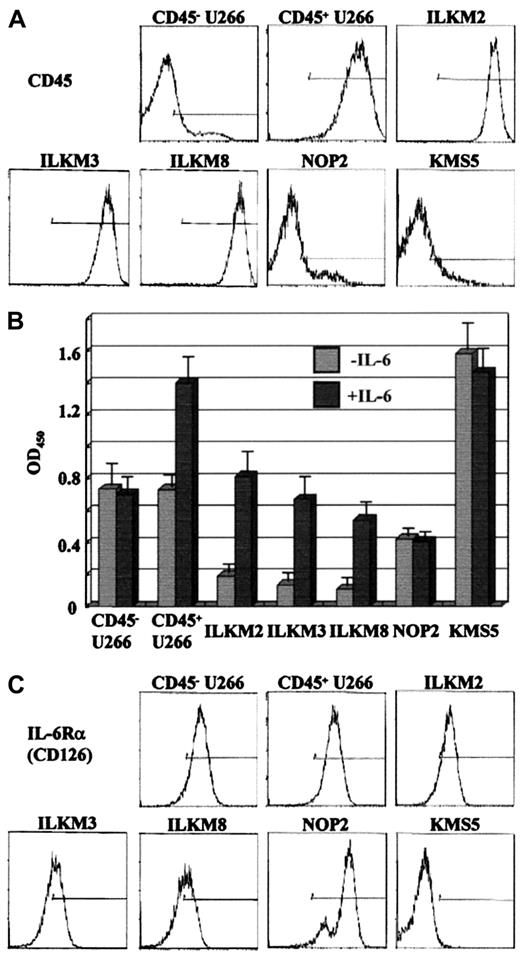
Although CD45 molecules are expressed on most hematopoietic cells including plasma cells, their expression on primary myeloma cells and cell lines is quite variable.19,24 The expression patterns of the CD45 antigens showed remarkable heterogeneity on a panel of myeloma cell lines. For example, strong CD45 expression was found on ILKM2, ILKM3, and ILKM8, but no CD45 expression was found on NOP2 and KMS5 (Figure 1A). In U266, there are only a few CD45+ cells among the majority of CD45− cells.24 Figure 1A also shows CD45 expression on each of the subpopulations of the U266 cell line isolated by cell sorting. IL-6 failed to enhance cell proliferation of NOP2, KMS5, and sorted CD45− U266, whereas ILKM2, ILKM3, ILKM8, and sorted CD45+ U266 expressing CD45 proliferated in response to IL-6 (Figure 1B). The same results were obtained using CD45+ U266 cells derived from CD45− U266 cells by IL-6 stimulation for 3 weeks,24 or CD45− U266 cells derived from CD45+ U266 cells by IL-6 withdrawal for 2 weeks,24 instead of direct sorting of CD45−or CD45+ subpopulations of the parental U266 cell line without any modification (data not shown). CD45− U266 and NOP2 showed no proliferative response to IL-6 (Figure 1B), whereas much more IL-6Rα was expressed on them than on ILKM2, ILKM3, and ILKM8 (Figure 1C). These results led us to speculate that CD45 molecules may play an indispensable role in myeloma cell proliferation enhanced by IL-6.
CD45+ myeloma cell lines proliferate in response to IL-6.
Flow cytometry of the indicated human myeloma cell lines stained for CD45 (A) or CD126 (IL-6Rα) (C) is shown. (B) Proliferation of human myeloma cell lines treated with (right bars) or without (left bars) IL-6 (2 ng/mL) was analyzed by BrdU incorporation, shown by mean values and SDs obtained from 3 independent experiments using triplicate samples. The CD45− or CD45+ U266 cells were isolated by a cell sorter from parental U266 cell line.
CD45+ myeloma cell lines proliferate in response to IL-6.
Flow cytometry of the indicated human myeloma cell lines stained for CD45 (A) or CD126 (IL-6Rα) (C) is shown. (B) Proliferation of human myeloma cell lines treated with (right bars) or without (left bars) IL-6 (2 ng/mL) was analyzed by BrdU incorporation, shown by mean values and SDs obtained from 3 independent experiments using triplicate samples. The CD45− or CD45+ U266 cells were isolated by a cell sorter from parental U266 cell line.
Activation of both STAT3 and MEK1/2-ERK1/2 was not sufficient for IL-6–responsive proliferation of myeloma cell lines
Because IL-6 is well known to activate STAT3 and ERK1/2 following activation of JAKs via gp130,4,5 we examined whether the 2 pathways were differentially activated in CD45− and CD45+ myeloma cell lines upon IL-6 stimulation. Surprisingly, IL-6 rapidly induced the activation of both STAT3 and ERK1/2 in CD45− U266 cells as well as in CD45+U266 cells (Figure 2A). We found in an IL-6–independent cell line, NOP2, the phosphorylation of both STAT3 and MEK1/2, which is the MAPK kinase, just upstream of ERK1/2 in response to IL-6 (Figure 2B), consistent with the results obtained from CD45− U266 cells. Similarly to there being almost no expression of IL-6Rα on KMS5 (Figure 1C), no phosphorylation of STAT3 and MEK1/2 in response to IL-6 was induced in this cell line (Figure2B). Other IL-6–dependent cell lines, such as ILKM2 and ILKM3, had activation of just STAT3 but not MEK1/2 by IL-6 stimulation of up to 90 minutes (Figure 2B), suggesting that the STAT3 pathway is likely to be primarily responsible for the IL-6–induced proliferation of these cell lines. In U266 cells IL-6 did not activate other members of the MAPK family, including SAPK/JNK and p38 MAPK (Figure 2C). As previously reported—that the Ras-ERK1/2 pathway is required for the gp130-mediated proliferation of a B-cell line5—the IL-6–mediated proliferation of CD45+ U266 cells was suppressed by a MEK1 inhibitor, PD98059 (Figure 2D), or by ERK1/2-specific antisense oligodeoxynucleotides (data not shown). Importantly, both STAT3 and MEK1/2-ERK1/2 were activated by IL-6 in CD45− U266 and NOP2, although these cells failed to proliferate in response to IL-6. Thus, the results suggest that these 2 pathways were not sufficient to induce the proliferation of myeloma cell lines by IL-6.
STAT3 and MEK1/2-ERK1/2 are activated in response to IL-6 in CD45− myeloma cell lines.
(A,B) Activation of STAT3 in myeloma cell lines stimulated with IL-6 for 0, 10, 30, or 90 minutes was determined by Western blot analysis (WB) using antibodies specific for phosphorylated STAT3 at the tyrosine residue 705 (STAT3-P). The activation of the Ras-ERK pathway was assessed by ERK1/2 kinase assay (KA) using Elk-1 as a substrate (A) and by Western blot analysis using the specific antibodies for the phosphorylated MEK1/2 at the serine residues 217 and 221 (MEK1/2-P) (B) for U266 cells (A) and for other myeloma cell lines (B), respectively. (C) Activation of SAPK/JNK and p38 MAPK was analyzed using phosphorylated SAPK/JNK at threonine 183 and tyrosine 185 (SAPK/JNK-P) and phosphorylated p38 MAPK at threonine 180 and tyrosine 182 (p38 MAPK-P) antibodies, respectively. (D) Proliferation of CD45+ U266 cells assessed by BrdU incorporation was suppressed by a MEK1 inhibitor, PD98059 (20 μM). Values of BrdU incorporation are indicated by means and SDs that were obtained from 3 independent experiments using triplicate samples.
STAT3 and MEK1/2-ERK1/2 are activated in response to IL-6 in CD45− myeloma cell lines.
(A,B) Activation of STAT3 in myeloma cell lines stimulated with IL-6 for 0, 10, 30, or 90 minutes was determined by Western blot analysis (WB) using antibodies specific for phosphorylated STAT3 at the tyrosine residue 705 (STAT3-P). The activation of the Ras-ERK pathway was assessed by ERK1/2 kinase assay (KA) using Elk-1 as a substrate (A) and by Western blot analysis using the specific antibodies for the phosphorylated MEK1/2 at the serine residues 217 and 221 (MEK1/2-P) (B) for U266 cells (A) and for other myeloma cell lines (B), respectively. (C) Activation of SAPK/JNK and p38 MAPK was analyzed using phosphorylated SAPK/JNK at threonine 183 and tyrosine 185 (SAPK/JNK-P) and phosphorylated p38 MAPK at threonine 180 and tyrosine 182 (p38 MAPK-P) antibodies, respectively. (D) Proliferation of CD45+ U266 cells assessed by BrdU incorporation was suppressed by a MEK1 inhibitor, PD98059 (20 μM). Values of BrdU incorporation are indicated by means and SDs that were obtained from 3 independent experiments using triplicate samples.
Activation of src family kinases in CD45+ but not CD45− myeloma cell lines
As shown in Figure 1, the proliferation of myeloma cell lines in response to IL-6 seems to be related to the expression of CD45 PTP-regulating activities of the src family PTKs.11-14Among the src family members, Lyn, Fyn, and Blk are shown to be expressed in a B-cell lineage.27-29 Lyn (p53 and p56) was similarly strongly expressed in all myeloma cell lines we examined (Figure 3A). In contrast, the expression of Fyn was quite variable and that of Blk barely detectable among these myeloma cell lines (Figure 3A). Elevated activities of Lyn or Fyn kinases were found in CD45+ U266, ILKM2, ILKM3, and ILKM8 but not in CD45− U266 and NOP2 (Figure 3B). The kinase activities of Lyn or Fyn, however, seem to be independent of IL-6 stimulation (Figure 3B). Because Fyn was absent in U266, only Lyn appeared to be constitutively associated with gp130 in both CD45− and CD45+ U266 cells and with CD45 molecules in CD45+ U266 cells (Figure 3C). In ILKM2, we also detected the association of both Lyn and Fyn with the gp130 or CD45 molecules independent of IL-6 stimulation (Figure 3C). Nevertheless, it remains unclear whether complexes consisting of gp130, Lyn, and CD45 molecules are present in these cells, because we failed to detect gp130 coprecipitated with CD45 in CD45+ U266 cells (data not shown). The SH2 domain–containing cytosolic PTPs, such as SHP-1 and SHP-2, have also been shown to play pivotal roles in signal transduction in lymphocytes.30 However, their expression was similar among these myeloma cell lines, except for ILKM8 (Figure 3A). Tyrosine phosphorylation of SHP-2 by IL-6 was similarly detected in these cell lines (data not shown). These results imply that the SH2 domain–containing cytosolic PTPs are unlikely to contribute to the differences between CD45− and CD45+myeloma cell lines, of src family PTK activity, and the proliferative responses to IL-6.
src family PTKs are activated in CD45+ but not CD45− myeloma cell lines.
(A) Western blot analysis shows the expression of Lyn (p53 and p56), Fyn, Blk, SHP-1, and SHP-2 in the myeloma cell lines indicated. (B) The kinase activity of Lyn or Fyn in myeloma cell lines stimulated with (+) or without (−) IL-6 was investigated by kinase assay using enolase as an exogenous substrate. (C) The immunoprecipitation (IP) and Western blot analysis with the indicated antibodies were used to examine the interaction of Lyn or Fyn kinases with CD45 or gp130 molecules in myeloma cell lines treated with (+) or without (−) IL-6.
src family PTKs are activated in CD45+ but not CD45− myeloma cell lines.
(A) Western blot analysis shows the expression of Lyn (p53 and p56), Fyn, Blk, SHP-1, and SHP-2 in the myeloma cell lines indicated. (B) The kinase activity of Lyn or Fyn in myeloma cell lines stimulated with (+) or without (−) IL-6 was investigated by kinase assay using enolase as an exogenous substrate. (C) The immunoprecipitation (IP) and Western blot analysis with the indicated antibodies were used to examine the interaction of Lyn or Fyn kinases with CD45 or gp130 molecules in myeloma cell lines treated with (+) or without (−) IL-6.
Activation of Lyn kinase is necessary for proliferation of CD45+ U266 cells by IL-6
To determine the physiologic roles of src family PTK activation in CD45+ myeloma cell lines, we used antisense oligodeoxynucleotides specific for Lyn in CD45+ U266 cells. Two different Lyn-specific antisense but not sense oligodeoxynucleotides inhibited the DNA synthesis of CD45+U266 cells promoted by IL-6 (Figure 4A). This result indicates that Lyn kinase is necessary for IL-6–induced proliferation of CD45+ U266 cells. The specificity of these antisense oligodeoxynucleotides was confirmed by the findings of the significantly reduced expression of Lyn but unaltered expression of STAT3, MEK1/2 (Figure 4C), and ERK1/2 proteins (data not shown). Furthermore, the PTK inhibitors, PP2 and herbimycin A, the former selective to the src family PTKs, also blocked enhancement of IL-6–induced proliferation of CD45+ U266 (Figure 4B) and ILKM2 (data not shown). Neither STAT3 nor MEK1/2 activation was affected by these antisense oligodeoxynucleotides (Figure 4C) or by src kinase inhibitor PP2 (Figure 4D). These data suggest that the proliferative defects of the blockage of Lyn expression or activation are independent of either STAT3 or ERK1/2 activation induced by IL-6.
Lyn PTK is required for proliferation of CD45+ U266 cells enhanced by IL-6.
(A) BrdU incorporation was used to determine the DNA synthesis of CD45+ U266 cells treated with Lyn-specific sense (S, left 2 bars) or antisense (AS, right 2 bars) oligodeoxynucleotides (10 μM) in the presence (the second and fourth bars) or absence (the first and third bars) of IL-6. The results obtained from the experiments using 2 different oligodeoxynucleotides 1 and 2 are indicated on the left and right, respectively. (B) The PTK inhibitors PP2 (300 ng/mL, middle) and herbimycin A (1 μg/mL, right) reduce the BrdU incorporation of CD45+ U266 cells by IL-6 (right bars; left bars are without IL-6). Control DMSO is shown on the left. (C) Western blot analysis shows the effect of antisense (AS) or sense (S) oligodeoxynucleotides specific for Lyn on the expression of Lyn, STAT3, and MEK1/2 in CD45+ U266 cells. Western blot analysis is also used to examine the phosphorylation of STAT3 (Tyr705) and MEK1/2 (Ser217/221) in CD45+ U266 cells treated with either Lyn-specific antisense or sense oligodeoxynucleotides in the presence of IL-6. (D) Western blot analysis shows the phosphorylation and expression of STAT3 and MEK1/2 in CD45+ U266 cells treated with PP2 or herbimycin A prior to IL-6 stimulation. Values of BrdU incorporation are indicated by the means and SDs resulting from 3 independent experiments using triplicate samples (A,B).
Lyn PTK is required for proliferation of CD45+ U266 cells enhanced by IL-6.
(A) BrdU incorporation was used to determine the DNA synthesis of CD45+ U266 cells treated with Lyn-specific sense (S, left 2 bars) or antisense (AS, right 2 bars) oligodeoxynucleotides (10 μM) in the presence (the second and fourth bars) or absence (the first and third bars) of IL-6. The results obtained from the experiments using 2 different oligodeoxynucleotides 1 and 2 are indicated on the left and right, respectively. (B) The PTK inhibitors PP2 (300 ng/mL, middle) and herbimycin A (1 μg/mL, right) reduce the BrdU incorporation of CD45+ U266 cells by IL-6 (right bars; left bars are without IL-6). Control DMSO is shown on the left. (C) Western blot analysis shows the effect of antisense (AS) or sense (S) oligodeoxynucleotides specific for Lyn on the expression of Lyn, STAT3, and MEK1/2 in CD45+ U266 cells. Western blot analysis is also used to examine the phosphorylation of STAT3 (Tyr705) and MEK1/2 (Ser217/221) in CD45+ U266 cells treated with either Lyn-specific antisense or sense oligodeoxynucleotides in the presence of IL-6. (D) Western blot analysis shows the phosphorylation and expression of STAT3 and MEK1/2 in CD45+ U266 cells treated with PP2 or herbimycin A prior to IL-6 stimulation. Values of BrdU incorporation are indicated by the means and SDs resulting from 3 independent experiments using triplicate samples (A,B).
Discussion
In this study, we found that IL-6 rapidly activated both STAT3 and ERK1/2 in CD45− U266 and NOP2 myeloma cell lines, although they failed to proliferate in response (Figures 1B and 2A-B). These myeloma cell lines lacked CD45 expression and src family PTK activation (Figures 1A and 3B). We also showed the relevance of the src family PTK, Lyn, to IL-6–enhanced proliferation of CD45+ U266 cells by using selective inhibitors (Figure 4). Therefore, we postulate that in myeloma cell lines the activation of STAT3 and ERK1/2 is not sufficient for cell proliferation enhanced by IL-6 and that myeloma cells require the cellular state of IL-6–independent activation of the src family PTKs, such as Lyn or Fyn, to proliferate in response to IL-6, which then further activates STAT3 and ERK1/2. Such a cellular context in myeloma cells would be contributed to by CD45 expression. Although the U266 cell line mainly used in the present study is genetically monoclonal, it contains both CD45− and CD45+ populations, which are reversible depending upon the availability of exogenous IL-6, and CD45 expression seems to correlate with the IL-6–responsive proliferation of the U266 cell line.24 It has been demonstrated by single-cell assays that a CD45+ population eventually accumulates during a 4-week culture of an initially CD45− U266 cell with IL-6.24 Conversely, CD45+ U266 cells lose CD45 expression after the removal of IL-6 for 2 weeks and subsequently act as CD45− U266 cells.24 In comparing the IL-6 signaling pathways, the use of the U266 cell line has a great advantage over different cell lines, which may differ in several aspects in addition to CD45 expression. Moreover, sorted CD45+ NOP2 cells that were found as a minor population in the NOP2 cell line (< 5%, Figure 1A) also proliferated in response to IL-6 (data not shown), suggesting that CD45 expression is related to IL-6–responsive cell proliferation of NOP2 as well as U266.
B cells lacking CD45 are incapable of proliferating in response to anti–immunoglobulin M stimulation, and T-cell maturation in thymus is impaired in CD45-deficient mice.31 These defects in lymphocytes are considered to be due to their inability to activate the src family PTKs.11-14 So, too, thymocyte development of CD45-deficient mice is rescued by expression of mutated Lck, which harbors the replacement of the COOH-terminal inhibitory phosphorylation site of a tyrosine residue with a phenylalanine.32Although it has been demonstrated that CD45 negatively regulated cytokine receptor signaling as a JAK phosphatase,33 we could not see down-regulation of tyrosine phosphorylation levels of Jak1, Tyk2, and gp130 in CD45+ U266 cells after IL-6 stimulation as compared with CD45− U266 cells (data not shown). In addition, CD45 molecules failed to coprecipitate with Hck, Jak1, Tyk2, the p85 subunit of phosphatidylinositol-3 kinase, STAT3, ERK1/2, MEK1/2, SAPK/JNK, or p38 MAPK (data not shown). Among the molecules we tested, only Lyn appeared to interact with CD45 in CD45+ U266 cells, as shown in Figure 3C—results suggesting that Lyn could be a major molecule regulated by the CD45 PTP in CD45+ U266 cells. Indeed, a PTP inhibitor, vanadate, suppressed IL-6–enhanced DNA synthesis of CD45+ U266 cells rather than CD45− U266 cells without interfering with STAT3 and MEK1/2 activation (data not shown), whereas the other PTPs were expressed similarly in CD45− and CD45+U266 cells (Figure 2A). Thus, with respect to the IL-6–induced proliferation of myeloma cells, CD45 could act as a positive regulator by controlling src family PTK activity, similarly to mitogenic signals via B-cell antigen receptor on B cells.
Several src family PTKs are reported to interact with cytokine receptors in either ligand-dependent or -independent manners. These include the association of Lck with IL-2Rβ34; Hck with gp130 35; Fyn, Lyn, or Hck with the common β chain of the IL-3R/IL-5R/granulocyte-macrophage colony-stimulating factor receptor (GM-CSFR)36-39; and Lyn with G-CSFR40or with the erythropoietin receptor.41 In myelomas, at least one report has shown that activation of src family PTKs was associated with IL-6 stimulation.42 In our study, Lyn PTK seemed to be activated independent of IL-6 stimulation (Figure 3B), whereas the interaction of Lyn with gp130 (Figure 3C) may have raised the possibility that Lyn activities are closely linked to IL-6 signaling. We found that the activities of the src family PTKs coprecipitated with gp130 in CD45− and CD45+U266 cells were barely detectable and were not altered by IL-6 stimulation (data not shown). These results imply that src family PTKs are unlikely to participate in the signaling pathway via gp130 in the U266 cell line. Antigen receptors on lymphocytes are considered to require src family PTKs as primary kinases responsible for initiating signals, whereas mitogenic signals via gp130 in myeloma cell lines required activation of src family PTKs that was independent of ligand stimulation. Accordingly, further studies will be necessary to determine the precise mechanisms of src family PTK activation in CD45+ myeloma cells.
STAT3 and ERK1/2 activated by IL-6 may be influenced by other elements. For example, the PC12 cell line can differentiate into neuronal cells in the presence of IL-6 together with or pretreated with nerve growth factor, which represses the STAT3 activation,43 and the IL-6–induced proliferation of prostate carcinoma cells requires ErbB2 that enhances the ERK1/2 activities.44 Although the src family PTKs have recently been shown to activate STATs,41,45 the inhibition of Lyn expression or kinase activity did not influence STAT3 activation in CD45+ U266 cells in our study (Figure 4C-D). In addition, the IL-6–induced activation of STAT3 was similar between CD45− and CD45+ cell lines, whereas there was a clear difference between them in the Lyn or Fyn activities (Figures 2A-B and 3B). Thus, our results suggest that in myeloma cells, src family PTKs are not directly involved in STAT3 and Ras-ERK1/2 activation by IL-6. Recent reports have supported our suggestion, showing that Lyn was required for G-CSF–induced cell proliferation46 and that the G-CSF–stimulated signals required src family PTK activities dissociated from the JAK-STAT pathway.47 The src family PTKs may phosphorylate several molecules, including adaptor molecules, enzymes, structural proteins, and transcription factors, resulting in a diversification of the initial signals.9 We also found that in CD45+ U266 cells Lyn kinase was likely to activate phospholipase Cγ–mediated signals, such as cellular calcium influxes and protein kinase C activation, independent of STAT3 and ERK1/2 pathways (H.I. and M.M.K., unpublished observations, May 2001).
A previous study48 using extremely high amounts of IL-6 (100 ng/mL) showed that ERK1/2 was activated by IL-6 in an IL-6–dependent myeloma cell line, B9, and that ERK1/2 activation was required for IL-6–induced proliferation of B9. In contrast, our study using a physiologic level of IL-6 (2 ng/mL) showed that MEK1/2-ERK1/2 activation was observed only in myeloma cell lines expressing high levels of IL-6Rα, such as U266 and NOP2, but was not related to IL-6 dependency (Figures 1 and 2A-B). Indeed, decreasing amounts of IL-6 (200 or 20 pg/mL) failed to induce MEK1/2 but did induce STAT3 activation in U266 (data not shown). Although PD98059 markedly suppressed cell proliferation of CD45+ U266 (Figure 2D), ILKM2, ILKM3, and ILKM8 in the presence of IL-6 (data not shown), their proliferation without IL-6 was also significantly impaired by PD98059 (Figure 2D and data not shown). ERK1/2 activities in cells may be influenced by serum components and, indeed, we found that under serum-free conditions the inhibitory effect of PD98059 on DNA synthesis of CD45+ U266 cells was IL-6 dependent (data not shown). Thus, ERK1/2 activities may partly result from serum components, whereas the further activation of ERK1/2 by IL-6 could contribute to enhancing the proliferation of CD45+ U266 cells. In contrast, both ILKM2 and ILKM3 underwent apoptosis without serum even in the presence of IL-6 (H.I. and M.M.K., unpublished observations, September 2001). Therefore, both the ERK1/2 pathway presumably activated by serum components and STAT3 activation by IL-6 may be essential to support the survival and proliferation of these IL-6–dependent cell lines.
Although IL-6 is a potent growth factor for myeloma cells both in vitro2 and in vivo,15 only a few subpopulations of tumor cells, such as CD45+MPC-1−CD49e−cells,19 proliferate in response to IL-6. Nevertheless, the molecular mechanisms underlying how IL-6 could induce the proliferation of CD45+ but not that of CD45−myeloma cells have not been elucidated. In this respect, our findings indicate that the activities of src family PTKs associated with the CD45 PTP seem to be a prerequisite for the myeloma cell proliferation that further requires the activation of STAT3 and ERK1/2 by IL-6. Finally, we also speculate that in myeloma cell lines, the proliferative responses induced by IL-6 are influenced by different cellular contexts, such as the presence or absence of the CD45 molecules and src family PTK activation, unrelated to IL-6–mediated signaling events.
We thank Dr S. Shimizu for the generous gift of ILKM2, ILKM3, and ILKM8 and Chugai Pharmaceutical Company for human recombinant IL-6. We also thank Ms R. Maemoto for her excellent secretarial assistance.
Supported in part by grants from the Ministry of Education, Science, Sports and Culture of Japan, the Ministry of Health and Welfare of Japan, the Japan Society for the Promotion of Science, and also by the Public Trust Haraguchi Memorial Cancer Research Fund, Sagawa Gann Research Foundation, the Yamanouchi Foundation for Research on Metabolic Disorders, Uehara Memorial Foundation, and the Osaka Cancer Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michio M. Kawano, Dept of Bio-Signal Analysis, Applied Medical Engineering Science, Graduate School of Medicine, Yamaguchi University, 1-1-1 Minami-kogushi, Ube, Yamaguchi 755-8505, Japan; e-mail: mkawano@po.cc.yamaguchi-u.ac.jp.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal