Abstract
Standard myeloablative conditioning prior to allogeneic hematopoietic stem cell (HSC) transplantation has been associated with significant toxicity in patients older than 45 years of age with myelofibrosis with myeloid metaplasia (MMM). We sought to evaluate the efficacy of a reduced-intensity conditioning regimen for allogeneic HSC transplantation in this setting. A regimen consisting of fludarabine (30 mg/m2 intravenously daily for 5 days) and melphalan (70 mg/m2 intravenously daily for 2 days) followed by transplantation of filgrastim-mobilized peripheral blood cells from HLA-identical siblings was administered to 4 older patients (median age, 56 years; range, 48-58 years) with advanced MMM. All patients achieved prompt neutrophil and platelet engraftment and have experienced a significant regression of splenomegaly and bone marrow fibrosis. All now have normal bone marrow cellularity. With a median follow-up of 13 months (range, 11-19 months), all 4 patients are alive with stable full-donor hematopoietic chimerism. These results support the feasibility and effectiveness of reduced-intensity conditioning prior to allogeneic HSC transplantation for older patients with advanced MMM.
Introduction
Myelofibrosis with myeloid metaplasia (MMM) is a clonal hematopoietic disorder characterized by bone marrow fibrosis, a leukoerythroblastic blood picture, splenomegaly, and extramedullary hematopoiesis.1,2 The average age at diagnosis of MMM is approximately 60 years, most patients being diagnosed at 50 to 69 years of age.3 With a median survival of between 3 to 5 years, it has the worst prognosis of all the chronic myeloproliferative diseases.3-5 Conventional therapies are often ineffective and only palliative.6-8 Recently, a report on 55 patients younger than age 55 years with MMM who underwent allogeneic hematopoietic stem cell (HSC) transplantation indicated that 48% of the recipients of an HLA-identical transplant survived event-free at 5 years.9 Nevertheless, the 1-year treatment-related mortality in this study was 27% in spite of the relatively young age (median, 42 years) of the patients receiving transplants. Further, a recent follow-up from this same group of investigators noted only a 14% 5-year overall survival in a subgroup of transplant recipients older than 45 years of age.10 Although selected older patients may fare well with standard myeloablative conditioning,11 such data raise concern that this conventional approach may be too toxic for the majority of patients with MMM owing to their advanced age. Recently, older recipients of allogeneic HSC transplantation have been treated successfully following a variety of less intensive nonmyeloablative conditioning regimens.12-14 On the basis of previous observations, we hypothesized that a reduced-intensity fludarabine-and-melphalan–containing conditioning regimen would promote initial engraftment and establish full-donor chimerism yet result in a lower risk of transplantation-related morbidity in older patients with MMM receiving an HSC transplant from an HLA-identical sibling.15 This approach could be questioned because of concerns that severe marrow fibrosis might adversely affect hematopoietic reconstitution.16 The preliminary results in 4 patients with MMM described in this report suggest both the feasibility and the effectiveness of this approach.
Study design
Patients were considered eligible for HSC transplantation with a nonmyeloablative conditioning regimen if they were older than age 45 years, had an HLA-identical sibling donor, and had a diagnosis of MMM based on the criteria proposed by Laszlo.17Furthermore, at the time of diagnosis, patients had to be categorized as having an intermediate or high risk for early disease-related mortality on the basis of the criteria of Dupriez et al.4All 4 patients were enrolled in a treatment protocol that was approved by the Institutional Review Board of the University of Illinois at Chicago. Written informed consent was obtained in all cases. HLA typing was based on serologic methods for HLA-A and HLA-B and low-resolution molecular typing at HLA-DRB1. Each donor received filgrastim (Neupogen) (Amgen, Thousand Oaks, CA) by subcutaneous injection at a dose of 5 μg/kg every 12 hours. Donor blood cells were collected via leukapheresis by means of a Cobe Spectra (version 5.0) (Cobe BCT, Lakewood, CO) with a target volume of 15 L commencing on day 5 of filgrastim administration. The CD34+ cell content of the leukapheresis product was enumerated by means of the ProCount method (Becton Dickinson, Mountain View, CA). The collection target was 4.0 × 106 CD34+ cells per kilogram of recipient weight. All leukapheresis products were collected prior to recipient conditioning and cryopreserved in 10% dimethyl sulfoxide. Recipient conditioning consisted of fludarabine at 30 mg/m2intravenously over 30 minutes daily for 5 days (days −6 to −2) and melphalan at 70 mg/m2 intravenously over 20 minutes daily for 2 days (days −3 and −2). The donor grafts were then thawed and infused on day 0. Graft-versus-host disease (GVHD) prophylaxis consisted of tacrolimus at 0.03 mg/kg/d by continuous intravenous infusion beginning on day −2 and methotrexate at 5 mg/m2 intravenously on days +1, +3, and +6. Filgrastim was given subcutaneously at a dose of 5 μg/kg/d beginning on day +7 and continuing until neutrophil engraftment was achieved.
Regimen-related toxicity was graded according to the criteria of Bearman et al.18 Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count (ANC) of at least 0.5 ×109/L (500/μL). Platelet engraftment was defined as the first day with a platelet count of at least 20 ×109/L (20 000/μL) without transfusion for a week. Acute and chronic GVHD were graded according to standard criteria.19,20 Posttransplantation donor-recipient chimerism was assessed by means of DNA microsatellite analysis as previously described.21 Hematologic response was defined as complete if all peripheral blood counts had become normal with or without the persistence of splenomegaly as long as 100% donor bone marrow chimerism was achieved. Bone marrow biopsies were performed at frequent intervals after transplantation to assess donor/host chimerism, the degree of bone marrow fibrosis, and overall bone marrow morphology. The degree of bone marrow fibrosis was graded according to the criteria of Bauermeister.22
Results and discussion
The clinical characteristics of the 4 patients included in this report are summarized in Table 1. All 4 were male with a median age at transplantation of 55.5 years (range, 48-58 years). The median time from diagnosis to transplantation was 9.5 months (range, 5-11 months). At diagnosis, all patients were considered to have intermediate-risk (n = 3) or high-risk (n = 1) disease. None had any history of antecedent myeloproliferative disorder. Three patients were moderately anemic (Hb lower than 10 g/dL), and 2 had required red blood cell transfusions. All 4 had between 1% and 9% circulating blasts prior to transplantation. All had experienced constitutional symptoms related to MMM. One patient had massive splenomegaly (spleen tip palpable in the right lower quadrant) at the time of transplantation. Morphologically, each patient had grade 4 bone marrow fibrosis on the basis of the reticulin and Masson trichrome stains. None of the patients had evidence of the BCR/ABLgene rearrangement by reverse-transcriptase–polymerase chain reaction performed on peripheral blood samples. Chromosomal analysis of bone marrow revealed normal metaphases (n = 2) or no growth (n = 2) prior to transplantation.
Patient and donor characteristics
| Clinical parameter . | Patient . | |||
|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | |
| Age/sex | 58/M | 54/M | 57/M | 48/M |
| Constitutional symptoms | Fatigue, night sweats | Weight loss, fever | Fatigue, night sweats | Fatigue, early satiety |
| WBC count, × 109/L | ||||
| At diagnosis | 1.7 | 3.8 | 3.3 | 5.3 |
| Pretransplant | 1.0 | 5.2 | 3.2 | 5.0 |
| ANC, × 109/L | ||||
| At diagnosis | 0.7 | 2.2 | 1.9 | 3.6 |
| Pretransplant | 0.4 | 3.5 | 1.8 | 3.7 |
| Myelocytes and metamyelocytes in peripheral blood, % | ||||
| At diagnosis | 3 | 14 | 13 | 7 |
| Pretransplant | 4 | 10 | 10 | 3 |
| Hb level, g/dL | ||||
| At diagnosis | 5.9 | 8.4 | 11.0 | 9.0 |
| Pretransplant | 7.2‡ | 7.8‡ | 10.2 | 8.9 |
| Nucleated red blood cells in peripheral blood, % | ||||
| At diagnosis | 1 | 2 | 5 | 2 |
| Pretransplant | 1 | 1 | 1 | 1 |
| Platelet count, × 109/L | ||||
| At diagnosis | 47 | 80 | 276 | 57 |
| Pretransplant | 23 | 34 | 209 | 63 |
| Circulating blasts, % | ||||
| At diagnosis | 1 | 3 | 6 | 1 |
| Pretransplant | 1 | 9 | 6 | 1 |
| Spleen size, cm below left costal margin | ||||
| At diagnosis | NP1-153 | 4 | 14 | 4 |
| Pretransplant | NP1-153 | 6 | 18 | 5 |
| Grade of BM fibrosis* | 4 | 4 | 4 | 4 |
| Prior RBC transfusions | Yes | Yes | No | No |
| Prior RBC transfusion units | 4 | 14 | 0 | 0 |
| Risk category† | High | Int | Int | Int |
| Donor age/sex | 54/F | 46/F | 55/M | 44/F |
| CD34+ cell dose transplanted, × 106/kg | 5.0 | 7.8 | 6.8 | 3.8 |
| Clinical parameter . | Patient . | |||
|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | |
| Age/sex | 58/M | 54/M | 57/M | 48/M |
| Constitutional symptoms | Fatigue, night sweats | Weight loss, fever | Fatigue, night sweats | Fatigue, early satiety |
| WBC count, × 109/L | ||||
| At diagnosis | 1.7 | 3.8 | 3.3 | 5.3 |
| Pretransplant | 1.0 | 5.2 | 3.2 | 5.0 |
| ANC, × 109/L | ||||
| At diagnosis | 0.7 | 2.2 | 1.9 | 3.6 |
| Pretransplant | 0.4 | 3.5 | 1.8 | 3.7 |
| Myelocytes and metamyelocytes in peripheral blood, % | ||||
| At diagnosis | 3 | 14 | 13 | 7 |
| Pretransplant | 4 | 10 | 10 | 3 |
| Hb level, g/dL | ||||
| At diagnosis | 5.9 | 8.4 | 11.0 | 9.0 |
| Pretransplant | 7.2‡ | 7.8‡ | 10.2 | 8.9 |
| Nucleated red blood cells in peripheral blood, % | ||||
| At diagnosis | 1 | 2 | 5 | 2 |
| Pretransplant | 1 | 1 | 1 | 1 |
| Platelet count, × 109/L | ||||
| At diagnosis | 47 | 80 | 276 | 57 |
| Pretransplant | 23 | 34 | 209 | 63 |
| Circulating blasts, % | ||||
| At diagnosis | 1 | 3 | 6 | 1 |
| Pretransplant | 1 | 9 | 6 | 1 |
| Spleen size, cm below left costal margin | ||||
| At diagnosis | NP1-153 | 4 | 14 | 4 |
| Pretransplant | NP1-153 | 6 | 18 | 5 |
| Grade of BM fibrosis* | 4 | 4 | 4 | 4 |
| Prior RBC transfusions | Yes | Yes | No | No |
| Prior RBC transfusion units | 4 | 14 | 0 | 0 |
| Risk category† | High | Int | Int | Int |
| Donor age/sex | 54/F | 46/F | 55/M | 44/F |
| CD34+ cell dose transplanted, × 106/kg | 5.0 | 7.8 | 6.8 | 3.8 |
WBC indicates white blood cell; ANC, absolute neutrophil count; Hb, hemoglobin; BM, bone marrow; RBC, red blood cell; NP, nonpalpable; Int, intermediate.
Marrow fibrosis according to the criteria of Bauermeister.22
Risk for disease progression according to the criteria of Dupriez et al.4
Received previous RBC transfusions.
Imaging studies revealed splenomegaly.
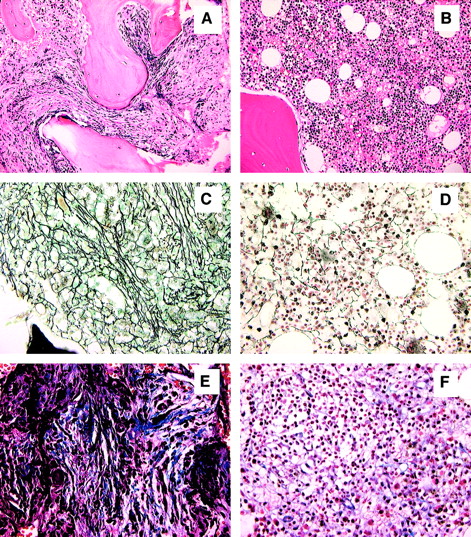
All patients tolerated conditioning well, and there were no grade 3 or 4 regimen-related toxicities. All patients developed transient but profound pancytopenia (ANC below 0.1 ×109/L [100/μL] and platelet count below 20 ×109/L [20 000/μL]) and required both red blood cell and platelet transfusion support. Neutrophil engraftment was achieved at a median of 14 days (range, 12-18 days). Platelet engraftment was achieved at a median of 28 days (range, 16-77 days). Three of 4 patients have achieved a sustained platelet count exceeding 100 ×109/L (100 000/μL). Red blood cell transfusion independence was achieved in all patients at a median of 45 days (range, 19-110 days). Only one patient experienced grade 1 acute GVHD and none grade 2-4 acute GVHD. Three patients have developed limited (n = 2) or extensive (n = 1) chronic GVHD that is completely responsive to prednisone and tacrolimus. One patient is off all immunosuppressive therapy without evidence of chronic GVHD. With a median follow-up of 13 months (range, 11-19 months), all patients have achieved either complete (n = 2) or marked (n = 2) resolution of splenomegaly, and all have achieved a normocellular bone marrow with marked regression of bone marrow fibrosis (from grade 4 to grade 1 in each) (Figure1). Full-donor chimerism was achieved by day +30 in all patients. All 4 patients have maintained full-donor chimerism within the bone marrow and peripheral blood compartments without requiring donor leukocyte infusion or rapid tapering of tacrolimus. Three recipients of sex-mismatched grafts underwent chromosomal analysis of bone marrow at approximately 1 year after transplantation, revealing a normal female complement in each. The 4 patients currently have Karnofsky performance scores of either 90% (n = 2) or 100% (n = 2).
Normalization of bone marrow morphology following reduced-intensity conditioning.
Hematoxylin and eosin stain of bone marrow prior to transplantation (A) and 11 months after transplantation (B). Reticulin stain of bone marrow prior to transplantation (C) and 11 months after transplantation (D). Masson-trichrome stain prior to transplantation (E) and 11 months after transplantation (F). Figures are from patient 4 and are representative of results observed in each patient. All magnifications are × 20.
Normalization of bone marrow morphology following reduced-intensity conditioning.
Hematoxylin and eosin stain of bone marrow prior to transplantation (A) and 11 months after transplantation (B). Reticulin stain of bone marrow prior to transplantation (C) and 11 months after transplantation (D). Masson-trichrome stain prior to transplantation (E) and 11 months after transplantation (F). Figures are from patient 4 and are representative of results observed in each patient. All magnifications are × 20.
Although follow-up is short, the results obtained in this small series of patients suggest that a fludarabine-and-melphalan–based conditioning regimen is well tolerated, is associated with a low risk of acute GVHD, promotes stable full-donor chimerism, and results in resolution of disease stigmata in older patients with advanced MMM receiving an HSC transplant from an HLA-identical sibling. If verified in a larger number of patients, the use of less intensive conditioning may extend the option of a potentially curative procedure to those individual patients who typically develop this disease rather than to a select group of younger individuals with MMM who happen to have an HLA-identical sibling.9 It remains unclear whether our patients have been “cured” given that splenomegaly, although markedly diminished, has persisted in 2 patients and that grade 1 bone marrow fibrosis was still present in each patient at approximately a year following transplantation. Nevertheless, the maintenance of full-donor hematopoietic chimerism in each patient at a median of 1 year is encouraging. The results suggest that reversal of the effects of the malignant clone following this conditioning regimen either occurs over a prolonged period of time or may not be entirely complete. In more practical terms, this approach has resulted in effective palliation of disease in a group of patients who would ordinarily be considered a poor risk for standard myeloablative conditioning. A similar argument has recently been made for the consideration of autologous HSC transplantation in older patients with MMM lacking an HLA-identical sibling.23 The fact that 3 of 4 patients have developed steroid-responsive chronic GVHD is notable given the risk of late complications associated with chronic GVHD. On the other hand, recent data suggest that a low level of GVHD may be beneficial since a graft-versus-myelofibrosis effect has recently been demonstrated in patients with MMM following donor leukocyte infusion.24 25
To our knowledge, this is the first description of the use of reduced-intensity conditioning for patients with MMM. In light of the relatively small number of transplantations that any one center performs each year for such patients, these encouraging preliminary results may form a reasonable basis for the development of a multicenter study designed to determine the ultimate impact of reduced-intensity conditioning prior to HSC transplantation in older patients with advanced MMM.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Steven M. Devine, 840 South Wood St, M/C 787, Chicago, IL 60612; e-mail: sdevine@uic.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal