Similar to endothelial cells (ECs), vascular endothelial growth factor (VEGF) induces Bcl-2 expression on VEGF receptor-positive (VEGFR+) primary leukemias and cell lines, promoting survival. We investigated the molecular pathways activated by VEGF on such leukemias, by performing a gene expression analysis of VEGF-treated and untreated HL-60 leukemic cells. One gene to increase after VEGF stimulation was heat shock protein 90 (Hsp90). This was subsequently confirmed at the protein level, on primary leukemias and leukemic cell lines. VEGF increased the expression of Hsp90 by interacting with KDR and activating the mitogen-activated protein kinase cascade. In turn, Hsp90 modulated Bcl-2 expression, as shown by a complete blockage of VEGF-induced Bcl-2 expression and binding to Hsp90 by the Hsp90-specific inhibitor geldanamycin (GA). GA also blocked the VEGF-induced Hsp90 binding to APAF-1 on leukemic cells, a mechanism shown to inhibit apoptosis. Notably, VEGF blocked the proapoptotic effects of GA, correlating with its effects at the molecular level. Earlier, we showed that in some leukemias, a VEGF/KDR autocrine loop is essential for cell survival, whereas here we identified the molecular correlates for such an effect. We also demonstrate that the generation of a VEGF/VEGFR autocrine loop on VEGFR+ cells such as ECs, also protected them from apoptosis. Infection of ECs with adenovirus-expressing VEGF resulted in elevated Hsp90 levels, increased Bcl-2 expression, and resistance to serum-free or GA-induced apoptosis. In summary, we demonstrate that Hsp90 mediates antiapoptotic and survival-promoting effects of VEGF, which may contribute to the survival advantage of VEGFR+cells such as subsets of leukemias.
Introduction
Vascular endothelial growth factor (VEGF165) regulates endothelial functions such as differentiation, migration, proliferation, and survival.1VEGF165 signals through its receptors VEGFR-1 (Flt-1) and VEGFR-2 (Flk-1/KDR), which are mainly expressed by endothelial cells, but also by subsets of tumor cells such as leukemias.2 3
Recent advances in the field of VEGF signaling identified the mitogen-activated protein (MAP) kinase pathway4 and also phosphatidylinositol 3 (PI3) kinase5,6 as being downstream of VEGF-induced receptor activation. However, the precise molecular mechanisms of VEGF signaling, particularly those involved in cell survival, are poorly understood. In this regard, it was recently shown that VEGF promotes survival of serum-starved endothelial cells (ECs) by increasing the levels of the antiapoptotic protein Bcl-2.7,8 Overexpression of Bcl-2 on human umbilical vein endothelial cells (HUVECs) was sufficient to prevent these cells from apoptotic death in the absence of VEGF.8 However, the molecular pathways involved in VEGF-mediated induction of Bcl-2, contributing to cell survival, are still unknown.
Previous studies have shown that VEGF and its receptors are expressed on a subset of leukemic cells. Earlier, we showed that VEGFR-2 (KDR) is functional on certain VEGF-producing leukemias, resulting in a VEGF/KDR autocrine loop, which mediates their proliferation and survival.3 In the present study, we sought to characterize the molecular mechanisms by which VEGF increases the survival of leukemic cells. First, we demonstrate that VEGF induces Bcl-2 expression on VEGFR+ primary leukemias and cell lines. Next, we exploited the molecular mechanisms of such induction. For this, we performed a gene expression analysis of HL-60 cells, treated or untreated with VEGF, and focused on the induction of genes that might be involved in apoptosis. One gene whose expression was significantly increased by VEGF was heat shock protein 90 (Hsp90). Because Hsps are involved in stress responses and are also linked with resistance to apoptosis,9-11 we hypothesized that this chaperone molecule might play an important role in mediating the survival effects of VEGF.
Hsp90 is a highly conserved, constitutively expressed protein, which represents about 2% to 5% of the total cellular proteins even in the absence of stress stimulation.12 Hsp90 has been shown to play a role in various cell regulation pathways by interacting with different proteins such as Erb-2, MEK, and Raf-1.13 Hsp90 acts by forming an association with a partner protein and preventing its degradation or participating in its folding.14 In addition, it was shown that an antibiotic, benzoquinone ansamycin, geldanamycin (GA),13 selectively blocks the activities of Hsp90, but does not interact with other members of the Hsp family. GA binds to Hsp90, preventing the formation of the Hsp90/partner protein complex and, therefore, resulting in the degradation of the partner protein. Particularly in what concerns apoptosis it was recently shown that Hsp90 blocks cytochrome c-mediated oligomerization of apoptotic protease-activating factor-1 (APAF-1) and subsequent activation of procaspase-9, thereby inhibiting apoptosis.11
In this study we demonstrate that VEGF induces Hsp90 expression on VEGFR+ leukemias. On VEGF stimulation, Hsp90 binds Bcl-2 and APAF-1, an effect mediated through VEGFR-2 (KDR) and involving the activation of the MAP kinase pathway. These actions of VEGF result in increased resistance to apoptosis in serum-free conditions or in response to high concentrations of GA. As suggested by its specificity, GA blocks VEGF-induced Bcl-2 and APAF-1 binding to Hsp90. Besides showing the effects of exogenous VEGF on leukemia survival, we demonstrate the generation of a VEGF/VEGFR autocrine loop on ECs results in Hsp90 up-regulation and increased resistance to serum deprivation or GA-induced apoptosis.
In summary, we identified some of the molecular events involved in the prosurvival effects of VEGF, in both malignant (leukemia) and also normal (EC) cells. The data shown here suggest that during leukemia progression, VEGF, either through the generation of an autocrine loop or acting in a paracrine fashion, may promote the survival of and protect VEGFR+ leukemias from apoptosis.
Materials and methods
Recombinant cytokines
The VEGF and placental-derived growth factor (PLGF) were obtained from R & D Systems (Minneapolis, MN). These were resuspended in mycoplasma-free phosphate-buffered saline (PBS) and used at different concentrations.
Antibodies and synthetic inhibitors
Monoclonal neutralizing antibodies to VEGFRs were used to identify which receptor was specifically activated in response to VEGF or PLGF: anti-KDR antibody (IMC-1C11) and anti-Flt-1 (6.12) were kindly provided by Imclone Systems (New York, NY). The antibodies were used at 1 μg/mL. PD-98059 (MAP kinase inhibitor) was obtained from Calbiochem (San Diego, CA) and used at a concentration of 30 μM. Wortmanin (PI3 kinase inhibitor) was obtained from Sigma (St Louis, MO) and used at a concentration of 30 nM and LY294002 (PI3 kinase inhibitor, Calbiochem), was used at a concentration of 3 μM.
Cell culture
Primary leukemia samples (a total of 4, diagnosed as acute myeloid leukemia) were obtained as described previously.3These as well as the promyelomonocytic leukemic cell line HL-60, the promonocytic cell line THP-1, and the megakaryocytic leukemic cell line HEL were used to study the effects of VEGF. Leukemic cells were cultured in RPMI (Gibco BRL, Rockville, MD) with 10% fetal calf serum (FCS), penicillin (100 U/mL), streptomycin (100 μg/mL), and Fungizone (0.25 μg/mL). For VEGFR expression analysis, cells were stained with human KDR-specific (clone 6.64) or Flt-1–specific (clone FB5) fluorescein isothiocyanate (FITC)–labeled monoclonal antibody (both obtained from ImClone Systems), and analyzed using a Coulter Elite Flow Cytometer, Miami, FL. HUVECs were also used in this study. These were cultured under standard endothelial culture conditions,15 and split on reaching confluency.
VEGF enzyme-linked immunosorbent assay
Supernatants from leukemic cultures (1 × 106cells/mL, in serum-free conditions) were collected after 24 hours, and analyzed for the presence of VEGF using a human VEGF-specific enzyme-linked immunosorbent assay (ELISA) and following the manufacturer's instructions (R & D Systems). Results are shown as picograms per milliliter of VEGF in culture supernatants, and each determination was done in triplicate.
RNA extraction
For messenger RNA (mRNA) extraction, and before incubation with VEGF, the cells were washed and serum starved for 4 hours in RPMI alone. Cells were left untreated or treated with VEGF (50 ng/mL) and were cultured for 12 hours before RNA extraction. Total RNA was isolated from both VEGF-treated (12 hours) and untreated HL-60 cells using HighPure RNA isolation kit (Roche Diagnostics, Indianapolis, IN). This procedure includes DNase-I treatment of RNA samples to prevent genomic DNA contamination. Poly A+ RNA was isolated using Dynabeads Oligo (dT)25 (Dynal Biotech, Lake Success, NY), using the manufacturer's protocol. RNA quality was verified by electrophoresis on a denaturing formaldehyde-agarose gel.
Gene expression analysis
Gene expression analysis was performed using the Atlas Human cDNA Expression Array (Clontech, San Diego, CA). Poly A+ RNA from both samples (1 μg each) was used for complementary DNA (cDNA) synthesis. cDNA synthesis, probe labeling, purification of labeled cDNA from unincorporated 32P-labeled nucleotides, and hybridization of samples to the cDNA expression array were performed according to the manufacturer's instructions. The images of hybridization were obtained by phosphorimager (Amersham Biosciences, Sunnyvale, CA) after 5 days of exposure. Signal intensities were estimated using the ImageQuant software (Molecular Dynamics). Results show the mean signal intensities determined for the genes differentially expressed in control versus VEGF-stimulated HL-60 cells.
Quantification of apoptotic cells in serum-free conditions
To determine survival in serum-free conditions, HL-60 (1 × 105/mL) or ECs (1 × 105/well of a 6-well plate) were cultured in serum-free medium for 72 hours. In the case of ECs, every 24 hours the cells were collected by gentle scraping of collagenase-treated cells (Gibco BRL)s, spun down at 1000 rpm, and analyzed for the presence of apoptotic cells using the annexin V staining kit (Immunotech, Beckman Coulter, Brea, CA), following the manufacturer's instructions, and using a Coulter Elite Flow Cytometer. In the HL-60 experiments, VEGF (20 ng/mL) was added every 24 hours, alone or together with GA, to the cultures. Results are shown as the percentage of early apoptotic cells (annexin V+). Triplicates were used in all experiments, and each experiment was repeated 3 times.
GA experiments
Geldanamycin was obtained from Sigma Chemical and diluted in dimethyl sulfoxide (DMSO) for all the in vitro experiments. For the Western blot analysis of Bcl-2 induction following VEGF stimulation, HL-60 cells were cultured in 6-well plates (Corning Costar, Cambridge, MA), at a cell density of 1 × 106 cells/mL, in serum-free RPMI and treated with VEGF alone, 0.02 μM GA or VEGF plus GA. For survival experiments, ECs were cultured in serum-free conditions and exposed to different concentrations of GA (0.02, 0.2, or 2 μM). In the case of HL-60 cells, a higher GA dose was also used (10 μM). The percentage of apoptotic cells in each culture condition was determined by annexin V staining, as above.
Protein extraction and Western blotting
To detect VEGF-induced changes in protein expression, HL-60 cells were stimulated with recombinant VEGF (50 ng/mL) or PLGF (100 ng/mL) for different time points (24 hours for Bcl-2 induction; 3 hours for protein binding following VEGF stimulation), at 37°C. For experiments using the MAP kinase or the PI3 kinase inhibitors, cells were stimulated with VEGF in the presence of each specific inhibitor for the duration of the treatment. After stimulation, cells were lysed in cold RIPA buffer (50 mM Tris, 5 mM EDTA, 1% Triton X-114, 0.4% sodium cacodylate, and 150 mM NaCl), in the presence of protease inhibitors (1 mg/mL aprotinin, 10 mg/mL leupeptin, 1 mM β-glycerophosphate, 1 mM sodium orthovanadate, and 1 mM phenylmethylsulfonyl fluoride). After centrifugation to remove cell debris, supernatants (a total protein minimum of 500 ng) were resuspended in loading buffer and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (7.5% gels) under reducing conditions (in the presence of β-mercaptoethanol). Proteins were subsequently blotted onto a nitrocellulose membrane following conventional protocols. Finally, blots were blocked in 1% bovine serum albumin (BSA)/PBS-1% Tween 20 for 1 hour at room temperature followed by incubation with primary and secondary antibodies. Goat polyclonal anti-Hsp90 (Santa Cruz Biotechnology, Santa Cruz, CA), mouse polyclonal antihuman Bcl-2, Bax (Oncogene Research Products, Calbiochem), and rabbit polyclonal antihuman APAF-1 (Santa Cruz Biotechnology) antibodies were used at a concentration of 1 μg/mL and secondary antigoat IgG-horseradish perioxidase (HRP; for Hsp90), antimouse IgG-HRP (for Bcl-2), or antirabbit IgG-HRP (for APAF-1) were used at 1:6000. For immunoprecipitation experiments, total cell lysates were incubated with 1 μg/mL primary antibody and protein-A agarose beads overnight at 4°C. Precipitated proteins were resolved by polyacrylamide gel electrophoresis. The enhanced chemiluminescence (ECL) detection system and ECL film (Amersham Pharmacia Biotech, Piscataway, NJ) were used to visualize the presence of proteins on the nitrocellulose blots. Intensity of Western blot bands was quantified by densitometry, using the Scion Image software (Scion, Frederick, MD).
Adenovirus experiments
Adenoviruses were kindly provided by Dr Neil Hackett (Gene Core Facility Weill Medical College of Cornell University, New York). Briefly, HUVECs were infected with (10 pfu) adeno-null or adeno-VEGF, by incubating the cells with the viral preparation overnight. Cells were then washed and placed in complete endothelium medium. They were subsequently cultured in serum-free conditions or in the presence of different concentrations of GA, as above. Results show the percentage of annexin-positive cells (early apoptotic), as determined after 72 hours. Each determination was done in triplicate.
Statistical analysis
To detect differences between data sets, specifically for cell survival (annexin V) data, a Student t test (2-tailed) was applied in which P < .05 was considered statistically significant. Western blot results are shown as the ratio between densitometry units (band intensity) in experimental conditions versus controls (untreated cells). These values were calculated from 3 independent experiments and are shown with SDs. A ratio of 1 (along the dotted line) indicates the experimental condition had identical Western blot results to the controls. For the annexin V data, a P < .05 was considered significant.
Results
VEGF165 promotes survival of leukemia by increasing Bcl-2 expression
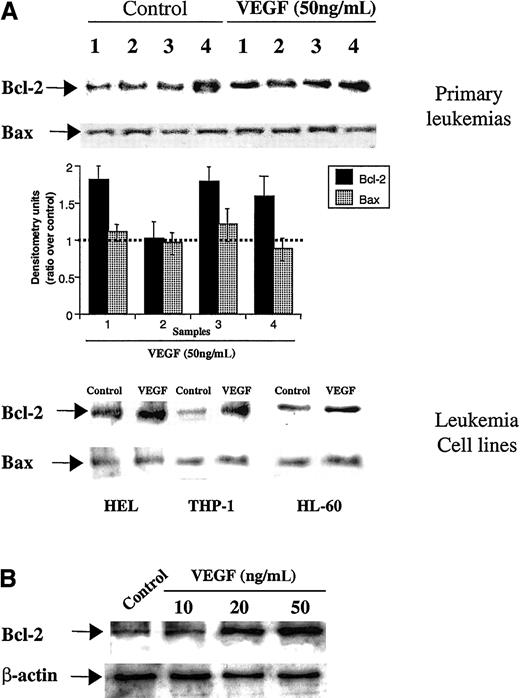
Given the involvement of Bcl-2 on VEGF-induced EC survival, we investigated whether it exerted a similar function on VEGFR+ leukemic cells. All leukemias and leukemic cell lines used in this study expressed either KDR or Flt-1, or both, as determined by flow cytometry (Table 1). As determined by Western blotting, on 3 primary leukemias (samples 1, 3, and 4) and on 2 cell lines (HEL and THP-1), there were also increased Bcl-2 levels after 24 hours of VEGF stimulation, whereas Bax showed modest changes over control (baseline) levels (Figure1A). This effect was dose-dependent (shown for HL-60 cells, Figure 1B) and is consistent with an increase in survival and proliferation of VEGFR+ leukemias, seen after longer incubations with VEGF (previously shown3).
Phenotype of the leukemic cell lines and primary leukemias described in this study
| Leukemic sample . | Flt-1 expression (%) . | KDR expression (%) . | VEGF (pg/mL) . |
|---|---|---|---|
| HL-60 | 7.92 ± 5.1 | 14.5 ± 5.4 | 144 ± 35 |
| HEL | 20 ± 3.9 | 6.1 ± 2.8 | 94 ± 15 |
| THP-1 | 6.8 ± 2.7 | 5 ± 2.3 | 90 ± 18 |
| 1 | 15.2 ± 2.9 | 10.4 ± 4.2 | 120 ± 16 |
| 2 | 8.8 ± 3.1 | — | 93 ± 21 |
| 3 | — | 7.3 ± 4.4 | 88 ± 18 |
| 4 | 21.4 ± 6.5 | 28 ± 8.1 | 196 ± 20 |
| Leukemic sample . | Flt-1 expression (%) . | KDR expression (%) . | VEGF (pg/mL) . |
|---|---|---|---|
| HL-60 | 7.92 ± 5.1 | 14.5 ± 5.4 | 144 ± 35 |
| HEL | 20 ± 3.9 | 6.1 ± 2.8 | 94 ± 15 |
| THP-1 | 6.8 ± 2.7 | 5 ± 2.3 | 90 ± 18 |
| 1 | 15.2 ± 2.9 | 10.4 ± 4.2 | 120 ± 16 |
| 2 | 8.8 ± 3.1 | — | 93 ± 21 |
| 3 | — | 7.3 ± 4.4 | 88 ± 18 |
| 4 | 21.4 ± 6.5 | 28 ± 8.1 | 196 ± 20 |
Cells were stained for surface VEGFR-1 (Flt-1) and VEGFR-2 (KDR), the level of surface expression determined by flow cytometry, as described in “Materials and methods,” and were also analyzed for VEGF production (by ELISA).
Dash indicates that there were no Flt-1+ or KDR+ cells detected in the total cell population.
Bcl-2 expression on primary leukemias, leukemic cell lines, and HL-60 cells.
(A) VEGF induces Bcl-2 expression on primary leukemias and leukemic cell lines. Cells were left untreated or stimulated with VEGF (50 ng/mL) for 24 hours. Total protein extracts were obtained and Bcl-2/Bax was detected by Western blotting (see “Materials and methods” for details). Western blotting results were quantified by densitometry and are shown as the ratio between densitometry values in treated versus untreated (control) cells. Each experiment was repeated 3 times, and the SD between the different ratios was calculated and is shown. (B) VEGF induces, in a dose-dependent manner, Bcl-2 expression on HL-60 cells. As a control for protein loading, β-actin levels are also shown.
Bcl-2 expression on primary leukemias, leukemic cell lines, and HL-60 cells.
(A) VEGF induces Bcl-2 expression on primary leukemias and leukemic cell lines. Cells were left untreated or stimulated with VEGF (50 ng/mL) for 24 hours. Total protein extracts were obtained and Bcl-2/Bax was detected by Western blotting (see “Materials and methods” for details). Western blotting results were quantified by densitometry and are shown as the ratio between densitometry values in treated versus untreated (control) cells. Each experiment was repeated 3 times, and the SD between the different ratios was calculated and is shown. (B) VEGF induces, in a dose-dependent manner, Bcl-2 expression on HL-60 cells. As a control for protein loading, β-actin levels are also shown.
VEGF165 induces expression of Bcl-2 through VEGFR-2 (KDR)
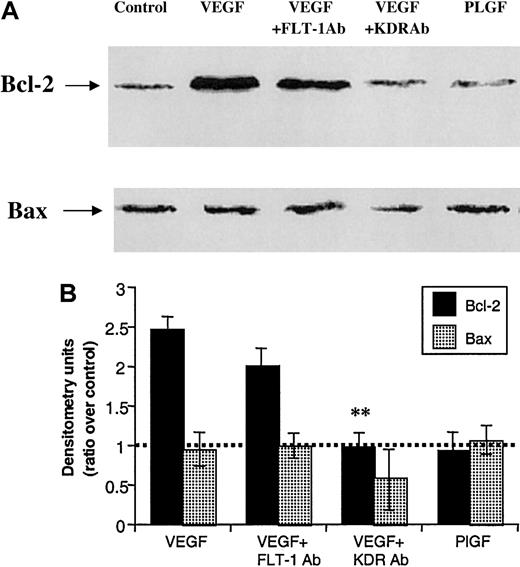
We used the HL-60 cell line as a model to investigate in detail the mechanisms by which VEGF promoted Bcl-2 expression and leukemic cell survival. First, KDR-specific (IMC-1C11) and Flt-1–specific (6.12) neutralizing monoclonal antibodies and PLGF (specific ligand for Flt-1) were used to examine the involvement of KDR and Flt-1 on VEGF-induced Bcl-2 induction (Figure2). VEGF induced a 2.5-fold increase in Bcl-2 expression on HL-60 cells, which could be prevented by coincubation with IMC-1C11 (Figure 2). Notably, treatment of HL-60 cells with the Flt-1–specific ligand PLGF had little effect on Bcl-2 expression (Figure 2), although incubation with the Flt-1–specific monoclonal antibody (6.12) reduced the VEGF-induced increases, but to a lesser extent than IMC-1C11. The levels of the proapoptotic Bax showed little change with any of the treatment conditions, decreasing only in the presence of IMC-1C11 (Figure 2).
VEGF increases Bcl-2, but not Bax, expression by interacting with KDR, but not FLT-1.
(A) Protein extracts were obtained from HL-60 cells, which were cultured in serum-free RPMI for 24 hours (control), in presence of 50 ng/mL VEGF, VEGF plus 1 μg/mL KDR antibody (clone IMC-1C11), VEGF plus 1 μg/mL FLT-1 antibody (clone 6.12), or 100 ng/mL (PLGF). (B) Western blotting results were quantified by densitometry and are shown as the ratio between densitometry values in treated versus untreated (control) cells. Each experiment was repeated 3 times, and the SD between the different ratios was calculated and is shown.
VEGF increases Bcl-2, but not Bax, expression by interacting with KDR, but not FLT-1.
(A) Protein extracts were obtained from HL-60 cells, which were cultured in serum-free RPMI for 24 hours (control), in presence of 50 ng/mL VEGF, VEGF plus 1 μg/mL KDR antibody (clone IMC-1C11), VEGF plus 1 μg/mL FLT-1 antibody (clone 6.12), or 100 ng/mL (PLGF). (B) Western blotting results were quantified by densitometry and are shown as the ratio between densitometry values in treated versus untreated (control) cells. Each experiment was repeated 3 times, and the SD between the different ratios was calculated and is shown.
VEGF165 activation of serum-starved HL-60 cells leads to multiple changes in gene expression
HL-60 cells were treated with VEGF for 12 hours to detect the expression of genes that could be involved in the antiapoptotic effects of VEGF. Subsequently, mRNA from treated and untreated cells was hybridized to an Atlas Human cDNA Expression Array, containing 588 known human genes. Analysis of the pattern of hybridization revealed a group of genes whose expression changed the most in response to VEGF stimulation (Table 2). Ninety genes were detected in HL-60 serum-starved cells at levels higher than background. We divided these genes into different groups, based on their level of expression related to the average level of the housekeeping genes present in the membrane (which was considered as 1.0). A difference in signal intensity of more than 2 times was considered significant and, therefore, corresponding genes were considered as differentially expressed.
Signal intensity (expression level) of differentially expressed genes on HL-60 cells with or without VEGF treatment
| Gene name . | Control . | VEGF . | Ratio (V/C) . |
|---|---|---|---|
| Prothymosin α | 9.23 | 3.81 | 0.41 |
| Diazepam-binding inhibitor | 3.59 | 8.21 | 2.29 |
| Hsp90 | 1.24 | 3.66 | 2.95 |
| Monocyte chemotactic and activating factor | 1.65 | 0.61 | 0.37 |
| S19 ribosomal protein | 1.00 | 0.48 | 0.48 |
| DNA-binding protein NF-E1 | 0.19 | 0.66 | 3.47 |
| Glutathione S-transferase pi | 0.65 | 0.15 | 0.23 |
| Transcriptional activator hSNF2a | 0.12 | 0.36 | 3.00 |
| Ribonuclease/angiogenin inhibitor RAI | 0.06 | 0.35 | 5.83 |
| Gene name . | Control . | VEGF . | Ratio (V/C) . |
|---|---|---|---|
| Prothymosin α | 9.23 | 3.81 | 0.41 |
| Diazepam-binding inhibitor | 3.59 | 8.21 | 2.29 |
| Hsp90 | 1.24 | 3.66 | 2.95 |
| Monocyte chemotactic and activating factor | 1.65 | 0.61 | 0.37 |
| S19 ribosomal protein | 1.00 | 0.48 | 0.48 |
| DNA-binding protein NF-E1 | 0.19 | 0.66 | 3.47 |
| Glutathione S-transferase pi | 0.65 | 0.15 | 0.23 |
| Transcriptional activator hSNF2a | 0.12 | 0.36 | 3.00 |
| Ribonuclease/angiogenin inhibitor RAI | 0.06 | 0.35 | 5.83 |
Control and VEGF columns contain the values related to the average signal intensity of housekeeping genes, which was 1.00. Only genes with a signal intensity ratio (V/C = VEGF/control) higher than 2 are shown. (Maximum signal intensity detected on HL-60 cells was 11.33, corresponding to nuclease-sensitive element DNA-binding protein.)
We found 4 highly abundant, differentially expressed genes on VEGF-treated HL-60 cells: prothymosin-α, Hsp90 (α subunit), monocyte chemotactic and activating factor (MCAF), and diazepam-binding inhibitor (Table 2). These genes are mostly involved in resistance to apoptosis and also in mechanisms of cellular differentiation.
VEGF165 induces Hsp90 in serum-starved leukemic cells
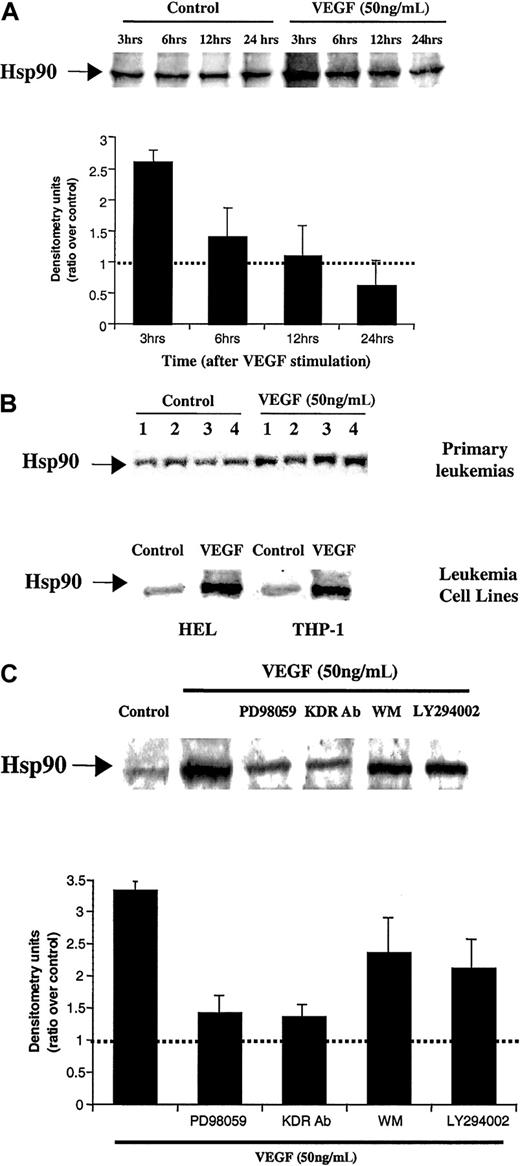
As determined by the cDNA array, Hsp90 mRNA levels increased approximately 3- fold in HL-60 cells treated with VEGF. These results were confirmed at the protein level, by Western blot analysis (Figure3A). The results show that on HL-60 cells cultured in the presence of VEGF, the expression of Hsp90 increased rapidly, reaching a maximum level after 3 to 12 hours (Figure 3A), and subsequently decreasing at later time points (last time point analyzed, 24 hours). VEGF also increased Hsp90 expression on 3 VEGFR+primary leukemias (samples 1, 3, and 4) and 2 other leukemic cell lines used in this study (Figure 3B).
VEGF induces Hsp90 expression on HL-60 cells and primary leukemias.
(A) VEGF induces Hsp90 expression on HL-60 cells. Results of Western blot analysis of Hsp90 expression level on HL-60 cells are shown. Protein extracts were obtained after 3, 6, 12, and 24 hours incubation of serum-starved HL-60 cells in the absence or presence of VEGF. Western blotting results were quantified by densitometry and are shown as the ratio between densitometry values in treated versus untreated (control) cells. Each experiment was repeated 3 times, and the SD between the different ratios was calculated and is shown. (B) VEGF induces Hsp90 expression also on primary leukemias and 2 other leukemic cell lines. Total protein extracts were obtained after 12 hours of VEGF stimulation, and Hsp90 was detected by Western blotting, as above. (C) VEGF increases Hsp90 expression by interacting with KDR and activating the MAP kinase pathway. Protein extracts were obtained from HL-60 cells, which were cultured in serum-free RPMI for 3 hours (control), in presence of 50 ng/mL VEGF, VEGF plus 1 μg/mL KDR antibody, or 100 ng/mL (PLGF). To determine the molecular pathways by which VEGF induced Hsp90 expression, protein extracts were also incubated with an MAP kinase inhibitor (PD98059, used at 30 μM), wortmanin (PI3 kinase inhibitor, used at 30 nM), or LY294002 (PI3 kinase inhibitor, used at 3 μM). Western blotting results were quantified by densitometry and are shown as the ratio between densitometry values in treated versus untreated (control) cells. Each experiment was repeated 3 times, and the SD between the different ratios was calculated and is shown.
VEGF induces Hsp90 expression on HL-60 cells and primary leukemias.
(A) VEGF induces Hsp90 expression on HL-60 cells. Results of Western blot analysis of Hsp90 expression level on HL-60 cells are shown. Protein extracts were obtained after 3, 6, 12, and 24 hours incubation of serum-starved HL-60 cells in the absence or presence of VEGF. Western blotting results were quantified by densitometry and are shown as the ratio between densitometry values in treated versus untreated (control) cells. Each experiment was repeated 3 times, and the SD between the different ratios was calculated and is shown. (B) VEGF induces Hsp90 expression also on primary leukemias and 2 other leukemic cell lines. Total protein extracts were obtained after 12 hours of VEGF stimulation, and Hsp90 was detected by Western blotting, as above. (C) VEGF increases Hsp90 expression by interacting with KDR and activating the MAP kinase pathway. Protein extracts were obtained from HL-60 cells, which were cultured in serum-free RPMI for 3 hours (control), in presence of 50 ng/mL VEGF, VEGF plus 1 μg/mL KDR antibody, or 100 ng/mL (PLGF). To determine the molecular pathways by which VEGF induced Hsp90 expression, protein extracts were also incubated with an MAP kinase inhibitor (PD98059, used at 30 μM), wortmanin (PI3 kinase inhibitor, used at 30 nM), or LY294002 (PI3 kinase inhibitor, used at 3 μM). Western blotting results were quantified by densitometry and are shown as the ratio between densitometry values in treated versus untreated (control) cells. Each experiment was repeated 3 times, and the SD between the different ratios was calculated and is shown.
Next, using HL-60 cells as a model, we investigated whether VEGF increased Hsp90 expression by interacting with VEGFR-1 (Flt-1) or VEGFR-2 (KDR). The VEGF-induced increase in Hsp90 expression was blocked by coincubating the HL-60 cells with VEGF plus IMC-1C11, PD-98059, but not wortmanin or LY20094 (PI3 kinase inhibitors) (Figure3C).
VEGF165 induces Bcl-2 expression through Hsp90 and promotes Bcl-2 binding to Hsp90
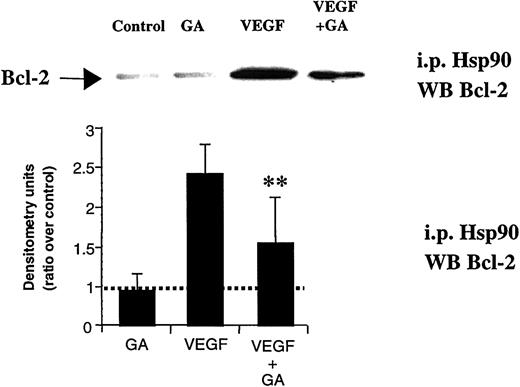
We hypothesized Hsp90 might be involved in the signaling cascade leading to the VEGF-induced changes in Bcl-2 expression. To verify this hypothesis, we used GA, an Hsp90-specific inhibitor. GA, even at low doses, blocked the VEGF-induced Bcl-2 expression on HL-60 leukemic cells, but did not affect the constitutive expression of other proteins, such as Hsp90 itself or Bax (data for Bax levels are shown, Figure 4). Similar results were obtained for the other leukemic cell lines and primary leukemias studied (data not shown).
GA blocks VEGF-induced Bcl-2 expression on HL-60 cells.
HL-60 cells were cultured in serum-free RPMI for 24 hours (control), in the presence of 50 ng/mL VEGF165 (VEGF) and combination of VEGF165 and 0.02 μM geldanamycin (VEGF + GA). Western blotting results were quantified by densitometry and are shown as the ratio between densitometry values in treated versus untreated (control) cells. Each experiment was repeated 3 times, and the SD between the different ratios was calculated and is shown. Double asterisks indicate that difference between VEGF plus GA and VEGF alone is significant (P < .05).
GA blocks VEGF-induced Bcl-2 expression on HL-60 cells.
HL-60 cells were cultured in serum-free RPMI for 24 hours (control), in the presence of 50 ng/mL VEGF165 (VEGF) and combination of VEGF165 and 0.02 μM geldanamycin (VEGF + GA). Western blotting results were quantified by densitometry and are shown as the ratio between densitometry values in treated versus untreated (control) cells. Each experiment was repeated 3 times, and the SD between the different ratios was calculated and is shown. Double asterisks indicate that difference between VEGF plus GA and VEGF alone is significant (P < .05).
Next, we demonstrated that after VEGF stimulation, Hsp90 interacts directly with Bcl-2. As shown in Figure5, stimulation of HL-60 cells with VEGF promoted Bcl-2 binding to Hsp90, an effect that was partially blocked by coincubating the cells with VEGF plus GA.
VEGF promotes Bcl-2 binding to Hsp90.
Cell extracts were immunoprecipitated with an antibody against Hsp90 and probed with an antibody against Bcl-2. After 12 hours of stimulation, VEGF promoted Bcl-2 binding to Hsp90, an effect that was partially blocked by coincubating the cells with GA (0.02 μM). Western blotting results were quantified by densitometry and are shown as the ratio between densitometry values in treated versus untreated (control) cells. Each experiment was repeated 3 times, and the SD between the different ratios was calculated and is shown. Double asterisks indicate GA significantly blocks Bcl-2 binding to Hsp90 (P < .05).
VEGF promotes Bcl-2 binding to Hsp90.
Cell extracts were immunoprecipitated with an antibody against Hsp90 and probed with an antibody against Bcl-2. After 12 hours of stimulation, VEGF promoted Bcl-2 binding to Hsp90, an effect that was partially blocked by coincubating the cells with GA (0.02 μM). Western blotting results were quantified by densitometry and are shown as the ratio between densitometry values in treated versus untreated (control) cells. Each experiment was repeated 3 times, and the SD between the different ratios was calculated and is shown. Double asterisks indicate GA significantly blocks Bcl-2 binding to Hsp90 (P < .05).
VEGF165 promotes Hsp90 binding to APAF-1
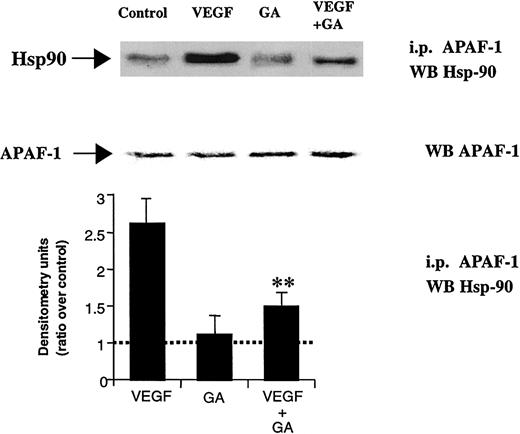
As seen for Hsp90 induction and binding to Bcl-2, VEGF promoted Hsp90 binding to APAF-1, an effect that was blocked by coincubating the cells with GA (Figure 6). In addition, as shown for Hsp90 itself, APAF-1/Hsp90 binding in response to VEGF could be blocked by IMC-1C11 (data not shown). Importantly, the basal levels of APAF-1 showed little change with any of the experimental conditions (Figure 6). These results suggest that on VEGFR+ leukemic cells, VEGF, by interacting with KDR and activating the MAP kinase pathway, leads to increased Hsp90 levels and promotes binding of Hsp90 to APAF-1, which may result in apoptosis blockade.
VEGF promotes Hsp90 binding to APAF-1.
Cell extracts were immunoprecipitated using an APAF-1–specific antibody and probed with a Hsp90-specific antibody. APAF-1 binding to Hsp90 was seen after VEGF stimulation for 12 hours. As a protein loading control, total APAF-1 levels are also shown. Coincubation with GA blocked the effects of VEGF, as seen for Bcl-2. Western blotting results were quantified by densitometry and are shown as the ratio between densitometry values in treated versus untreated (control) cells. Each experiment was repeated 3 times, and the SD between the different ratios was calculated and is shown. Double asterisks indicate GA significantly blocks APAF-1 binding to Hsp90.
VEGF promotes Hsp90 binding to APAF-1.
Cell extracts were immunoprecipitated using an APAF-1–specific antibody and probed with a Hsp90-specific antibody. APAF-1 binding to Hsp90 was seen after VEGF stimulation for 12 hours. As a protein loading control, total APAF-1 levels are also shown. Coincubation with GA blocked the effects of VEGF, as seen for Bcl-2. Western blotting results were quantified by densitometry and are shown as the ratio between densitometry values in treated versus untreated (control) cells. Each experiment was repeated 3 times, and the SD between the different ratios was calculated and is shown. Double asterisks indicate GA significantly blocks APAF-1 binding to Hsp90.
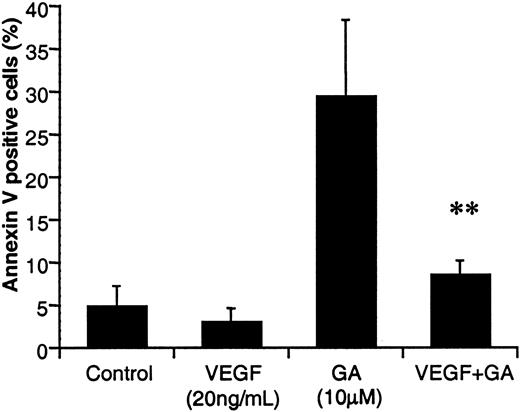
VEGF protects HL-60 cells from apoptosis in vitro
We have previously shown that subsets of leukemic cells produce VEGF and express its receptors, which results in an autocrine loop that mediates leukemia survival. Here we demonstrate that VEGF induces Hsp90, which in turn binds Bcl-2 and APAF-1, and thus may protect cells from apoptosis. Next, we showed that VEGF protected HL-60 cells from both serum-free (not shown) and GA-induced apoptosis. As seen in Figure7, when cultured in the presence of 10 μM GA for 72 hours, HL-60 undergoes a 6-fold increase in apoptosis (measured by annexin V+ cells) when compared to control (untreated) cells. In contrast, coincubation of HL-60 cells with GA plus VEGF decreased the percentage of apoptotic HL-60 cells by 3.5-fold, to levels similar to controls (Figure 7). The reduced sensitivity of HL-60 cells to GA, seen in the presence of VEGF, supports our in vitro data demonstrating that VEGF up-regulates Hsp90 levels. Similarly, addition of VEGF protected HL-60 cells from serum-free–induced apoptosis (not shown).
VEGF protects HL-60 cells from GA-induced apoptosis.
HL-60 cells were cultured in serum-free RPMI, left untreated or treated with VEGF (20 ng/mL), GA (10 μM), or VEGF plus GA every 24 hours, for 72 hours. Apoptotic cells were determined by annexin V staining, as described in “Materials and methods.” Results are shown as a percentage of apoptotic (annexin V+) cells. Each determination was done in triplicate. Double asterisks indicate VEGF significantly protects HL-60 cells from GA-induced apoptosis.
VEGF protects HL-60 cells from GA-induced apoptosis.
HL-60 cells were cultured in serum-free RPMI, left untreated or treated with VEGF (20 ng/mL), GA (10 μM), or VEGF plus GA every 24 hours, for 72 hours. Apoptotic cells were determined by annexin V staining, as described in “Materials and methods.” Results are shown as a percentage of apoptotic (annexin V+) cells. Each determination was done in triplicate. Double asterisks indicate VEGF significantly protects HL-60 cells from GA-induced apoptosis.
Generation of a VEGF/VEGFR autocrine loop protects ECs from apoptosis in vitro
Earlier, we demonstrated the generation of a VEGF/KDR autocrine loop was essential for leukemia growth in vivo.16 As shown above, addition of VEGF to VEGFR-expressing leukemic cells protects them from apoptosis in vitro. Next, we investigated whether the generation of a VEGF/VEGFR autocrine loop conferred resistance to apoptosis also on normal cells such as ECs. First, we compared the sensitivity of HUVECs to GA, in a dose-response experiment. As determined by annexin V staining of the cells in the presence of different concentrations of GA (0.02, 0.2, and 2 μM), most ECs die over a 48-hour period (Figure 8A).
Effect of VEGF/VEGFR autocrine loop on ECs.
(A) EC survival in serum-free conditions or in the presence of different concentrations of GA is shown. ECs were cultured in serum-free RPMI and left untreated or treated with GA, for a 72-hour period. Apoptotic cells were determined by annexin V staining, as described in “Materials and methods.” Results are shown as a percentage of apoptotic (annexin V+) cells. Each determination was done in triplicate. (B) Infection of ECs with adeno-VEGF induces Hsp90 expression. Total protein extracts were obtained from noninfected (control), adeno-null–infected, or adeno-VEGF–infected ECs, after 12 hours of culture in serum-free conditions. Results show Hsp90 levels, as determined by Western blotting. (C) ECs infected with adeno-null or adeno-VEGF are resistant to serum-free and GA-induced apoptosis. Noninfected, adeno-null–infected or adeno-VEGF–infected ECs were cultured for 72 hours in serum-free conditions, or with increasing concentrations of GA (0.02, 0.2, and 2 μM). Apoptotic cells (as determined by annexin V staining) were quantified after 72 hours. Results are shown as a percentage of apoptotic cells, and each determination was done in triplicate. Adeno-VEGF–infected cells survived significantly longer in serum-free conditions or after treatment with GA (**P < .05).
Effect of VEGF/VEGFR autocrine loop on ECs.
(A) EC survival in serum-free conditions or in the presence of different concentrations of GA is shown. ECs were cultured in serum-free RPMI and left untreated or treated with GA, for a 72-hour period. Apoptotic cells were determined by annexin V staining, as described in “Materials and methods.” Results are shown as a percentage of apoptotic (annexin V+) cells. Each determination was done in triplicate. (B) Infection of ECs with adeno-VEGF induces Hsp90 expression. Total protein extracts were obtained from noninfected (control), adeno-null–infected, or adeno-VEGF–infected ECs, after 12 hours of culture in serum-free conditions. Results show Hsp90 levels, as determined by Western blotting. (C) ECs infected with adeno-null or adeno-VEGF are resistant to serum-free and GA-induced apoptosis. Noninfected, adeno-null–infected or adeno-VEGF–infected ECs were cultured for 72 hours in serum-free conditions, or with increasing concentrations of GA (0.02, 0.2, and 2 μM). Apoptotic cells (as determined by annexin V staining) were quantified after 72 hours. Results are shown as a percentage of apoptotic cells, and each determination was done in triplicate. Adeno-VEGF–infected cells survived significantly longer in serum-free conditions or after treatment with GA (**P < .05).
The generation of a VEGF/VEGFR autocrine loop on ECs was achieved by infecting ECs with an adenovirus expressing human VEGF. Adeno-VEGF–infected ECs had higher constitutive levels of Hsp90 (Figure 8B) and Bcl-2 (not shown) and were thus 5- to 10-fold more resistant to GA-induced and serum-free–induced apoptosis than their normal, noninfected endothelial counterpart (shown for the 72-hour time point, Figure 8C, P < .05). ECs infected with adeno-null (nonmodified adenovirus) also had increased Hsp90 levels, although, relative to the controls, the increase was less profound than in the adeno-VEGF–infected ones (Figure 8B). Likewise, adeno-null infection also improved EC survival in serum-free conditions and augmented their resistance to GA-induced apoptosis, although to a lesser extent than that seen with the adeno-VEGF–infected ones. Taken together, the data shown here suggest that VEGF, either exogenous or acting in a paracrine manner, stimulates cell survival of both leukemia and ECs, by up-regulating Hsp90, Bcl-2 induction, and apoptosis inhibition.
Notably, as seen in Figure 7, HL-60 cells required doses of GA that were 5-fold higher than those for ECs to die by apoptosis over a 72-hour period.
Discussion
Vascular endothelial growth factor is one of the most potent proangiogenic factors. It regulates differentiation, migration, proliferation, and survival of ECs by interacting with its receptors VEGFR-1 (Flt-1) and VEGFR-2 (Flk-1/KDR). In addition, it has recently been shown that VEGF protects ECs from apoptosis, by inducing the antiapoptotic gene, Bcl-2.7 However, detailed molecular analysis of the specific pathway(s) leading to Bcl-2 induction has not been done. Furthermore, besides ECs, it was recently shown that subsets of leukemias expresses Flt-1 and KDR.2,3 Earlier, we demonstrated these receptors are functional on certain VEGF-producing leukemias, resulting in the generation of an autocrine loop that promotes their proliferation and migration.3 Previous studies have also shown VEGF protects hematopoietic cells and a leukemia cell line from chemotherapy-induced apoptosis, an effect that involved induction of MCL1.17 In the present study we investigated in detail the molecular pathways induced by VEGF on VEGFR+ leukemias, which contribute toward leukemic cell survival, by focusing on Bcl-2.
As shown for ECs, VEGF induced Bcl-2 expression on both primary leukemias and cell lines. To find the molecular pathways by which VEGF promotes leukemia survival, we used the HL-60 cell line as a model. We started by showing that VEGF induces Bcl-2 expression by interacting with KDR on the cell surface. Next, we performed a cDNA expression array analysis of VEGF-treated and nontreated HL-60 cells. About 15% of the total number of genes included on the cDNA array were expressed in HL-60 serum-starved cells. Considering the number of genes represented in the gene array (588 genes), this percentage fits the estimation that one cell type expresses 15% to 20% of the total number of genes in the genome. As shown in Table 1, 3 of the genes whose expression changed the most between treated and untreated cells, were prothymosin-α (ProT-[alpha]), diazepam-binding inhibitor (DBI), and the α subunit of Hsp90. Several groups have previously shown prothymosin-α is involved in chromatin remodeling, its cellular levels strongly correlating with cell proliferation and differentiation.18 Some information is also available for DBI, connecting it to cell proliferation.19
A plethora of information is available regarding Hsp90 and other members of the Hsp family and their importance for cell proliferation and differentiation. Furthermore, and of particular interest to the present studies, Hsps play an important role in apoptosis.9-11,14 For instance, it was recently shown that Hsp60 promotes apoptosis by accelerating the maturation of procaspase-3.20 On the other hand, Hsp70 and Hsp27 block apoptosis by binding to APAF-1 and cytochrome c, respectively, therefore preventing the maturation of caspase-9.10,21 It has also been shown that Hsp90 may prevent apoptosis by stabilizing the antiapoptotic protein RIP-114 and binding to APAF-1, preventing its oligomerization.11 Considering their involvement in the regulation of apoptosis, we investigated whether VEGF mediated its survival-promoting effects through one or more Hsps. In the cDNA array used in these studies we verified the level of expression of 5 Hsps. As mentioned above, Hsp90 was up-regulated 3-fold in leukemic cells treated with VEGF. However, using this method, there were no significant differences in the expression level of Hsp60 and Hsp27, whereas Hsp40 and Hsp70 were not detected at all. Therefore, we hypothesized that Hsp90 might be involved in the VEGF-induced up-regulation of Bcl-2 and regulation of leukemic cell survival.
We demonstrate on VEGFR+ primary leukemias and cell lines that VEGF, through KDR, leads to activation of the MAP kinase cascade and results in up-regulation of Hsp90 levels. Previous studies had shown Hsp90 plays an important role in regulating VEGF-induced endothelial cell migration in vitro.22 Here, we show that Bcl-2 induction in response to VEGF is completely abrogated by cotreating cells with the Hsp90-specific antibiotic GA. Because Hsp90 stabilizes different proteins, thereby preventing their degradation, we hypothesized that, in the presence of GA, Hsp90 may fail to bind Bcl-2, and as a result Bcl-2 is rapidly degraded. In fact, coimmunoprecipitation experiments showed that, following VEGF stimulation, Bcl-2 binds Hsp90, an effect that is abrogated by GA. Similarly, VEGF not only increased Hsp90 and Bcl-2 levels but also promoted binding of Hsp90 to APAF-1, a mechanism previously shown to block apoptosis.11 Whether there is a direct interaction between Hsp90, APAF-1, and Bcl-2, leading to inhibition of apoptosis, is not established. Given that GA blocks Bcl-2 expression also on HUVECs (data not shown), the present work indicates that leukemic and endothelial cells may share common molecular pathways that mediate VEGF-induced survival, and that VEGF up-regulates Hsp90/Bcl-2 levels on both cell types.
It is well established that circulating VEGF levels are increased in patients with different hematologic diseases, namely, leukemia.23 Moreover, we have shown that an autocrine VEGF/KDR loop is functional in subsets of leukemias,3 and Ferrajoli et al24 showed the level of KDR expression by circulating leukemic cells correlated with poor survival of chronic lymphocytic leukemia patients. Similarly, Fusetti et al25demonstrated that the level of VEGF production by different leukemias determines their capacity to engraft and form tumors in vivo. Here we suggest that increases in circulating VEGF, acting in a paracrine or autocrine fashion, may in fact promote survival of subsets of leukemic cells through Hsp90 regulation. Indeed, following VEGF stimulation, HL-60 cells resisted serum-free–induced and also GA-induced apoptosis in vitro.
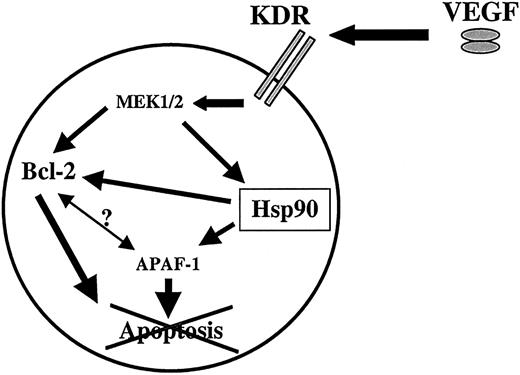
Next, using ECs as a model of nonmalignant, VEGFR-expressing cells, we determined whether the survival-promoting effects of VEGF were unique to leukemias. We hypothesized that cells that produce VEGF and express at least one of its receptors, such as some of the leukemias described above, may have elevated Hsp90 levels and thus be more resistant to apoptosis than the VEGFR− counterparts. In response to exogenous or endogenous VEGF, Hsp90 would prevent the degradation of Bcl-2 and APAF-1, resulting in increased survival of VEGFR-expressing cells (a proposed model is shown in Figure9). Therefore, generation of a VEGF/VEGFR autocrine loop on ECs should also result in increased resistance to serum-free and GA-induced apoptosis. This was achieved by infecting ECs with an adenovirus expressing human VEGF165. Normal ECs died in serum-free conditions after 2 to 3 days. However, as a result of the adenovirus infection, VEGF-producing ECs survived significantly longer (beyond 7 days), and actually proliferated (data not shown), in serum-free conditions, compared to the noninfected or adeno-null infected counterparts. As predicted, these cells had higher Hsp90 and also Bcl-2 (data not shown) levels. As a consequence of this increase in Hsp90 levels, adeno-VEGF–infected ECs were also significantly more resistant to GA-induced and serum-free–induced apoptosis.
Proposed model of the effects of VEGF on VEGFR+ cells such as subsets of leukemias.
Patients with leukemia have elevated circulating VEGF levels, which, in an autocrine or paracrine fashion, may stimulate VEGFR-2 (KDR) resulting in activation of the MAP kinase pathway and increased Hsp90 levels. Hsp90 in turn promotes survival of leukemic cells by binding APAF-1 and Bcl-2, thereby inhibiting apoptosis. Whether there is a direct interaction between Hsp90, APAF-1, and Bcl-2 is still not established.
Proposed model of the effects of VEGF on VEGFR+ cells such as subsets of leukemias.
Patients with leukemia have elevated circulating VEGF levels, which, in an autocrine or paracrine fashion, may stimulate VEGFR-2 (KDR) resulting in activation of the MAP kinase pathway and increased Hsp90 levels. Hsp90 in turn promotes survival of leukemic cells by binding APAF-1 and Bcl-2, thereby inhibiting apoptosis. Whether there is a direct interaction between Hsp90, APAF-1, and Bcl-2 is still not established.
The present report reveals, for the first time, that Hsp90 is an important mediator of VEGF-induced cell survival, a mechanism that involves up-regulation and binding of Bcl-2 and APAF-1 to Hsp90. Whether acting in a paracrine or autocrine manner, here we demonstrated that elevated circulating VEGF levels will confer VEGFR-expressing tumor cells with greater survival potential and resistance to apoptosis. Furthermore, on VEGF-producing, VEGFR-expressing tumor cells, such as leukemias, the existence of a VEGF/VEGFR autocrine loop may result in the maintenance of Hsp90 at high levels, stabilization of Bcl-2/APAF-1, and apoptosis blockade. Whether VEGF-induced Hsp90 also leads to the stabilization or increased production of other antiapoptotic proteins, remains to be determined.
It is now established that angiogenesis plays an important role in the growth of solid tumors.26 However, only recently it was suggested that angiogenesis might be critical for proliferation of liquid tumors as well.2,27 ECs may support leukemic cell growth through the release of different cytokines, and leukemic cells in turn support endothelial growth by releasing VEGF or other proangiogenic factors.2 Previously, we have shown that As2O3 causes apoptosis of human ECs, and suggested its reported antileukemic effects might involve inhibition of angiogenesis.28 In the present report, given the greater sensitivity of ECs to GA-mediated apoptosis, we hypothesize that GA may exert similar effects. The potent antitumor effects of GA, which led to its use in phase 2 and 3 trials of a variety of malignancies, may thus involve inhibition of angiogenesis, a mechanism that has not been demonstrated to date.
S.D. and S.V.S. contributed equally to this work.
S. Rafii is supported by a Translational Research Award from The Leukemia & Lymphoma Society; National Heart, Lung, and Blood Institute (NHLBI) grants R01s HL-58707, HL-61849, HL-66592, HL-67839; and a Research Scholar Grant from American Cancer Society (RSG-01-091-01) and the Lupin Foundation. S.D. was supported by Portuguese Science and Technology Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shahin Rafii, Division of Hematology/Oncology, Rm C-606, Weill Medical College of Cornell University, 1300 York Ave, New York, NY 10021; e-mail: srafii@mail.med.cornell.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal