Clinical trials of thrombopoietin (TPO), the central regulator of megakaryocytopoiesis, have revealed few side effects associated with its use. We here report a case of pancytopenia associated with the development of neutralizing antibodies to TPO that occurred in a patient who had undergone multicycle chemotherapy with multiple cycles of subcutaneous administration of pegylated recombinant human megakaryocyte growth and development factor. Samples of the patient's bone marrow showed trilineage hypoplasia with absence of myeloid, erythroid, and megakaryocyte progenitor cells but with elevated endogenous levels of erythropoietin, granulocyte colony-stimulating factor, and stem-cell factor. To our knowledge, this is the first report of an aplastic anemia–like syndrome associated with neutralizing antibodies to TPO.
Introduction
Two different forms of thrombopoietin (TPO) have entered clinical trials.1 One is a recombinant form of the native molecule (rhTPO) and the other a pegylated, truncated version (pegylated recombinant human megakaryocyte growth and development factor [PEG-rHuMGDF]) with biologic activity similar to that of the native molecule. Early studies showed that both rhTPO2 and PEG-rHuMGDF3 are potent stimulators of thrombopoiesis and enhance platelet recovery when given after chemotherapy.4,5 Both agents were reported to have minimal toxic effects; in particular, platelets produced after their administration function normally and have no evidence of activation.2,5,6 Although antibodies to TPO were observed in the initial study of rhTPO, they were nonneutralizing and transient.2 Here, we describe a case in which prolonged pancytopenia with neutralizing antibodies to TPO developed in a woman with ovarian cancer who had undergone 6 cycles of chemotherapy associated with administration of PEG-rHuMGDF.
Study design
Granulocyte-macrophage colony-forming cells, erythroid burst-forming units, and megakaryocyte colony-forming cells were assayed as described previously7-9 by using freshly obtained bone marrow cells. Serum from the patient (stored at −20°C) was incubated with growth factors for in vitro cultures at 37°C for 60 minutes and examined for its activity on normal bone marrow cells. The highest concentration of patient's serum used was 10% of the final culture volume.
Standard solid-phase sandwich enzyme immunoassays were used to measure serum levels of TPO,10 granulocyte colony-stimulating factor (G-CSF), and erythropoietin (EPO) (R&D Systems, Minneapolis, MN). Cytokine levels were calculated from a standard curve generated by analysis of recombinant cytokines. Stem-cell factor (SCF) was assayed as described previously.11 More than 200 samples of normal human serum were used to establish the SCF standard of 0.78 ± 0.25 ng/mL (range, 0.29-1.62 ng/mL).
Two established assays were used to detect neutralizing antibodies. The first was a solid-phase radioimmunoassay (RIA).12 Reactivity was determined on the basis of the ratio of counts per minute in the posttreatment sample to the counts per minute in the pretreatment (normal control) sample. A ratio of 2.0 or higher derived from 2 independent assays was considered to indicate reactivity. Reactive samples were titrated. The use of controls demonstrated specificity; for example, samples of antiserum to EPO, G-CSF, SCF, and keratinocyte growth factor (KGF) were unreactive and anti-TPO antiserum samples did not cross-react with EPO, G-CSF, SCF, or KGF. The second assay was a neutralizing-antibody bioassay using Mpl-transfected 32D cells. Again, specificity was demonstrated: there was no inhibition of control cultures.12 Serum samples were considered inhibitory if cell growth was less than 50% with 250 pg/mL TPO. Results were expressed as the titer of serum at which inhibition occurred.12 Both assays were done on all serum samples and on several separate occasions.
Results and discussion
A 58-year-old woman with stage 3 ovarian adenocarcinoma underwent debulking laparotomy in December 1997. Her past medical history included hypertension and depression, controlled with propanolol and alprazolam, respectively. In January 1998, she began treatment in a phase 1 trial of PEG-rHuMGDF (Amgen, Thousand Oaks, CA).13 At that time, blood counts and renal and hepatic function were normal, and her performance status was 0 according to Eastern Cooperative Oncology Group criteria. Neither RIA nor bioassay detected any antibodies to megakaryocyte growth and development factor (MGDF) or TPO.
A single dose of PEG-rHuMGDF (3 μg/kg of body weight; total, 200 μg) was given 7 days before the first dose of chemotherapy. The first cycle of chemotherapy (consisting of 600 mg/m2 of body-surface area of carboplatin [total, 980 mg] and 1200 mg/m2 cyclophosphamide [total, 1950 mg]) was followed by administration of filgrastim (5 μg/kg per day; Amgen) but not PEG-rHuMGDF. For cycles 2 to 6, the patient received the same chemotherapy regimen with filgrastim, along with PEG-rHuMGDF (5 μg/kg per day; total, 332 μg) for 3 days. Chemotherapy was given at 28-day intervals. There were no substantial delays in treatment; the last course was given 5 months after the first.
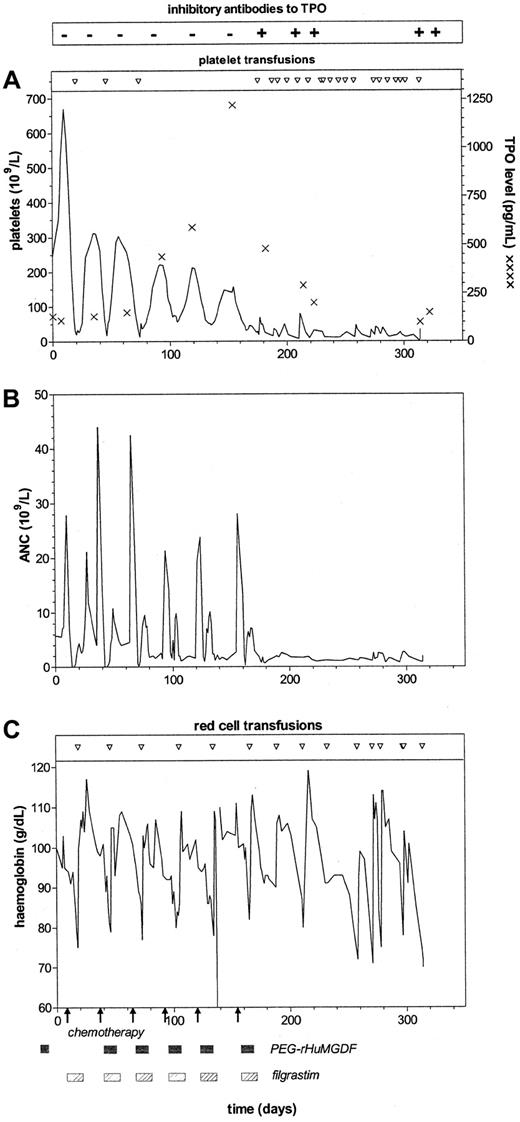
Blood counts were done at least every other day after chemotherapy until hematopoietic recovery and thereafter as clinically indicated. Platelets were transfused (5 units, random donor) when the platelet count was under 20 × 109/L, and red blood cells were given when the hemoglobin level was less than 90 g/L. The chemotherapy dose was reduced by 25% after the third cycle of chemotherapy because of fever with neutropenia and by an additional 25% after the fourth cycle because of thrombocytopenia. Transfusion of platelets was required after cycles 1, 2, 3, and 6; transfusion of red blood cells was necessary after every cycle. After the first 5 cycles of chemotherapy, blood counts became normal (Figure1). The results of RIA and bioassays were negative for antibodies to MGDF and TPO throughout the chemotherapy regimen.
Development of anti-TPO antibodies and association with thrombocytopenia, neutropenia, and anemia.
Shown is the development of anti-TPO antibodies in the patient in relation to administration of PEG-rHuMGDF (shaded boxes) with chemotherapy (arrows) and G-CSF. Inhibitory antibody to TPO was not detected by RIA or bioassay on 6 occasions (minus signs). Inhibitory antibody to TPO was detected by RIA on 5 occasions (plus signs) and also by bioassay on 4 of these. The first sample positive on RIA was negative on bioassay. In the 4 samples positive on bioassay, inhibitory antibody to TPO was detected at serum dilutions of 1:200, 1:100, 1:100, and 1:100. (A) Platelet count, platelet transfusion requirements, and serum TPO levels (asterisks). (B) ANC. (C) Hemoglobin levels and red blood cell transfusion requirements.
Development of anti-TPO antibodies and association with thrombocytopenia, neutropenia, and anemia.
Shown is the development of anti-TPO antibodies in the patient in relation to administration of PEG-rHuMGDF (shaded boxes) with chemotherapy (arrows) and G-CSF. Inhibitory antibody to TPO was not detected by RIA or bioassay on 6 occasions (minus signs). Inhibitory antibody to TPO was detected by RIA on 5 occasions (plus signs) and also by bioassay on 4 of these. The first sample positive on RIA was negative on bioassay. In the 4 samples positive on bioassay, inhibitory antibody to TPO was detected at serum dilutions of 1:200, 1:100, 1:100, and 1:100. (A) Platelet count, platelet transfusion requirements, and serum TPO levels (asterisks). (B) ANC. (C) Hemoglobin levels and red blood cell transfusion requirements.
After the sixth cycle of chemotherapy, platelet and hemoglobin levels failed to return to normal. Although the absolute neutrophil count (ANC) initially reached normal levels, it decreased to subnormal levels after filgrastim treatment was discontinued. At that time, neutralizing TPO antibodies were first detected by RIA, although bioassay results were negative. On 4 subsequent occasions, both RIA and bioassay indicated the presence of neutralizing antibodies (Figure 1). After the antibodies were detected, serum TPO levels fell despite persistent thrombocytopenia, and regular transfusions were required.
It was surprising that the thrombocytopenia was associated with neutropenia, anemia, and bone marrow changes of trilineage hypoplasia. Consistent with this finding, assays of bone marrow progenitor cells from the patient revealed an almost complete absence of erythroid, myeloid, and megakaryocytic precursors. The patient's serum was examined by using in vitro cultures with normal bone marrow cells as the target. Rather than inhibiting the growth of bone marrow progenitor cells, the serum dramatically stimulated the growth of myeloid and erythroid colonies (Table 1). Specific assays confirmed elevated levels of EPO (723.9 arb U/L; normal, 3.3-16.6 arb U/L), G-CSF (39 ng/L; normal, 35 ng/L), and SCF (1.21 ng/mL; normal, 0.53-1.03 ng/mL). The ANC at the time of the G-CSF assay was 2.4 × 109/L.
Results of analysis of colony-stimulating activity in the patient's serum
| Stimulus . | GM-CFCs . | Meg-CFCs . | Erythroid colonies . | |||
|---|---|---|---|---|---|---|
| Donor 1 . | Donor 2 . | Donor 1 . | Donor 2 . | Donor 1 . | Donor 2 . | |
| None | 0 | 0 | 0 | 0 | 0 | 0 |
| None + patient's serum | 7 ± 1 | 5 ± 2 | 0 | 0 | 2 ± 1 | 15 ± 4 |
| Growth factors | 30 ± 2 | 21 ± 1 | 2.7 ± 0.2 | 1.7 ± 0.1 | 34 ± 1 | 58 ± 1 |
| Growth factors + patient's serum | 44 ± 4 | 60 ± 3 | 1.6 ± 0.1 | 4.2 ± 0.2 | 51 ± 5 | 65 ± 2 |
| Stimulus . | GM-CFCs . | Meg-CFCs . | Erythroid colonies . | |||
|---|---|---|---|---|---|---|
| Donor 1 . | Donor 2 . | Donor 1 . | Donor 2 . | Donor 1 . | Donor 2 . | |
| None | 0 | 0 | 0 | 0 | 0 | 0 |
| None + patient's serum | 7 ± 1 | 5 ± 2 | 0 | 0 | 2 ± 1 | 15 ± 4 |
| Growth factors | 30 ± 2 | 21 ± 1 | 2.7 ± 0.2 | 1.7 ± 0.1 | 34 ± 1 | 58 ± 1 |
| Growth factors + patient's serum | 44 ± 4 | 60 ± 3 | 1.6 ± 0.1 | 4.2 ± 0.2 | 51 ± 5 | 65 ± 2 |
Shown are results from replicate cultures using bone marrow samples from 2 healthy donors. Cells (104/mL) were incubated with no stimulus, 10% patient's serum, a combination of growth factors (granulocyte-macrophage [GM] colony-stimulating factor, granulocyte colony-stimulating factor, erythropoietin, interleukin 6, stem-cell factor, and pegylated recombinant human megakaryocyte growth and development factor), or a mixture of these stimuli. Erythroid-stimulating activity and GM-stimulating activity were present in the patient's serum samples.
GM-CFCs indicates GM colony-forming cells; Meg-CFCs, megakaryocyte colony-forming cells.
During the 6 to 12 months after the patient completed chemotherapy, when neutralizing antibodies present, the mean (± SD) platelet count was 27 ± 17 × 109/L with transfusion support, the hemoglobin level was 95 ± 13 g/L with red blood cell transfusions, and the ANC was 1.6 ± 0.6 × 109/L. A bone marrow aspirate and trephine sample were obtained 7 and 11 months after chemotherapy. On each occasion, the aspirates were aparticulate, but both trephine samples showed trilineage hypoplasia primarily affecting erythroid and megakaryocytic cells. There were no increases in reticulin and no cytogenetic abnormalities. During this time, the patient had lethargy, easy bruising, and occasional epistaxis but no episodes of fever. No alterations in renal or hepatic function were observed at any time.
The patient was given prednisolone (50 mg/day for 21 days) beginning 6 months after chemotherapy, but the platelet count did not increase and the antibody titer did not change. Ten months after completing chemotherapy, the patient still required platelet and red blood cell transfusions for maintenance of adequate blood counts. At that time, the level of CA 125 antigen (a marker of ovarian cancer), which had been mildly elevated before chemotherapy (40 U/mL), was normal and there was no evidence of ovarian cancer.
Several findings suggest the existence of a causal relation between the appearance of the neutralizing antibody and the development of trilineage bone marrow hypoplasia in this patient. First, the patient had not previously received chemotherapy and had no known condition associated with a predisposition to prolonged chemotherapy-induced myelosuppression. Second, prolonged myelosuppression was not observed in other patients treated with the same chemotherapy regimen, although lower peak platelet counts with successive cycles are characteristic of this treatment.5,13-15 Third, the development of neutralizing TPO antibody was coincident with the development of pancytopenia and was specific (eg, anti-EPO antibodies were never detected). Fourth, the elevated levels of EPO, G-CSF, and SCF indicated the presence of an aplastic anemia–like, multilineage, stem cell defect that is known to be mediated by TPO.7 16-19
Development of neutralizing antibodies to endogenous cytokines after administration of growth factors occurs rarely. One patient with chronic renal failure was observed to have onset of EPO-resistant anemia after an initial response to EPO.20 Multicycle GM-CSF therapy has resulted in anti–GM-CSF antibodies that blunted the GM-CSF response.21 Development of antibodies to G-CSF did not alter resting blood counts or G-CSF response.22
The mechanism for the immune response to PEG-rHuMGDF and TPO is unknown. Although protein conjugation with polyethylene glycol was previously found to reduce antibody responses compared with results with the unmodified molecule,23 the prolonged, repeated exposure of skin dendritic cells to PEG-rHuMGDF likely assisted in the development of an immune response.24 This was probably exacerbated by the 36-hour half-life of PEG-rHuMGDF.25 It is also possible that truncation of the native TPO molecule to generate MGDF exposed a unique and antigenic carboxyl-terminal or that the absence of serine-linked carbohydrate altered the immunogenicity of PEG-rHuMGDF compared with native TPO. Whatever the mechanism of the response, our case illustrates a potential obstacle to additional clinical development of this cytokine and reveals an interesting clinical consequence of the multilineage action of TPO.
We thank Linda Shaner, Amgen, Thousand Oaks, CA, for assistance in conducting the cytokine assays. This work is dedicated to the memory of Dora Menchaca, who was tragically killed in the events of September 11, 2001.
Supported in part by grants from the Anti-Cancer Council of Victoria, Carlton; the National Health and Medical Research Council, Canberra; the Cooperative Research Centre for Cellular Growth Factors, Parkville, Australia; and Amgen, Thousand Oaks, CA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Russell Basser, CSL Ltd, 45 Poplar Rd, Parkville, Victoria, 3052, Australia; e-mail: russell_basser@csl.com.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal