Epstein-Barr virus (EBV)–associated posttransplantation lymphoproliferative disorders (PTLDs) are a well-recognized complication of immunosuppression in solid organ transplant recipients. The reported therapeutic approaches are frequently complicated by rejection, toxicity, and other infectious pathologies, and overall mortality in patients with unresponsive PTLD remains high. Thus, low-toxicity treatment options or, preferably, some form of prophylactic/preemptive intervention are warranted to improve PTLD outcome in this setting. We assessed whether transfer of EBV-specific cytotoxic T lymphocytes (CTLs) generated in vitro from the peripheral blood of allograft recipients receiving immunosuppression could increase EBV-specific killing in vivo without augmenting the probability of graft rejection. Autologous EBV-specific CTLs were generated for 23 patients who were identified as being at risk of developing PTLD through the finding of elevated EBV DNA load. Of the 23 patients, 7 received 1 to 5 infusions of EBV-specific CTLs. CTL transfer was well tolerated, and none of the patients showed any evidence of rejection. An increase of the EBV-specific cytotoxicity was observed after infusion, notwithstanding continuation of immunosuppressive therapy. EBV DNA levels had a 1.5- to 3-log decrease in 5 patients, whereas in the other 2 graft recipients CTL transfer had no apparent stable effect on EBV load. Our data suggest that the infusion of autologous EBV-specific CTLs obtained from peripheral blood mononuclear cells recovered at the time of viral reactivation is able to augment virus-specific immune response and to reduce viral load in organ transplant recipients. This approach may, therefore, be safely used as prophylaxis of EBV-related lymphoproliferative disorders in these patients, following a strategy of preemptive therapy guided by EBV DNA levels.
Introduction
Posttransplantation lymphoproliferative disorder (PTLD) is a potentially lethal complication of immunosuppression in solid organ transplant (SOT) and bone marrow transplant (BMT) recipients,1-3 mostly associated with the proliferation of Epstein-Barr virus (EBV)–infected B cells whose expansion in immunocompetent individuals is controlled by cytotoxic T lymphocytes (CTLs).4 There is ample evidence that an adoptive immunotherapy approach with transfer of lymphocytes or EBV-specific CTLs of donor origin is effective as prophylaxis and/or treatment of EBV-related PTLD in recipients of T-cell–depleted BMT5-8; data have shown that anti-CD20 monoclonal antibody can be an equally useful therapeutic strategy when donor-derived EBV CTLs are not available.9 10
Conversely, treatment of PTLD in patients receiving organ allografts who fail to respond to reduction or discontinuation of immunosuppression,11-13 is still controversial. Antiviral drugs,12 α interferon,12-14 anti–B-cell monoclonal antibodies,12,15 and chemotherapy13,16,17 have all been used and reported to induce remission in selected cases, and, as described for BMT recipients, also in the setting of SOT the use of anti-CD20 has proved effective in resolving established PTLD.18 Notwithstanding the 50% to 85% remission rate attained by the reported therapeutic approaches, frequent complications such as rejection, toxicity, and other infectious pathologies heavily compromise graft and host survival; moreover, overall mortality in patients with unresponsive PTLD remains high.
Therefore, alternative low-toxicity treatment options or, preferably, some form of prophylactic/preemptive intervention are warranted to improve PTLD outcome after organ transplantation. Application of a cellular immunotherapy approach similar to that used in BMT recipients is an appealing strategy, as it has been demonstrated that high levels of EBV DNA in peripheral blood predict development of PTLD also in organ transplant recipients.19,20 However, PTLD in the latter setting are mainly of recipient origin,21 and an HLA-matched donor is usually unavailable.22 The choice of HLA-haploidentical donor-derived virus-specific CTLs has proved only transiently successful in inducing remission of PTLD after organ transplantation,23 possibly because of a rapid immunologic clearance of CTLs secondary to allorecognition.
For these reasons, the use of autologous CTLs is probably a better option in this setting. It has been shown that autologous CTLs generated from pretransplant blood samples of SOT recipients are effective in controlling EBV replication and enhancing EBV-specific cellular responses after transplantation.24 More importantly, it has been demonstrated that EBV-specific cellular memory response can be expanded in vitro from peripheral blood samples of SOT recipients even when they are receiving immunosuppressive therapy and that CTLs thus reactivated display effective antiviral activity and have therapeutic potential against PTLD.25 26 This strategy has the advantage of allowing treatment of recipients who were seronegative before transplantation and, thus, at greater risk of developing PTLD after primary EBV infection in the posttransplantation period.
Two open questions about the use of autologous EBV-specific CTLs for the treatment of PTLD in allograft recipients concern the possibility of maintaining long-term therapeutic levels of CTLs23,26 and the well-described potential danger of inducing massive inflammatory reactions in the affected organ in patients with bulky disease.8 26 In particular, the reported dramatic systemic adverse effects of CTL expansion and activity in the case of bulky disease could be lessened or, at best, avoided by a preemptive strategy based on single or repeated CTL infusion on the finding of EBV-polymerase chain reaction (PCR) positivity and subsequent monitoring of viral load in blood and EBV-specific cellular responses.
Therefore, we assessed the feasibility of generating autologous EBV-specific CTLs from the peripheral blood of organ transplantation patients receiving in vivo immunosuppression for prevention of graft rejection and proceeded to verify whether transfer of recipient's virus-specific CTLs in this setting could increase in vivo EBV-specific killing and cellular immune responses without consistently increasing the probability of graft rejection.
Patients, materials, and methods
Patients
Approval for this study was obtained from the Ethical Committee at IRCCS Policlinico S. Matteo, Pavia, Italy, and patients or guardians gave written informed consent before entry into the study. Asymptomatic SOT recipients found positive for 1000 genome copies of EBV DNA/105 on 2 sequential occasions, values considered being predictive for a high risk of developing symptomatic EBV infection,19 were enrolled into the study. Sixty patients fulfilled these criteria, and peripheral blood samples were collected to generate EBV-transformed B-lymphoblastoid cell lines (EBV-LCLs) to serve as stimulators to reactivate EBV-specific CTLs.
EBV CTL lines were reactivated from 23 EBV-seropositive allograft recipients, and 7 were subsequently infused. Of these 7 patients, 3 were adult men who were given heart transplants, whereas 4 were children, 2 boys and 2 girls, who received heart (n = 2), liver (n = 1), and kidney (n = 1) allografts. Other clinical features of the 7 cases are summarized in Table 1.
Characteristics of the 7 transplant recipients treated with Epstein-Barr virus-specific cytotoxic T-lymphocyte lines
| Patient . | Sex/age . | Treatment . | Interval treatment/EBV DNA positivity (y) . | Immunosuppression at time of cell harvest and infusion . | Clinical effects of CTL infusion(s) . |
|---|---|---|---|---|---|
| 1 | M/60 | Heart | 9 | CsA (1.5 mg/Kg/d) | EBV DNA fell below detectable levels |
| No evidence of rejection | |||||
| 2 | M/51 | Heart | 3 | FK506 (0.06 mg/Kg/d)* | EBV DNA fell below detectable levels |
| No evidence of rejection | |||||
| 3 | M/58 | Heart | 0.5 | CsA (4 mg/Kg/d) | EBV DNA reduced > 2 logs |
| Steroids (0.06-0.15 mg/Kg/d) | No evidence of rejection | ||||
| 4 | M/7 | Heart | 1 | CsA (10 mg/Kg/d) | EBV DNA levels still fluctuating |
| Azathioprine (0.7 mg/Kg/d) | No evidence of rejection | ||||
| 5 | F/7 | Heart | 5 | CsA (7 mg/Kg/d) | EBV DNA levels reduced > 2 logs |
| No evidence of rejection | |||||
| 6 | F/4 | Liver | 1 | CsA (4 mg/Kg/d) | EBV DNA levels still fluctuating |
| Steroids (0.4 mg/Kg every other d) | No evidence of rejection | ||||
| 7 | M/5 | Kidney | 2 | CsA (5.8 mg/Kg/d) | EBV DNA levels reduced > 2 logs |
| Steroids (0.2 mg/Kg/d) | No evidence of rejection |
| Patient . | Sex/age . | Treatment . | Interval treatment/EBV DNA positivity (y) . | Immunosuppression at time of cell harvest and infusion . | Clinical effects of CTL infusion(s) . |
|---|---|---|---|---|---|
| 1 | M/60 | Heart | 9 | CsA (1.5 mg/Kg/d) | EBV DNA fell below detectable levels |
| No evidence of rejection | |||||
| 2 | M/51 | Heart | 3 | FK506 (0.06 mg/Kg/d)* | EBV DNA fell below detectable levels |
| No evidence of rejection | |||||
| 3 | M/58 | Heart | 0.5 | CsA (4 mg/Kg/d) | EBV DNA reduced > 2 logs |
| Steroids (0.06-0.15 mg/Kg/d) | No evidence of rejection | ||||
| 4 | M/7 | Heart | 1 | CsA (10 mg/Kg/d) | EBV DNA levels still fluctuating |
| Azathioprine (0.7 mg/Kg/d) | No evidence of rejection | ||||
| 5 | F/7 | Heart | 5 | CsA (7 mg/Kg/d) | EBV DNA levels reduced > 2 logs |
| No evidence of rejection | |||||
| 6 | F/4 | Liver | 1 | CsA (4 mg/Kg/d) | EBV DNA levels still fluctuating |
| Steroids (0.4 mg/Kg every other d) | No evidence of rejection | ||||
| 7 | M/5 | Kidney | 2 | CsA (5.8 mg/Kg/d) | EBV DNA levels reduced > 2 logs |
| Steroids (0.2 mg/Kg/d) | No evidence of rejection |
EBV, Epstein-Barr virus; CTL, cytotoxic T lymphocyte; CsA, cyclosporin A.
This patient had FK506 blood levels around 9 ng/mL.
Harvest and isolation of peripheral blood mononuclear cells
Peripheral blood samples were collected from the patients at the time of enrollment. Part of the harvested peripheral blood mononuclear cells (PBMCs) was used to generate EBV-induced B-lymphoblastoid cell line (B-LCL), and the remaining cells were cryopreserved for later use to activate CTLs. In the case of insufficient recovery, a second sample for CTL reactivation was obtained once B-LCLs had expanded.
PBMCs were isolated by Ficoll-Hypaque density gradient centrifugation, resuspended in RPMI 1640 medium (Gibco, Paisley, Scotland) supplemented with 2 mM L-glutamine and 10% heat-inactivated fetal calf serum (FCS; RPMI-FCS; Hyclone, Logan, UT), and used fresh or cryopreserved.
Detection of EBV DNA
EBV DNA was quantified by using an originally developed quantitative PCR.19 Briefly, PBMC samples were amplified in the presence of an internal reaction control and in parallel with serial dilutions of external standards (consisting of EBVEBNA1 gene PCR target sequence cloned in PCR 2000 plasmid). Gel densitometry was then used to quantitate PCR signals and to generate a standard curve for calculation of EBV DNA copy number.
B-LCL production
PBMCs were incubated with EBV-containing supernatant from the B95-8 cell line (American Type Culture Collection, Rockville, MD) in the presence of 800 ng/mL cyclosporin A (Sandoz Pharmaceuticals, Basel, Switzerland) in RPMI-FCS. Cells were continuously cultured for 3 to 4 weeks, following a protocol previously described.6
Preparation and administration of EBV-specific T-cell lines
EBV-specific CTLs were reactivated and expanded in vitro according to a method previously reported for preparation of BMT donor-derived T-cell lines, following general manufacturing practice standard procedures.6 In summary, EBV-specific CTLs were prepared from fresh or frozen PBMCs, plated in 2 mL RPMI-FCS at 2 × 106 cells per well, and stimulated with irradiated autologous B-LCL at a responder-to-stimulator (R:S) ratio of 40:1. After 10 days, cultures were restimulated with irradiated autologous B-LCL at a R:S ratio of 4:1. Starting on day 14, 20 U/mL recombinant interleukin 2 (rIL-2; Hoffman-La Roche, Basel, Switzerland) was added to the wells, and the cultures were subsequently restimulated weekly with irradiated autologous B-LCL in the presence of rIL-2. Before cryopreservation, T cells were examined for EBV specificity in a standard 51Cr-release assay against a panel of targets, including recipient B-LCL and phytohemagglutinin (PHA) blasts, HLA-mismatched allogeneic B-LCL and PHA blasts, and the lymphokine-activated killer–permissive Daudi cell line. Cell lines were also evaluated for immunophenotype and for sterility. Samples with satisfactory test results were thawed and administered intravenously to patients. The patients received a first dose of 2 × 107CTL/m2, and subsequent dose schedule was decided on the basis of clinical evaluation, EBV PCR levels, and immunologic follow-up.
The introduction of new regulations in the culture of cellular products for in vivo use since the beginning of the study prompted us to verify the feasibility of modifying the culture conditions at first use. These further studies provided evidence that the procedure can be performed by using X-VIVO 20 medium (BioWhittaker, Walkersville, MD) with 2% autologous plasma, and EBV CTL lines are now activated and expanded with the modified protocol.
Flow cytometry
Monoclonal antibodies used to characterize cultured cells were CD3 (anti-Leu-4) fluorescein isothiocyanate (FITC), anti–HLA-DR phycoerythrin (PE), CD8 (anti-Leu-2a) FITC and PE, CD56 (anti-Leu-19) PE, anti-TCRγδ FITC, CD4 (anti-Leu-3a) PE, CD19 (anti-Leu-12) FITC, CD20 (anti-Leu-16) PE, and CD45 (anti-HLe-1) FITC (Becton Dickinson, Mountain View, CA). Appropriate isotype-matched controls were included. Cytofluorimetric analysis was performed by means of direct immunofluorescence on a FACScan flow cytometer (Becton Dickinson).
Cytotoxicity assay
Cytotoxic activity was measured as previously described.27 Spontaneous release from the target cells was consistently less than 25%. Results were expressed as the percentage of specific lysis.
Limiting dilution assay for evaluation of EBV-specific cytotoxic T-cell precursors
To evaluate cytotoxic T-cell precursor (CTLp) frequency to EBV, responder cells were stimulated with autologous irradiated B-LCL according to a limiting dilution assay partially derived from a previously described method.28 Briefly, 24 replicates of decreasing numbers (8 × 104, 6 × 104, 4 × 104, 2 × 104, 104, 5 × 103, and 2.5 × 103) of cryopreserved PBMCs used as responder cells were seeded in 96-well round-bottom microplates in a final volume of 200 μL RPMI-FCS in the presence of 104 autologous irradiated (7000 rads) B-LCL. On days 7 and 14, the cultures were restimulated with 104 autologous irradiated B-LCL. On day 21, the plates were split and tested against autologous or HLA-mismatched B-LCL in a standard cytotoxicity assay.
Calculation of CTLp frequency
Assay wells were defined as positive when 51Cr release exceeded the mean + 3 SD of control wells. The frequency of responding cells was determined by maximum likelihood estimation, using a statistical program, and the variance by the use of 95% confidence limits.
Results
Generation of autologous EBV-specific CTL lines
The most compelling issue in designing an immunotherapy protocol to prevent or treat EBV-related complications in SOT patients is to assess the probability of reactivating in vitro an EBV-specific CTL response from the PBMCs of these immunosuppressed hosts. In particular, it is important to understand whether most graft recipients are able to mount a memory EBV CTL response in vitro, and whether this response bears the characteristics observed in healthy donors.
To address these points, autologous EBV-specific CTL lines were generated at our institution for SOT recipients who were identified as being at risk of developing EBV-related PTLD through the finding of elevated numbers of EBV DNA genome copies on 2 or more consecutive samples. EBV-LCLs were initiated from PBMCs of the 60 graft recipients enrolled in the study. We succeeded in expanding and cryopreserving EBV-LCLs for 55 of the 60 patients, whereas in 5 cases initial growth was followed by rapid apoptosis and loss of the EBV-transformed lines. We then proceeded to activate autologous EBV-specific CTL lines in the first 23 of the 55 patients whose LCLs had expanded, using the method described by Rooney et al6,8 for BMT donors. CTL lines were successfully generated in all 23 patients. Phenotypic analysis indicated that the 23 lines were 90% ± 8% CD3+, 60% ± 21% CD8+, and 30% ± 23% CD4+ and contained 10% ± 8% cells that were CD3+/CD8+/CD56+ and 9% ± 9% cells of natural killer phenotype (CD56+/CD3−). EBV specificity of the CTL lines was indicated by the fact that all CTL lines showed strong lysis of the autologous EBV-LCL, whereas little or no reactivity was observed against HLA-mismatched LCLs, autologous PHA blasts, allogeneic HLA-mismatched PHA blasts, and Daudi cell line. Moreover, antibody inhibition studies showed that cytotoxicity was mostly class I HLA-restricted (excluding the case of a CTL line that was more than 70% CD4+ and showed a class II HLA-restriction). Table2 and Table3 report phenotype and cytotoxicity data obtained from 7 CTL lines subsequently infused to patients. These data were little different to those described for healthy donors by other groups6 8 and in EBV CTLs generated from a group of 20 BMT donors at our institution; the exception being that in the organ graft recipient group 17% of the lines contained more than 70% CD4+ cells (versus 5% observed in the donors) and 30% of the CTLs showed a high percentage (> 20%) of T-cell receptor γδ+ cells (versus 0% found in the donors; data not shown).
Phenotypic analysis of the infused Epstein-Barr virus-specific cytotoxic T-lymphocyte lines
| Patient . | CD3+ . | CD8+ . | CD4+ . | γδ+ . | CD56+/CD3− . | CD3+/CD8+/CD56+ . |
|---|---|---|---|---|---|---|
| 1 | 82 | 80 | 4 | 3 | 10 | 6 |
| 2 | 90 | 84 | 7 | 33 | 8 | 10 |
| 3 | 98 | 84 | 22 | 2 | 2 | 13 |
| 4 | 92 | 22 | 70 | 8 | 5 | 5 |
| 5 | 99 | 96 | 4 | 1 | 1 | 39 |
| 6 | 98 | 8 | 90 | 0 | 2 | 4 |
| 7 | 68 | 40 | 28 | 20 | 28 | 21 |
| Patient . | CD3+ . | CD8+ . | CD4+ . | γδ+ . | CD56+/CD3− . | CD3+/CD8+/CD56+ . |
|---|---|---|---|---|---|---|
| 1 | 82 | 80 | 4 | 3 | 10 | 6 |
| 2 | 90 | 84 | 7 | 33 | 8 | 10 |
| 3 | 98 | 84 | 22 | 2 | 2 | 13 |
| 4 | 92 | 22 | 70 | 8 | 5 | 5 |
| 5 | 99 | 96 | 4 | 1 | 1 | 39 |
| 6 | 98 | 8 | 90 | 0 | 2 | 4 |
| 7 | 68 | 40 | 28 | 20 | 28 | 21 |
Data are reported as the percentage of positive cells.
Cytotoxic activity of the infused Epstein-Barr virus-specific cytotoxic T-lymphocyte lines
| Patient . | Auto B-LCL . | Mismatched B-LCL . | Auto B-LCL + α class I . | Auto B-LCL + α class II . | Daudi cells . | Auto B-LCL CD4−effectors . | Auto B-LCL CD8− effectors . |
|---|---|---|---|---|---|---|---|
| 1 | 43 | 16 | 19 | 46 | ND | ND | ND |
| 2 | 49 | 18 | 21 | 51 | ND | ND | ND |
| 3 | 79 | 11 | 19 | ND | ND | ND | ND |
| 4 | 53 | 7 | 21 | 50 | ND | 45 | 263-150 |
| 5 | 35 | 9 | 8 | ND | 15 | ND | ND |
| 6 | 40 | 4 | 14 | 30 | ND | 22 | 35 |
| 7 | 36 | 14 | ND | ND | 10 | ND | ND |
| Patient . | Auto B-LCL . | Mismatched B-LCL . | Auto B-LCL + α class I . | Auto B-LCL + α class II . | Daudi cells . | Auto B-LCL CD4−effectors . | Auto B-LCL CD8− effectors . |
|---|---|---|---|---|---|---|---|
| 1 | 43 | 16 | 19 | 46 | ND | ND | ND |
| 2 | 49 | 18 | 21 | 51 | ND | ND | ND |
| 3 | 79 | 11 | 19 | ND | ND | ND | ND |
| 4 | 53 | 7 | 21 | 50 | ND | 45 | 263-150 |
| 5 | 35 | 9 | 8 | ND | 15 | ND | ND |
| 6 | 40 | 4 | 14 | 30 | ND | 22 | 35 |
| 7 | 36 | 14 | ND | ND | 10 | ND | ND |
Data are reported as the percentage of specific lysis at an effector-to-target ratio of 10:1. B-LCL, B-lymphoblastoid cell line; ND, not done.
A residual population of CD8dim + cells was present in the CD8− fraction.
Effect of CTL transfer on EBV DNA load
Of the 23 EBV-specific CTL lines activated in vitro, 7 were administered to patients on detection of persistent high levels of EBV DNA. Their phenotype analysis and specificity characteristics are reported in Tables 2 and 3.
No modification of immunosuppressive therapy was introduced between the time of cell harvest, CTL generation, and the time before or during CTL infusion with the exception of those described later for patient 3.
Autologous EBV-specific CTL infusions were well tolerated in all patients, as no toxic effects could be attributed to the T-cell therapy. None of the patients had clinical signs of graft rejection, and organ biopsies, when performed, showed no evidence of rejection after CTL infusions.
The patients received an initial infusion of 2 × 107/m2 CTLs, and 2 to 4 subsequent doses were administered on the basis of EBV DNA follow-up and after having assessed the absence of signs of rejection. In patients 1 and 2, infusion of a single CTL dose was followed by a rapid decrease of EBV DNA titers to reach levels below the detection threshold within 1 to 8 weeks from CTL therapy (Figure 1A). The EBV load remained undetectable during the 46-month follow-up period in both patients. Figure 2B shows data from patients 3, 5, and 7. In patients 5 and 7, the first CTL infusion did not produce a stable decrease of EBV load, and, therefore, further CTL doses (3 and 2, respectively) were administered. In these 2 recipients, EBV DNA levels showed a stable 1- to 1.5-log decrease, and during the 26 and 19 months of follow-up EBV load remained well below the values observed before CTL infusion.
Sequential analysis of EBV DNA levels in the peripheral blood of the 7 patients treated with EBV-CTL lines.
(A) This panel shows EVB DNA dynamics in patients 1 (□) and 2 (▴), (B) refers to patients 3 (●), 5 (▵), and 7 (■); and (C) refers to patients 4 (□) and 6 (▴). Arrows indicate CTL infusions.
Sequential analysis of EBV DNA levels in the peripheral blood of the 7 patients treated with EBV-CTL lines.
(A) This panel shows EVB DNA dynamics in patients 1 (□) and 2 (▴), (B) refers to patients 3 (●), 5 (▵), and 7 (■); and (C) refers to patients 4 (□) and 6 (▴). Arrows indicate CTL infusions.
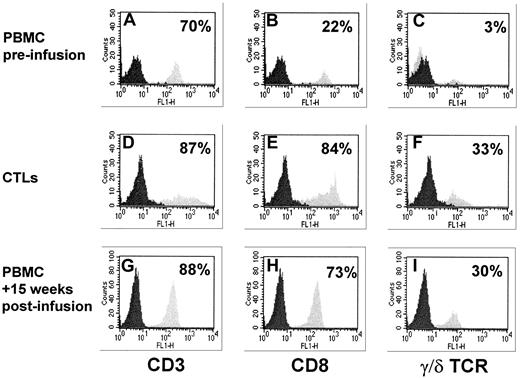
T-cell phenotype analysis in patient 2.
Results obtained from PBMCs collected at +15 weeks from infusion are compared with phenotype analysis performed on the transferred CTL line and on the patient's preinfusion PBMCs. (A,D,G) These panels refer to CD3+ cells, (B,E,H) to CD8+ subset, and (C,F,I) to T-cell receptor γδ+ population. An impressive peripheral expansion of CD8+ and T-cell receptor γδ+ T cells is observed in the patient after CTL infusion. Data are expressed as the percentage of positive cells.
T-cell phenotype analysis in patient 2.
Results obtained from PBMCs collected at +15 weeks from infusion are compared with phenotype analysis performed on the transferred CTL line and on the patient's preinfusion PBMCs. (A,D,G) These panels refer to CD3+ cells, (B,E,H) to CD8+ subset, and (C,F,I) to T-cell receptor γδ+ population. An impressive peripheral expansion of CD8+ and T-cell receptor γδ+ T cells is observed in the patient after CTL infusion. Data are expressed as the percentage of positive cells.
Patient 3 initially responded to the first CTL infusion with a decrease in EBV DNA levels. However, the patient received an increase in daily steroid dosage, and a new EBV DNA peak of 100 000 copies per 105 PBMCs was observed. Antiviral therapy was initiated with no effect on viral load. The infusion of 3 further CTL doses at weekly intervals succeeded in lowering the viral load by 2 logs, and DNA levels have now stabilized at 103 copies per 105 PBMCs notwithstanding continuation with the higher steroid schedule (Figure 1B).
Administration of EBV-CTLs in patient 6 resulted in a small and temporary decrease in EBV DNA titer, but new peaks occurred after the first infusion. Therefore, this patient received 2 additional CTL infusions, but each T-cell line transfer was equally ineffective in obtaining a stable decrease in EBV load (Figure 1C). Comparable poor results were also observed in patient 4, who received a total of 5 CTL infusions with no apparent consistent effect on EBV DNA levels (Figure1C).
All 7 patients remain alive and with no sign of EBV-PTLD 4 to 46 months (median, 25) after CTL infusion.
Effect of CTL transfer on T-cell immunity
To determine the effect of CTL infusions on specific immune reconstitution of treated patients, the pattern of CTL regeneration and CTL precursor frequency was evaluated on peripheral blood samples obtained after CTL transfer.
Measurement of CTLp frequency was performed in patients 4 to 7 (Table4). In preinfusion samples obtained from patient 5, EBV CTLp levels were detectable, although at values in the lower end of the normal range. CTLp levels increased after each transfer and maintained stable levels throughout the study period. In patients 4, 6, and 7, EBV CTLp levels were low to undetectable before immunotherapy. In patient 4, CTLp levels remained unchanged after the first infusion but increased after each subsequent transfer. However, in this patient, the precursor frequency gradually decreased between infusions. In patient 6, CTL transfer caused a 1.5-log increase in cytotoxic precursor frequency, and, although CTLp numbers showed a slight decrease between infusions, posttransfer levels remained high during the follow-up months.
Cytotoxic T-lymphocyte (CTL) precursor frequency in peripheral blood of patients before and after CTL infusion
| Patient . | Before infusion . | Post-I dose 2 wk . | Post-I dose 4 wk . | Post-II dose 2 wk . | Post-II dose 8 wk . | Post-III dose 2 wk . | Post-III dose 12 wk . |
|---|---|---|---|---|---|---|---|
| 4 | 1/37 658 | 1/40 689 | — | 1/22 094 | 1/27 075 | 1/20 787 | 1/22 276 |
| 5 | 1/15 764 | — | — | 1/10 069 | — | 1/6 243 | 1/6 209 |
| 6 | 1/832 786 | 1/23 845 | 1/28 210 | 1/30 454 | 1/28 230 | — | — |
| 7 | 1/64 040 | 1/42 785 | — | 1/45 067 | — | — | — |
| Patient . | Before infusion . | Post-I dose 2 wk . | Post-I dose 4 wk . | Post-II dose 2 wk . | Post-II dose 8 wk . | Post-III dose 2 wk . | Post-III dose 12 wk . |
|---|---|---|---|---|---|---|---|
| 4 | 1/37 658 | 1/40 689 | — | 1/22 094 | 1/27 075 | 1/20 787 | 1/22 276 |
| 5 | 1/15 764 | — | — | 1/10 069 | — | 1/6 243 | 1/6 209 |
| 6 | 1/832 786 | 1/23 845 | 1/28 210 | 1/30 454 | 1/28 230 | — | — |
| 7 | 1/64 040 | 1/42 785 | — | 1/45 067 | — | — | — |
Unfortunately, because of a lack of sufficient numbers of preinfusion PBMCs, evaluation of CTLp frequency could not be performed in patients 1 to 3. Nevertheless, data on CTL regeneration from postinfusion peripheral blood samples are available for patients 1 and 2. These data indicate that, after each EBV CTL transfer, an increase in EBV-specific lytic activity was observed, accompanied by a decrease in cytotoxicity directed against HLA-mismatched targets (data not shown).
A comparative phenotype analysis performed on the infused CTL line and on peripheral blood collected before and 4 months after infusion from patient 2 is shown in Figure 2. Although preinfusion peripheral blood phenotype showed only 22% CD8+ and 3% γδ+cells, PBMCs collected after infusion included 73% CD8+lymphocytes with 30% γδ+ cells. These values are very close to those observed in the original CTL line (84% CD8+lymphocytes with 33% γδ+ cells).
Discussion
Our experience is in line with previously reported preliminary data25,26 on the feasibility of reactivating and expanding autologous EBV-specific CTLs from samples of patients receiving immunosuppression after SOT. The strategy of using PBMCs obtained at the time of EBV DNA detection rather than before the graft24 makes a prophylactic/preemptive intervention more advantageous in this setting, as it removes the need for storage of mononuclear cells from candidates to organ transplantation before the procedure and extends applicability to patients who develop EBV-related complications many years from transplantation and are, therefore, not likely to have pretransplantation samples stored. The method used, derived from Rooney et al,6,8 allowed us to generate EBV CTLs with characteristics comparable to those observed in EBV-specific lines from healthy donors.8 Furthermore, the CTLs could be expanded to reach values higher than 108 in all cases.
Infusion of EBV-specific CD8+ CD4+ polyclonal T cells caused an increase of EBV-specific immunity in all evaluable patients, with stable decrease in viral load in 5 of 7 treated patients. Patient 3, who experienced an early increase of EBV load associated with the presence of fever and malaise and responded to each CTL infusion with a substantial reduction of EBV DNA levels, exemplifies the possible negative effects of steroids on CTL therapy. Because of the increased prednisone dosage, EBV load in this patient transiently reached a higher peak compared with preinfusion levels, probably because of inactivation or destruction of specific CTLp secondary to the action of the immunosuppressive agent. Nonetheless, the combination of antiviral therapy and repeated CTL infusions caused a decrease in viral load, which has remained below 104/105 cells notwithstanding maintenance of heavily immunosuppressive regimen. None of the patients showed evidence of graft rejection.
Patients 4 and 6, who did not respond to repeated CTL infusion with a stable decrease in EBV DNA levels, received T-cell lines with more than 70% CD4+ cells. In the case of patient 4, cytotoxicity assays showed that specific killing was mediated by the 20% CD8+ cell population included in the CTL line, whereas in patient 6 specific cytotoxicity was mediated by both CD8+and CD4+ cells. The low percentage of CD8+cells infused in the former patient and the presence of CD4+ cytotoxic populations, whose specific activity is less efficient, in the second case could be deemed responsible for the poor results in terms of reduction of viral load. In both these patients an increase in CTLp frequency was observed after each CTL infusion. One may argue that the combination of lower immunologic efficacy and persistent immunosuppression might cause a rapid CTLp decrease between infusions. However, although a tendency to decrease was indeed observed, EBV-specific CTLp levels in the follow-up period were constantly higher than those observed before immunotherapy. More importantly, neither patient, including patient 4 who consistently showed high levels of EBV DNA during the 30-month observation time, has developed PTLD. The achievement and maintenance of a higher “set point” for EBV-specific T-cell precursor frequency is likely an important endpoint for any prophylactic therapy to reduce the risk of PTLD imposed on these patients by their continuing high virus load.
Recent work may explain this discrepancy.29 30EBV-infected resting memory B cells, expressingLMP2A as the only viral latency gene, accumulate in the peripheral blood of immunosuppressed patients and are associated with high EBV DNA levels. Only very small numbers of proliferating infected lymphoblasts are normally present in the peripheral circulation of these individuals, whereas lytically infected cells account for the portion of viral DNA levels not ascribable to resting B cells in 50% of the patients. Infused CTLs may clear EBV-transformed B cells carrying the full array of latency antigens, and consequently prevent PTLD development, but can only recognize and kill EBV-infected resting B cells through the subdominant LMP2A-specific response. Hence, the residual EBV loads observed in our patients may indicate not cell therapy failure but rather reflects the different patterns of EBV CTL reactivation and antiviral immunologic surveillance. In some patients, the presence ofLMP2A-specific and/or lytic cycle–specific cells in the CTL line might control resting B cells carrying the antigen and lytically infected B cells, respectively; additionally, the in vivo expansion and cytokine production of transferred CTLs because of encounter with EBV might drive the expansion of endogenous cellular immune response directed against LMP2A and lytic cycle proteins, thus reaching the goal of a complete decline of EBV DNA levels.
The question of CTL duration in these immunosuppressed patients, central to the design of a prophylactic treatment schedule, remains open. Direct evidence of CTL persistence in the peripheral blood of these patients could not be obtained, as cells had not been marked before infusion. Yet, the sequential peripheral blood phenotype analysis performed in patient 2 was suggestive of an impressive in vivo expansion of infused CTLs that persisted for several months after infusion. An expansion of CD8+/CD56−lymphocyte subset was also observed for as long as 3 months after CTL infusion in peripheral blood samples collected from patient 1. These data are not conclusive, and further studies are awaited to define infusion requirement in SOT recipients.
In a general strategy of PTLD management, we favor a preemptive approach over the treatment of established disease, in the attempt to reduce the risk of therapy-related complications. As to the best strategy, at present the available preemptive options include temporal reduction of immune suppression, antiviral drugs, anti-CD20 monoclonal antibodies, and CTLs. Reduction of immunosuppression is associated with an augmented risk of graft rejection and can, therefore, be acceptable only in non–life-saving transplantations, whereas antiviral drugs may cause direct organ toxicity, rendering their use advisable only for a short time span. The use of anti-CD20 for prophylaxis is to be considered with caution, as administration may cause prolonged profound B-cell depletion and hypogammaglobulinemia that exacerbates immunodeficiency in transplantation patients.10,31Moreover, the repeated use may select a population of CD20− EBV-transformed proliferating B cells, thus rendering its subsequent use ineffective.32 The toxicity problems described in association with CTL therapy refer mainly to the possibility of inducing massive inflammatory reactions in the presence of bulky disease.8 26 The theoretical concern of causing graft rejection because of the presence of residual alloreactive populations in the cell lines has not been confirmed, as indicated also by our present experience.
Overall, our findings suggest that the infusion of autologous EBV-specific CTLs obtained from PBMCs recovered at the time of viral reactivation augments virus-specific immune responses and reduces viral load in organ transplant recipients and can, therefore, be safely used as prophylaxis of EBV-related lymphoproliferative disorders in these patients, following a strategy of preemptive therapy guided by EBV DNA levels. The effectiveness of EBV CTL infusion in terms of PTLD prevention cannot be assessed in such a small cohort of patients. However, most of the untreated patients show persistently detectable EBV DNA levels, and the calculated incidence of PTLD in a historical control group of 74 SOT recipients with detectable EBV DNA levels was 8%. The positive predictive value of EBV DNA levels more than 1000 copies in the cohort of 120 studied patients was 64% for symptomatic EBV infection.19 A prospective randomized trial to compare CTL therapy with other prophylactic interventions is warranted to assess the real efficacy in terms of PTLD prevention and graft survival. On the basis of the issues discussed above, we propose to adopt, as cell therapy arm of the trial, a sequential CTL infusion approach.
We thank the patients who contributed; the Heart Transplant, Liver Transplant, and Kidney Transplant Units in Pavia, Bergamo, Milano, and Genova; and the Nord Italia Transplant program for their cooperation.
Supported in part by grants from the Associazione Italiana Ricerca sul Cancro (AIRC) to P.C., F.L., and R.M. and by grant RFM/97 from IRCCS Policlinico S. Matteo.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Patrizia Comoli, Laboratorio di Immunologia, Dipartimento di Scienze Pediatriche, IRCCS Policlinico S. Matteo, P.le Golgi 2, 27100 Pavia, Italia; e-mail: pcomoli@smatteo.pv.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal