Gastric marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT)–type can regress after anti–Helicobacter pyloritreatment. The International Extranodal Lymphoma Study Group, the United Kingdom Lymphoma Group, and the Groupe d'Etude des Lymphomes de l'Adulte have conducted a trial to ascertain whether the addition of chlorambucil is of benefit after anti–H pylori therapy. At the last interim analysis, 105 (55%) of 189 patients had achieved a complete histologic remission after anti-Helicobactertherapy. To further assess the ability of treatment to eradicate the lymphoma clone, we analyzed the gastric biopsies from a subset of the patients by polymerase chain reaction (PCR) targeted to the immunoglobulin heavy chain genes as a molecular marker for minimal residual disease. Sixty-two cases were examined at diagnosis. Fifty-four cases were monoclonal by PCR. Forty-two of these patients achieved histologic complete remission (hCR) after anti-Helicobacter treatment: 34 cases underwent molecular follow-up analysis. Fifteen patients (44%) were in molecular remission with a median follow-up of 2 years after antibiotic treatment and of 1 year after the achievement of hCR. Less than half of the patients with MALT lymphoma can achieve sustained molecular remission after anti-Helicobacter therapy. The presence of molecular disease in the absence of histologic disease does not appear to be associated with histologic relapse, but, given the indolent nature of MALT lymphomas, a longer follow-up is needed.
Introduction
Localized gastric marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT)–type is an indolent disease with characteristic features.1,2 Gastric MALT lymphoma is usually preceded by the occurrence of the Helicobacter pylori–associated chronic gastritis, and it can regress after antibiotic treatment. It is still not known whether antibiotics alone might induce definitive cure of the lymphoma. The International Extranodal Lymphoma Study Group (IELSG) and the United Kingdom Lymphoma Group (UKLG), together with the Groupe d'Etude des Lymphomes de l'Adulte (GELA), have conducted a trial to ascertain whether the addition of chlorambucil is of benefit after anti–H pyloritherapy. At the last interim analysis, 105 (55%) of 189 patients had achieved a complete histologic remission after antibiotics, and 15 lymphoma histologic relapses were observed in the total group.3
To further assess the ability of treatment to eradicate the lymphoma clone, we analyzed the gastric biopsies from a subset of the patients by polymerase chain reaction (PCR) targeted to the immunoglobulin heavy chain (IgH) genes.
Patients, materials, and methods
Patients with stage I primary gastric low-grade MALT lymphoma were eligible. Histologic diagnosis was made according to the criteria described in Isaacson and Norton4 and in the REAL/WHO classification.5 6H pylori infection was assessed at histologic examination (Giemsa stain or other specific methods) or by serology (presence of immunoglobulin G anti–H pylori antibodies). Baseline evaluations included patient history, physical examination, routine laboratory tests, chest X-ray, computed tomography scan of abdomen and pelvis, bone marrow aspirate and biopsy, and gastric endoscopy with multiple biopsies. Patients were treated with one of the following anti-Helicobacter regimens: omeprazole 20 mg daily + clarithromycin 250 mg twice daily + tinidazole 500 mg twice daily for 1 week, omeprazole (40 mg twice daily for 2 weeks) + amoxicillin (1 g 3 times daily for 2 weeks) + clarithromycin (250 mg 3 times daily for 2 weeks) or 2 weeks' treatment with colloidal bismuth (120 mg 4 times daily) + metronidazole (400 mg 3 times daily) + tetracycline (500 mg 4 times daily) or amoxicillin (500 mg 4 times daily). Endoscopy was repeated 2 to 3 months after treatment for H pyloriinfection. If the latter had been eradicated, a further 4 to 6 months had to elapse before assessment of tumor response by endoscopy. Persistent H pylori infection was retreated with further antibiotic treatment, with a maximum of 3 courses being given.
Patients were registered at the time of diagnosis, and, when in complete remission, were assigned randomly to observation or to chlorambucil (6 mg/m2 daily orally for 14 days repeated every 28 days for 6 cycles). The trial had been approved by local ethics committees, and written informed consent was obtained from all the enrolled patients. Follow-up endoscopies had to be performed at 6-month intervals for the first 2 years, thereafter at yearly intervals. At least 8 gastric biopsies had to be performed during follow-up endoscopy.
Histologic examination of follow-up biopsies was performed by applying the score proposed by Wotherspoon et al.7 Score 0-2 was defined as histologic complete response (hCR), histologic score 3 as partial response, and score 4-5 as persistent lymphoma. A panel of pathologists reviewed all biopsies. Patients who failed to achieve hCR after eradication of H pylori could still be randomly assigned to observation or chlorambucil if the physician felt it was appropriate to do so. Otherwise, the nonresponders could be treated with chemotherapy (chlorambucil as first choice).
DNA extraction, consensus IgH PCR amplification, and allele-specific oligonucleotide PCR were performed as previously described.8 9 Briefly, at diagnosis, samples were analyzed with a combination of 2 different PCR assays to detect B-cell monoclonality as molecular marker for follow-up tests. The third complementarity-determining region was first amplified by seminested PCR using the FR3A primer for the conserved framework region-3 (FR3) segment of the variable region and the LJH and VLJH primers for the joining region. A seminested PCR using the FR2A consensus primer was then applied for the cases apparently polyclonal with FR3A-directed PCR.
PCR products were visualized on 10% and 7% nondenaturing polyacrylamide gels for FR3A and FR2A amplicons, respectively. The presence of a distinct single band was considered diagnostic for monoclonality, and the presence of a smear of amplified products was considered diagnostic for polyclonality. In case of no PCR products, samples were assessed for the presence of amplifiable DNA with a PCR using primers for the beta-globin gene. We defined molecular complete remission (mCR) as the disappearance, in the DNA extracted from follow-up samples in hCR, of the lymphoma monoclonal population present in the diagnostic sample. We defined molecular relapse as the reappearance, having achieved a mCR, of the lymphoma monoclonal band in a follow-up sample, still in hCR. Two different follow-up calculations were performed: from antibiotic treatment and starting from the achievement of hCR.
Results
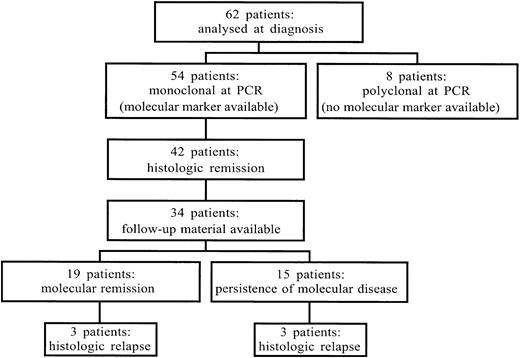
Sixty-two cases were analyzed at diagnosis (Figure1). Fifty-four (87%; 95% confidence interval [CI], 76%-94%) cases were monoclonal and 8 polyclonal by PCR for the IgH genes: 40 were FR3A+, 14 were FR3A−/FR2A+, and 8 were FR3A−/FR2A−. To assess the reason of false-negative FR3A result, the VDJ region was sequenced in the FR3A−/FR2A+ group of cases (Table1). Somatic mutations, or polymorphisms, were detected within the DNA sequence recognized by the FR3A primer in 10 (71%; 95% CI, 42%-92%) of 14. These data showed that the false polyclonal PCR results with FR3A alone may be due to somatic mutations within the VDJ regions that decrease the annealing ability of the consensus primer.
Flowchart showing the number of patients analyzed by PCR assay for the rearrangement of the IgH genes as molecular marker.
The 62 patients represent the group of cases among the patients enrolled in the study, whose diagnostic material had been sent for molecular assays. See text for further details.
Flowchart showing the number of patients analyzed by PCR assay for the rearrangement of the IgH genes as molecular marker.
The 62 patients represent the group of cases among the patients enrolled in the study, whose diagnostic material had been sent for molecular assays. See text for further details.
DNA sequence recognized by FR3A primer in 14 cases of mucosa-associated lymphomic tissue lymphomas, apparently polyclonal with FR3A/JH seminested polymerase chain reaction, but monoclonal with FR2A/JH
| Patient . | FR3A DNA sequence . |
|---|---|
| 2 | ACACGGCCGTATATTACTGT |
| 8 | ACACGGCTGTGTATTACTGT |
| 9 | ACACAGCTTTGTATTATTGT |
| 11 | AGATGGCGGGATATTGTAGT |
| 14 | ACACGGCCCTGTATTACTGT |
| 15 | ACACGGCCGTCTATTACTGT |
| 22 | ACACGGCAATCTACTACTGT |
| 26 | ACACGGCTGTATATTACTGT |
| 28 | ACACGGCCGTTTATTACTGT |
| 29 | ACACGGCCGTGTATTACTGT |
| 38 | ACACAGCCGTGTATTACTGT |
| 55 | ACACGGCTGTGTATTACTGT |
| 56 | ACACAGGCACATATTTTTGT |
| 67 | ACACGGCTGTGTATTATTGT |
| FR3A primer sequence | ACACGGCYSTGTATTACTGT* |
| Patient . | FR3A DNA sequence . |
|---|---|
| 2 | ACACGGCCGTATATTACTGT |
| 8 | ACACGGCTGTGTATTACTGT |
| 9 | ACACAGCTTTGTATTATTGT |
| 11 | AGATGGCGGGATATTGTAGT |
| 14 | ACACGGCCCTGTATTACTGT |
| 15 | ACACGGCCGTCTATTACTGT |
| 22 | ACACGGCAATCTACTACTGT |
| 26 | ACACGGCTGTATATTACTGT |
| 28 | ACACGGCCGTTTATTACTGT |
| 29 | ACACGGCCGTGTATTACTGT |
| 38 | ACACAGCCGTGTATTACTGT |
| 55 | ACACGGCTGTGTATTACTGT |
| 56 | ACACAGGCACATATTTTTGT |
| 67 | ACACGGCTGTGTATTATTGT |
| FR3A primer sequence | ACACGGCYSTGTATTACTGT* |
Bold fonts represent the somatic mutation present in lymphoma clone, discordant from FR3A primer sequence.
Nucleotide ambiguity codes: Y represents C or T, S represents C or G.
Forty-six (74%; 95% CI, 62%-84%) of the 62 cases achieved hCR. The rate of hCR was 77% (95% CI,64%-84%) in monoclonal and 50% (95% CI, 16%-84%) in polyclonal cases: 42 of 54 and 4 of 8, respectively.
Follow-up material for molecular biology assays was available in 34 cases among the 42 monoclonal cases that had achieved hCR. The molecular follow-up data obtained in these cases is shown in Figure2.
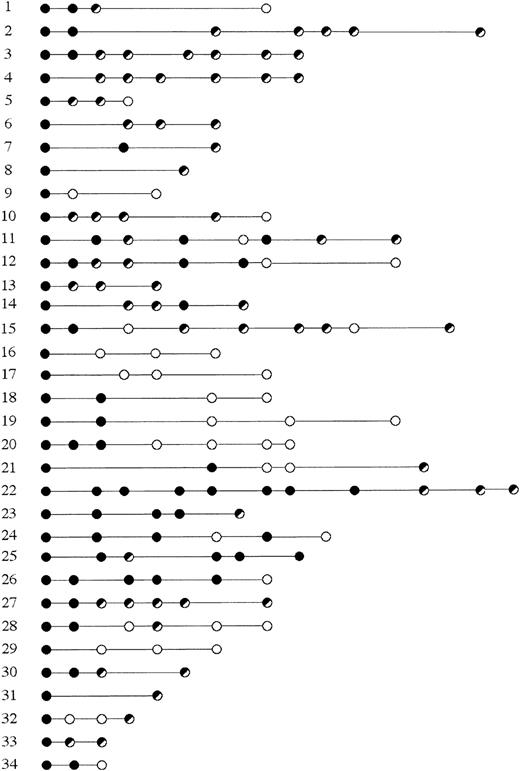
Molecular follow-up in 34 patients with MALT lymphoma.
Black circles represent histologic disease; white, mCR; black and white, persistent molecular disease in hCR. The follow-up is expressed in months; the first time point is at the diagnosis.
Molecular follow-up in 34 patients with MALT lymphoma.
Black circles represent histologic disease; white, mCR; black and white, persistent molecular disease in hCR. The follow-up is expressed in months; the first time point is at the diagnosis.
Fifteen (44%; 95% CI, 27%-62%) of the 34 patients failed to achieve mCR. Of the 19 (56%; 95% CI, 38%-73%) patients who achieved mCR, 16 had their mCR documented within 1 year after hCR, and 3 needed a longer period of time (up to 2 years) to convert their hCR into a molecular one. There were 6 molecular relapses, with 2 patients achieving a second mCR. Fifteen patients (44%; 95% CI, 27%-62%) were in mCR with a median follow-up of 2 years (6-72 months) after antibiotic treatment and of 1 year (0-48 months) after the achievement of hCR.
Five patients (15%; 95% CI, 5%-31%) experienced histologic relapses. Case 11 had a first histologic relapse in the presence of molecular disease, achieved a second remission (both histologic and molecular), followed by a second transient histologic relapse, which resolved again, but now with persistent molecular disease. Cases 12 and 14 showed a transient histologic relapse in the presence of molecular disease. In case 14, the relapse was followed by the persistence of the lymphoma clone. Case 24 had histologic relapses after mCR, then followed by a second mCR. Case 25 had persistent B-cell monoclonality. As a whole, there were 6 histologic relapses in 5 patients: 2 of them occurred in mCR, 4 in the presence of molecular disease. In all the cases, the lymphoma at relapse showed the same IgH monoclonal band detected by PCR at the time of diagnosis.
Discussion
The indolent nature of the disease in most cases of MALT lymphoma permits a conservative approach with antibiotic therapy, as the sole initial treatment. It is still not clear if antibiotic therapy alone can achieve complete lymphoma eradication. Thus, the IELSG, the GELA, and the UKLG have conducted a trial to determine whether the addition of chlorambucil is of benefit after anti–H pylori therapy. The aim of this preliminary report on the molecular follow-up is not to analyze the effect of chlorambucil after antibiotic treatments but rather to determine the ability to correlate molecular residual disease to clinical status. We present the molecular follow-up data obtained in a series of patients enrolled in the trial. Of the patients treated with anti–H pylori therapy, 74% achieved hCR, and 56% of these patients had a mCR; 44% were still in mCR at the last follow-up biopsy. Our data are similar to those reported by other groups.10-12 After antibiotic treatment, it is common to observe the persistence of the lymphoma clone, even in the absence of histologic disease. Thiede et al11 found that the persistent monoclonality might be due to aggregates of morphologically unsuspicious lymphocytes in the basal gastric mucosa. The relevance of these cells bearing the same immunoglobulin rearrangement of the lymphoma clone is not yet clear. Monoclonal populations have been described in other situations with apparently no increased risk of relapse or tumor occurrence. B-cell clones bearing the lymphoma-associated t(14;18)(q32;q21) can be detected in patients apparently cured for localized follicular lymphoma,13 and tumor-associated translocations can be detected, even if at low levels, in healthy individuals.14-16 Monoclonal B-cell populations can be seen in benign H pylori–associated chronic gastritis, Sjögren syndrome, and Hashimoto thyroiditis.17 However, in all of the study patients the clonal IgH rearrangement is initially detected from the malignant B cells, and, as such, it may be regarded that the subsequent clonal detection represents a marker from the initial malignant cells and, thus, representative of minimal residual disease. Because the follow-up of all published studies in MALT lymphoma is short, due to the indolent natural history of the localized forms, it is not possible to rule out a higher risk of relapse in cases that fail to achieve mCR.
Two of the 6 histologic relapses observed occurred despite the previous achievement of an mCR. This finding might be explained by unrepresentative biopsy samples, resulting in false-negative histologic and molecular results. We agree with Thiede et al11 who suggested 2 consecutive examinations are required before stating that a patient is in complete remission to avoid misinterpretation.
In conclusion, the molecular follow-up analysis in gastric MALT lymphoma reveals the persistence of the malignant clone in more than half of the cases in hCR after antibiotic treatments. The significance of this finding is still unclear. It is likely that the addition of further genetic tests, such as the demonstration of the t(11;18) or nuclear localization of the BCL10 protein,18 might improve our understanding of the response of MALT lymphoma clone to antibiotic treatment. Improved clinical and histologic criteria in assessing the response to antibiotics and further follow-up and monitoring of additional cases will define the significance of the persistent B-cell monoclonality occurring in hCR and the role of chlorambucil after antibiotic treatment in eradicating the lymphoma clone.
We thank Miss Michela Gisi for her expert technical assistance.
Supported by grants from the Swiss National Science Foundation (No. 32-45993.95) and from the Schweizerische Krebsliga/Krebsforschung Schweiz (No. AKT 623).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Francesco Bertoni, International Extranodal Lymphoma Study Group (IELSG), Oncology Institute of Southern Switzerland, Ospedale San Giovanni, 6500 Bellinzona, Switzerland; e-mail: frbertoni@tin.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal