Abstract
Costimulatory molecules B7-1 and B7-2 (hereby collectively called B7) interact with CD28 and CTLA4 on T cells and promote antitumor immunity. The function of B7-CTLA4 interaction in antitumor CTL response remains controversial. Here we used CD28−/− and CD28+/− or CD28+/+ transgenic mice that express the T-cell receptor specific for an unmutated tumor antigen, P1A, and for tumor cells expressing a CTLA4-specific B7 mutant to evaluate the function of CD28-B7 and CTLA4-B7 interactions in induction and effector phases of antitumor immunity. We report that B7-CD28 and B7-CTLA4 interactions promote tumor rejection. However, this is achieved by distinct mechanisms. B7-CD28 interaction enhances T-cell clonal expansion, though a role for this interaction in the effector phase cannot be ruled out. In contrast, B7-CTLA4 interaction enhances the CTL-mediated destruction of tumors, but not T-cell clonal expansion.
Introduction
The identification of B7-11,2 and B7-21-6 and their receptors, CD28 and CTLA4,2,7,8 as prototypic costimulatory molecules has led to several novel approaches in tumor immunotherapy, including the expression of B7 on tumors9-12 and the use of antireceptor antibodies.12 13 Elucidation of the function of B7 receptors on T cells would facilitate the development of additional therapeutic strategies targeted at this pathway. Despite extensive analysis on the function of B7-CD28/CTLA4 interaction in the last decade, it is still unresolved whether CD28 and CTLA4 have opposite functions in immune regulation.
The prevailing notion that CTLA4 is a negative regulator during T-cell activation was based on 3 lines of circumstantial evidence, namely, the effect of anti-CTLA4 monoclonal antibodies (mAbs),14,15 the fatal lymphoproliferative diseases in CTLA4-deficient mice,16-18 and the fact that CTLA4 associates with a tyrosine phosphatase, SHP-2.19,20 Recent studies, however, have raised questions about this interpretation. For example, because CTLA4 is expressed in developing thymocytes and because of the effect of anti-CTLA4 mAb on anti-CD3–induced cell death and deletion of myelin-basic protein-specific T cells,21,22 one potential explanation of lymphoproliferative disease is an alteration of T-cell repertoire in CTLA4-deficient mice.23 Consistent with this, recent studies indicate that the lymphoproliferative diseases can be cured by eliminating potential autoreactive T-cell repertoires.24,25 In addition, the effect of anti-CTLA4 mAbs depends on the condition of T-cell receptor (TCR) engagement rather than the valence of the antibodies, and it is independent of the cytoplasmic domain of CTLA4 that is involved in association with SHP-2.26,27 Moreover, with one exception,28most groups have failed to observe any inhibitory effect of B7 when CD28−/− T cells are used.29-31 Direct comparison between CTLA4−/− and CTLA4+/+ T cells in vivo revealed no advantage of CTLA4−/− T cells during immune responses.32
Using B7-transfected cell lines and tumors, we have reported that B7 can promote the activation of CD28−/− T cells in vitro and promote tumor rejection in vivo.33 34 The cellular basis for enhanced tumor rejection has not been clearly elucidated in vivo. To address the function of B7 receptors during antitumor CTL response, we developed an in vivo model involving the adoptive transfer of tumor-specific naive T cells into RAG-2−/− syngeneic mice. We analyzed clonal expansion and effector function of CD28+/− and CD28−/− T cells in the tumor-bearing RAG-2−/− mice. Our results demonstrate that B7-CD28 and B7-CTLA4 interactions play different roles in the antitumor immune response. At the inductive phase, B7 on either tumor or host antigen-presenting cells (APCs) interacts with CD28 on T cells to promote clonal expansion. However, at the effector phase, CTLA4 on T cells interacts with B7 on tumor cells to promote the cognate destruction of tumor cells in vivo, though a role for CD28 in this process cannot be ruled out.
Materials and methods
Experimental animals
Transgenic mice expressing the TCR specific for tumor antigen P1A35-43:Ld complex have been described.35 TCR transgenes were back-crossed with BALB/cByJ for at least 6 generations before they were used for this study. BALB/c mice with a targeted mutation of CD28 were purchased from Jackson Laboratories (Bar Harbor, ME). BALB/c mice with a targeted mutation of RAG-2 were purchased from Taconic (Germantown, NY). CD28+/− and CD28−/− P1CTL mice were derived from breeding between CD28−/− mice and CD28+/− P1CTL+ transgenic mice—the F1 of CD28−/− BALB/c and BALB/c P1CTL. Cell line plasmocytoma J558 transfected either with vector alone (J558-Neo), wild-type B7-1 (J558-B7), or B7-1 with a mutation from W to A at position 88 (J558-B7W) has been described.34
Antibodies and fusion protein
Anti–B7-1 (3A12 and 10.16A)6,36 and anti–B7-2 (GL-1) mAbs4 were purified from the hybridoma supernatants using a protein G affinity column. For in vitro analysis, the antibodies were biotinylated. Biotinylated or phycoerythrin (PE)–conjugated anti-Vα8, anti-CD28, and anti-CTLA4 mAbs were purchased from PharMingen (San Diego, CA). Fusion proteins CD28 immunoglobulin and CTLA4 immunoglobulin, composed of the immunoglobulin (Ig)G1 Fc portion and extracellular domains of CD28 or CTLA4, were produced using the pIg vector (Clontech, San Diego, CA) according to the manufacturer's instructions. Murine B7 immunoglobulin, which consists of the extracellular domain of murine B7-1 and the Fc portion of the murine IgG2a, was produced according to a described procedure.37 Ascites of anti-CD28 mAb 37N38and anti-CTLA4 mAb 4F1015 were produced using hybridomas kindly provided by Drs James P. Allison (University of California, Berkeley) and Jeffery A. Bluestone (University of Chicago, IL), respectively.
Flow cytometry
Cell surface expression of B7-1 was detected with mAb 10.16A. Unlabeled anti–B7-1 was detected using goat–antihamster IgG–fluorescein isothiocyanate (FITC) (Caltag, Mountain View, CA). Biotinylated anti–B7-1 and anti–B7-2 were detected using PE-streptavidin.
Spleen cells from P1CTL transgenic mice were used directly without in vitro stimulation or were stimulated with P1A peptide (AA35-43, 0.1 μg/mL) for 3 days before analysis. In addition, single-cell suspension was prepared from J558-B7 tumors surgically isolated from RAG-2−/− BALB/c mice at 4 days after adoptive transfer of CD28+/+, CD28+/−, or CD28−/−P1CTL. CD8+ T cells were marked with FITC-labeled anti-CD8 mAbs. Cell surface expression of CTLA4 was measured using PE-conjugated anti-CTLA4 mAb 4F10 (PharMingen). To detect the expression of intracellular CTLA4, T cells were treated with perm/wash buffer (PharMingen) before the addition of PE-labeled anti-CTLA4 mAbs. For blocking studies, intact or permeabilized cells were preincubated with a 1:10 dilution of anti-CD8, anti-CD28, or anti-CTLA4 mAb for 1 hour at 4°C. Biotinylated anti-B7 immunoglobulin or heat-stable antigen (HSA)–immunoglobulin fusion protein39 was added at a final concentration of 25 μg/mL and was incubated for 2 hours. After 4 washes with perm/wash buffer, the cell-bound fusion protein was detected using PE-conjugated streptavidin. Fluorescence was analyzed using a Coulter XL analyzer (Beckman Coulter, CA). List mode data were analyzed using the Flowjo 3.4 (Tree Star, La Jolla, CA).
Adoptive transfer of purified transgenic T cells
Pools of spleen and lymph node cells from the P1CTL-transgenic mice were incubated with a cocktail of mAbs (anti-CD4 mAb GK1.5, anti-FcR mAb 2.4G2, and anti-CD11c mAb N418). After the removal of unbound mAbs, the cells were incubated with anti-immunoglobulin–coated magnetic beads. Antibody-coated cells were removed with a magnet. Unbound cells consisted of more than 90% CD8 T cells with no detectable CD4 T cells. All CD8 T cells expressed the transgenic receptor as revealed by staining with anti-Vα8 mAb. Purified T cells were adoptively transferred by intravenous injection into RAG-2−/− mice with established tumors or had received tumor cells on the day of adoptive transfer. In some experiments, the CD8 T cells were labeled with carboxylfluoresceindiacetate succinimidyl ester (CFSE) before adoptive transfer, as described.40
Tumorigenicity assay 5 × 106 J558 cells were injected in the flanks as described.11 Tumor size and incidence were determined by physical examination.
Results
Inside P1CTL or on the cell surface, CTLA4 is the only detectable non-CD28 receptor for B7-1 and is induced by CD28-independent mechanisms
Two assumptions must be verified before one can use CD28−/− T cells to study the function of B7-CTLA4 interaction. First, CTLA4 expression must be autonomous from that of CD28. Second, in addition to CD28 and CTLA4, activated T cells must express no other B7 receptor. CTLA4 is inducible during T-cell activation. It is known that anti-CD28 can enhance the expression of CTLA441; however, it is unclear whether CD28 is required for CTLA4 expression. We analyzed the expression of CTLA4 among resting and activated P1CTL by flow cytometry. Profiles of CTLA4 expression on gated CD8 T cells are presented in Figure1 and Figure2.
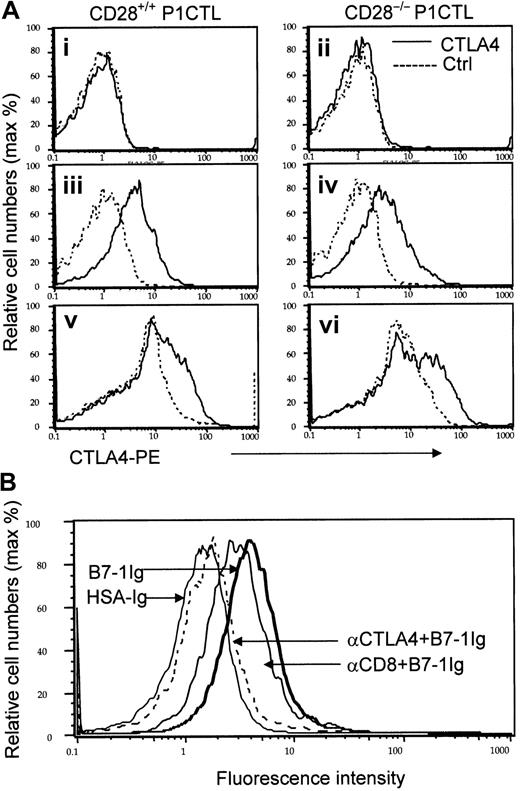
Expression and identity of a non-CD28 receptor for B7-1 on the cell surface of P1CTL.
(A) CD28-independent expression of CTLA4 on activated T cells. Naive (i-ii), in vitro–activated (iii-iv), and ex vivo–activated (v-vi) P1CTL were stained with either PE-conjugated anti-CTLA4 mAb (solid lines) or isotype control (dotted lines). Data shown were gated CD8 T cells, marked by FITC-conjugated anti-CD8 mAb. (B) Blocking of B7-1 immunoglobulin binding to activated CD28−/− P1CTL by anti-CTLA4, but not anti-CD8 mAbs. CD28−/− P1CTL were stimulated for 3 days in vitro with P1A peptide and were stained with biotinylated B7-1 immunoglobulin or control HSA immunoglobulin followed by PE-conjugated streptavidin. To verify the involvement of CTLA4, half the cells were pretreated with either anti-CD8 or anti-CTLA4 mAb (100 μg/mL) for 30 minutes before the addition of biotinylated fusion proteins. Data shown are normalized histograms using Flowjo software (version 3.4). Essentially identical numbers of cells were analyzed to produce the overlaid histograms. The number of gated CD8 T cells analyzed were naive, 10 000 events; in vitro activated, 5000 events; ex vivo activated CD28−/− T cells, 4000 events; ex vivo– activated WT CD8 T cells, 5000 events. These experiments were repeated twice with similar results.
Expression and identity of a non-CD28 receptor for B7-1 on the cell surface of P1CTL.
(A) CD28-independent expression of CTLA4 on activated T cells. Naive (i-ii), in vitro–activated (iii-iv), and ex vivo–activated (v-vi) P1CTL were stained with either PE-conjugated anti-CTLA4 mAb (solid lines) or isotype control (dotted lines). Data shown were gated CD8 T cells, marked by FITC-conjugated anti-CD8 mAb. (B) Blocking of B7-1 immunoglobulin binding to activated CD28−/− P1CTL by anti-CTLA4, but not anti-CD8 mAbs. CD28−/− P1CTL were stimulated for 3 days in vitro with P1A peptide and were stained with biotinylated B7-1 immunoglobulin or control HSA immunoglobulin followed by PE-conjugated streptavidin. To verify the involvement of CTLA4, half the cells were pretreated with either anti-CD8 or anti-CTLA4 mAb (100 μg/mL) for 30 minutes before the addition of biotinylated fusion proteins. Data shown are normalized histograms using Flowjo software (version 3.4). Essentially identical numbers of cells were analyzed to produce the overlaid histograms. The number of gated CD8 T cells analyzed were naive, 10 000 events; in vitro activated, 5000 events; ex vivo activated CD28−/− T cells, 4000 events; ex vivo– activated WT CD8 T cells, 5000 events. These experiments were repeated twice with similar results.
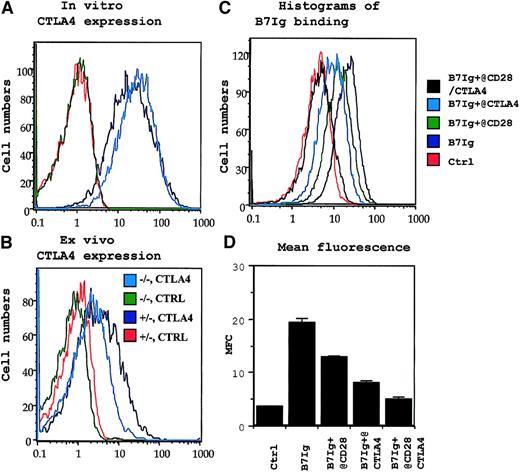
CD28-independent expression of CTLA4 and absence of B7 receptors other than CD28 and CTLA4 in permeabilized P1CTL T cells.
(A) CD28-independent expression of intracellular CTLA4 in activated P1CTL. (B) CD28-independent expression of intracellular CTLA4 in tumor-infiltrating P1CTL. The genotype of CD28 locus (+/−or −/−) and the antibodies (CTLA4 or ctrl for isotype control) used for panels A and B are shown in the legends in panel B. (C, D) Blocking of B7- immunoglobulin binding to activated T cells by anti-CD28 or anti-CTLA4. Activated CD28+/− P1CTL were incubated first with either anti-CD28 or anti-CTLA4 mAb ascites. They were then incubated with biotinylated B7 immunoglobulin or a murine IgG2a mAb isotype control (ctrl). The amount of biotinylated B7 immunoglobulin or ctrl was determined by PE-conjugated streptavidin. Data in panels A, C, and D were repeated at least 5 times, and those in panel B were repeated twice.
CD28-independent expression of CTLA4 and absence of B7 receptors other than CD28 and CTLA4 in permeabilized P1CTL T cells.
(A) CD28-independent expression of intracellular CTLA4 in activated P1CTL. (B) CD28-independent expression of intracellular CTLA4 in tumor-infiltrating P1CTL. The genotype of CD28 locus (+/−or −/−) and the antibodies (CTLA4 or ctrl for isotype control) used for panels A and B are shown in the legends in panel B. (C, D) Blocking of B7- immunoglobulin binding to activated T cells by anti-CD28 or anti-CTLA4. Activated CD28+/− P1CTL were incubated first with either anti-CD28 or anti-CTLA4 mAb ascites. They were then incubated with biotinylated B7 immunoglobulin or a murine IgG2a mAb isotype control (ctrl). The amount of biotinylated B7 immunoglobulin or ctrl was determined by PE-conjugated streptavidin. Data in panels A, C, and D were repeated at least 5 times, and those in panel B were repeated twice.
Naive CD28+/+ and CD28−/− P1CTL had no detectable cell-surface CTLA4, as expected (Figure 1Ai-ii). To test whether CTLA4 can be induced on activated T cells, we activated CD28+/+ and CD28−/− transgenic T cells with their specific antigen, P1A, for 3 days in vitro. Regardless of CD28 expression, the transgenic T cells had low, but detectable, cell surface CTLA4 (Figure 1Aiii-iv). To determine whether CTLA4 expression is autonomous of CD28 during an in vivo immune response, we adoptively transferred CD28+/+ and CD28−/− P1CTL into RAG-2–deficient syngeneic mice that bore J558-B7+ tumors. Three days later, the expression of CTLA4 in the tumor-infiltrating T cells was analyzed. As shown in Figure 1Av-vi, substantial populations of tumor-infiltrating P1CTL isolated from J558-B7 tumors expressed CTLA4. Levels of CTLA4 were comparable between CD28+/+ and CD28−/− P1CTL. Thus, on P1CTL, CTLA4 is expressed by CD28-independent mechanisms.
Because the overwhelming proportion of steady-state CTLA4 resides within the cells and translocates to the site of TCR engagement,42 we included 0.1% saponin in the staining buffer to measure cell surface and intracellular CTLA4 molecules simultaneously. Again, naive T cells did not express any detectable intracellular CTLA4 (data not shown). As shown in Figure 2A, CD28+/− P1CTL expressed a high level of CTLA4, as expected. Interestingly, similar levels of CTLA4 were detected in CD28−/− T cells. Thus, in the presence of high doses of antigen, CTLA4 expression does not depend on CD28. Although the ex vivo P1CTL expressed lower CTLA4 than that activated by a high dose of peptide in vitro, significant levels of CTLA4 were observed among the CD28+/− and CD28−/− P1CTL. A comparison of the CTLA4 levels between the CD28+/− and CD28−/− T cells indicated that, to some extent, CD28-B7 interaction can enhance intracellular CTLA4 expression, as has been reported.41 43
CD28 and CTLA4 are 2 known receptors for B7-1.2,7Recently, genetic evidence was reported that implied the existence of additional B7 receptor(s), though no data to date support the direct binding of B7 to CD28−/− CTLA4−/− T cells.44 To test whether CD28 and CTLA4 are the only receptors for B7-1 on P1CTL, we activated CD28+/− and CD28−/− P1CTL cells in vitro with the specific peptide and tested their binding to B7-1 immunoglobulin. As shown in Figures 1B and 2C-D, activated T cells bound significantly to the biotinylated B7-1 immunoglobulin, but not to control fusion protein or mouse IgG. Thus, biotinylated B7-1 immunoglobulin, but not control biotinylated HSA immunoglobulin, bound to intact CD28−/− T cells. This binding was almost completely blocked by anti-CTLA4 mAbs. The specificity of the blocking is confirmed because a control anti-CD8 mAb had only a small effect. These results indicated that most B7-1 binding to CD28−/− T cells was mediated by CTLA4.
Because most CTLA4 on P1CTL resided intracellularly (Figure 2A-B), we also tested B7-1 binding after permeabilization. Either anti-CD28 or anti-CTLA4 mAbs partially blocked B7-1 immunoglobulin binding to CD28+/− T cells. Importantly, a combination of the 2 mAbs completely blocked B7-1 immunoglobulin binding to activated T cells. These results confirmed that CD28 and CTLA4 account for all B7-1 receptors in activated P1CTL. Thus, one can use CD28−/−P1CTL to study the function of CTLA4.
B7 on host antigen-presenting cells, but not on tumors, are responsible for T-cell clonal expansion: roles for CD28 and CTLA4
We first compared CD28+/− and CD28−/−P1CTL for their proliferation and cytokine production in response to the P1A antigen in vitro. As shown in Figure3A, CD28+/− and CD28−/− T cells proliferated vigorously to P1A antigen stimulation, though CD28+/− T cells required 100-fold less P1A antigen to achieve maximal proliferation. In the presence of anti–B7-1 and anti–B7-2 mAbs, CD28+/− and CD28−/− T cells responded equally well to P1A peptide. Because anti–B7-1 and anti–B7-2 mAbs did not inhibit the proliferation of CD28−/− T cells, B7 expressed on the APCs was insufficient to costimulate T-cell proliferation through a pathway other than CD28. This was most likely attributed to the relatively low level of B7 on the APCs given that B7-1 can costimulate the clonal expansion of CD28−/− CD4 T cells when it is overexpressed on Chinese hamster ovary cells.33
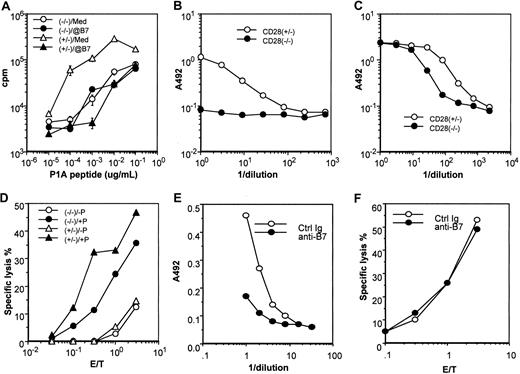
Functional characterization of the CD28+/−and CD28−/− P1CTL.
(A) Proliferative response of transgenic spleen cells to varying concentrations of the P1A peptide was measured by pulsing the culture for 6 hours with 3H-TdR, starting at 42 hours of culture. A mixture of anti–B7-1 and anti–B7-2 (1 μg/mL) mAbs was added at the beginning of the culture. Data presented are means and SE of triplicates of cpm. (B, C) Role of CD28 in production of cytokines, IL-2 (B) and IFN-γ (C) in response to antigenic P1A peptide (0.1 μg/mL). Cytokines released into the supernatants at 48 hours after antigenic stimulation were measured by sandwich ELISA. (D) Cytotoxicity of activated CD28+/− and CD28−/− P1CTL. Spleen cells activated in vitro for 4 days were used as the effectors, whereas the macrophage cell line P388D1, pulsed with (+P) or without (−P) P1A peptide (1 μg/mL) was used as the target. (E) Anti-B7 mAbs inhibit IFN-γ production by CD28−/− P1CTL, as detailed in panel C, except that anti–B7-1 and anti–B7-2 mAbs (10 μg/mL) were added into the culture. (F) Anti-B7 mAbs (added before the addition of effector T cells and present during CTL assay only) did not inhibit the cytolysis of P1A peptide–pulsed P388D1 target cells by P1CTL. Data shown are representative of at least 2 independent experiments.
Functional characterization of the CD28+/−and CD28−/− P1CTL.
(A) Proliferative response of transgenic spleen cells to varying concentrations of the P1A peptide was measured by pulsing the culture for 6 hours with 3H-TdR, starting at 42 hours of culture. A mixture of anti–B7-1 and anti–B7-2 (1 μg/mL) mAbs was added at the beginning of the culture. Data presented are means and SE of triplicates of cpm. (B, C) Role of CD28 in production of cytokines, IL-2 (B) and IFN-γ (C) in response to antigenic P1A peptide (0.1 μg/mL). Cytokines released into the supernatants at 48 hours after antigenic stimulation were measured by sandwich ELISA. (D) Cytotoxicity of activated CD28+/− and CD28−/− P1CTL. Spleen cells activated in vitro for 4 days were used as the effectors, whereas the macrophage cell line P388D1, pulsed with (+P) or without (−P) P1A peptide (1 μg/mL) was used as the target. (E) Anti-B7 mAbs inhibit IFN-γ production by CD28−/− P1CTL, as detailed in panel C, except that anti–B7-1 and anti–B7-2 mAbs (10 μg/mL) were added into the culture. (F) Anti-B7 mAbs (added before the addition of effector T cells and present during CTL assay only) did not inhibit the cytolysis of P1A peptide–pulsed P388D1 target cells by P1CTL. Data shown are representative of at least 2 independent experiments.
When stimulated by optimal amounts of P1A peptides, CD28+/− T cells produced a large amount of interleukin-2 (IL-2). In contrast, no IL-2 was detectable from the culture of CD28−/− T cells, even when a sufficient dose of antigen was used to induce maximal proliferation (Figure 3B). In comparison with CD28+/− T cells, CD28−/− T cells produced a significant, though 3- to 5-fold lower, amount of interferon (IFN)–γ (Figure 3C) and IL-4 (not shown). Moreover, regardless of the CD28 genotype, activated P1CTL was cytotoxic to a macrophage cell line pulsed with specific antigen. Cytotoxicity levels appeared to be 3- to 5-fold lower in CD28−/− T cells (Figure 3D). Both properties—the essential role in IL-2 production and the ability to reduce the amounts of antigen required for proliferation—are consistent with known functions of CD28.31 45
Interestingly, anti–B7-1 plus anti–B7-2 significantly inhibited the production of IFN-γ by CD28−/− P1CTL (Figure 3E). These results suggest that B7-CTLA4 interaction promotes cytokine production by T cells. However, blocking with anti-B7 had no effect on the cytotoxicity of CD28−/− P1CTL (Figure 3F).
We labeled transgenic T cells with CFSE and injected them into RAG-2−/− mice that bore either J558-B7 or J558-Neo tumors. T-cell division was analyzed at 4 days after adoptive transfer. As shown in Figure 4, both CD28+/− and CD28−/− P1CTL proliferated vigorously in tumor-bearing mice. Within the same time frame, little proliferation was observed in mice that bore no tumors. Thus, the bulk of the proliferation was tumor driven.
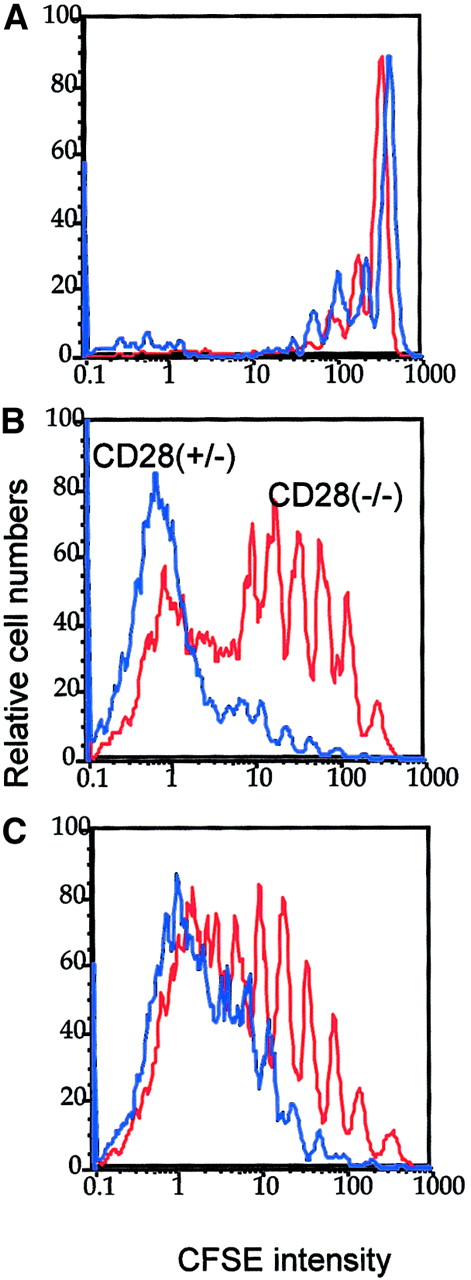
Optimal in vivo clonal expansion of P1CTL requires CD28 on the T cells.
Purified CD28+/− (blue lines) or CD28−/−(red lines) CD8+ P1CTL were labeled with CFSE and were adoptively transferred into RAG-2−/− mice that bore either J558-B7 (C) or J558-Neo (B) tumors or into RAG-2−/− mice that received no tumor cells (A). Mononuclear cells were harvested on day 4 after transfer. Division of P1CTL was analyzed by flow cytometry. Data shown are CFSE intensity of gated Vα8+ T cells, as measured by flow cytometry and analyzed by Flowjo software, as detailed in the legend to Figure 1. Five thousand gated P1CTL cells were analyzed. The increased proliferation of CD28+/− T cells over that of the CD28−/− T cells was reproduced in 3 independent experiments.
Optimal in vivo clonal expansion of P1CTL requires CD28 on the T cells.
Purified CD28+/− (blue lines) or CD28−/−(red lines) CD8+ P1CTL were labeled with CFSE and were adoptively transferred into RAG-2−/− mice that bore either J558-B7 (C) or J558-Neo (B) tumors or into RAG-2−/− mice that received no tumor cells (A). Mononuclear cells were harvested on day 4 after transfer. Division of P1CTL was analyzed by flow cytometry. Data shown are CFSE intensity of gated Vα8+ T cells, as measured by flow cytometry and analyzed by Flowjo software, as detailed in the legend to Figure 1. Five thousand gated P1CTL cells were analyzed. The increased proliferation of CD28+/− T cells over that of the CD28−/− T cells was reproduced in 3 independent experiments.
In the spleens, CD28+/− T cells proliferated substantially faster than the CD28−/− T cells. The difference was more striking in mice bearing J558-Neo tumors than in those that bore J558-B7 tumors. Similar results were observed in the draining lymph nodes (data not shown). Thus, CD28 can enhance the clonal expansion of P1CTL in vivo. It is worth noting that in this experiment, the division of CD28+/− T cells was not accelerated in the J558-B7 tumor-bearing mice. However, in other experiments, B7-1 on tumor cells enhanced T-cell division (data not shown). This was perhaps influenced by the number of tumor cells in the spleen given that considerable variations in the number were observed among individual mice.46 J558 tumor cells induce direct priming and cross-priming in this model.46 Direct priming by tumor cells requires the expression of B7 on the tumor cells. However, cross-priming is mediated by B7-expressing host APCs and may not necessarily be enhanced by B7 expression on the tumor cells. It is therefore not surprising to observe variations with regard to the effect of tumor-expressed B7 on the rate of T-cell division in vivo.
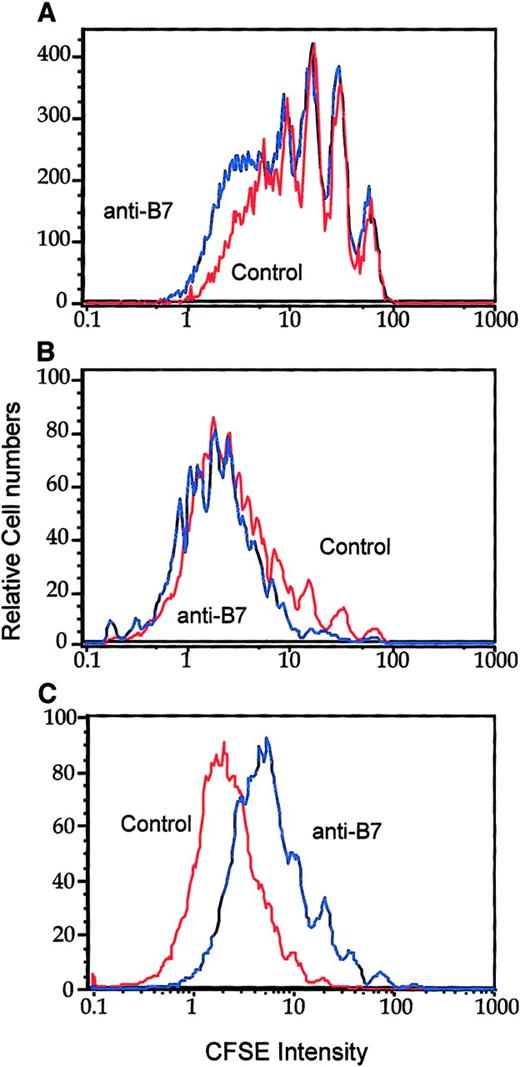
To address the role of B7-CTLA4 interactions in T-cell clonal expansion, we injected anti–B7-1 and anti–B7-2 mAbs into the J558-Neo tumor-bearing mice on days 0, 1, and 2 of adoptive transfer of CFSE-labeled T cells. On day 3, T-cell division was analyzed by flow cytometry. Interestingly, the division of CD28−/− T cells was neither enhanced nor reduced by an effective dose of anti–B7-1 and anti–B7-2 mAbs (Figure 5A-B). The mAbs blocked B7-1 and B7-2 as they substantially reduced the proliferation of WT T cells (Figure 5C). The difference between the anti-B7 and control immunoglobulin groups (Figure 5) was not as dramatic as that between the CD28+/− and CD28−/− groups (Figure 4). It is possible that the blockade by anti-B7 is incomplete in vivo. Nevertheless, the complete lack of effect of anti-B7 on the division of CD28−/− T cells supports the conclusion that B7-CTLA4 interaction does not play a significant role in T-cell division in vivo.
B7-CTLA4 interaction does not contribute to T-cell proliferation in vivo.
CD28+/+ or CD28−/− P1CTL were labeled with CFSE and injected into mice that had J558-Neo tumors. On days 0, 1, and 2, the tumor-bearing mice were injected with a mixture of either control rat/hamster immunoglobulin or anti–B7-1 and anti–B7-2 mAbs intraperitoneally (100 μg/antibody per mouse per injection). On day 3, spleen (A, C) or tumor (B) cells were harvested and analyzed. (A) Effect of anti-B7 mAb on the division of CD28−/− P1CTL accumulated in the spleen. (B) Effect of anti-B7 mAb on the division of tumor-infiltrating P1CTL. (C) Anti-B7 mAb blocks the division of CD28+/+ T cells. Data shown were CFSE intensity of gated Vα8+ T cells, as measured by flow cytometry, and were analyzed by Flowjo software, as detailed in legend to Figure 1. Five thousand gated P1CTL cells were analyzed. Data shown are representative of 3 independent experiments.
B7-CTLA4 interaction does not contribute to T-cell proliferation in vivo.
CD28+/+ or CD28−/− P1CTL were labeled with CFSE and injected into mice that had J558-Neo tumors. On days 0, 1, and 2, the tumor-bearing mice were injected with a mixture of either control rat/hamster immunoglobulin or anti–B7-1 and anti–B7-2 mAbs intraperitoneally (100 μg/antibody per mouse per injection). On day 3, spleen (A, C) or tumor (B) cells were harvested and analyzed. (A) Effect of anti-B7 mAb on the division of CD28−/− P1CTL accumulated in the spleen. (B) Effect of anti-B7 mAb on the division of tumor-infiltrating P1CTL. (C) Anti-B7 mAb blocks the division of CD28+/+ T cells. Data shown were CFSE intensity of gated Vα8+ T cells, as measured by flow cytometry, and were analyzed by Flowjo software, as detailed in legend to Figure 1. Five thousand gated P1CTL cells were analyzed. Data shown are representative of 3 independent experiments.
Role for CTLA4 in cognate destruction of tumor cells by CTL
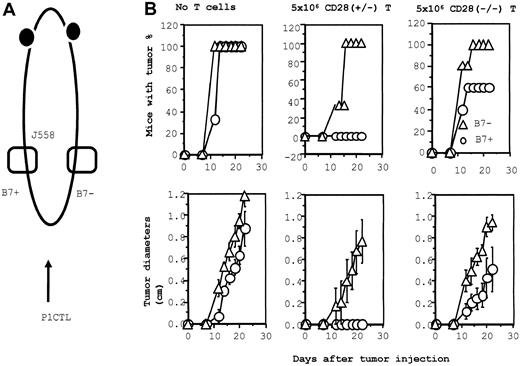
We have recently demonstrated that B7-1 also plays a major role for the effector function of P1CTL for several lineages of P1A-expressing tumors.47 To analyze the function of CD28 and CTLA4 at the effector phase, we injected J558-B7 and J558-Neo tumor cells at separate flanks of the same RAG-2−/− mice. Some of the tumor-bearing mice were then adoptively transferred with either CD28+/− or CD28−/− P1CTL (Figure6A). As shown in Figure 6B, J558-Neo and J558-B7 grew at comparable rates in RAG-2−/− mice that received no T cells. The comparable growth kinetics of the 2 tumor cells in RAG-2−/− mice have been verified in more than 20 experiments (data not shown). Thus, NK cells by themselves do not preferentially reject J558-B7 tumors in syngeneic mice. In mice that received CD28+/− P1CTL, J558-B7 tumors failed to develop, whereas J558-Neo tumors grew progressively. This is consistent with our previous report that WT P1CTL preferentially rejects J558-B7 tumors over J558-Neo tumors.47
B7-dependent rejection of J558 tumor: role of CD28 and CTLA4.
(A) Diagram of experimental design. J558-B7 and J558 tumor cells were injected at separate flanks of the RAG-2−/− BALB/c mice. These mice then either received no T cells or they received CD28+/− or CD28−/− T cells on the day of tumor injection. The tumor incidence (top panels) and growth kinetics were monitored. (B) Tumor rejection by 5 × 106 of CD28+/− or CD28−/− P1CTL. Groups that received no T cells or CD28+/− T cells had 3 mice per group; the group that received CD28−/− T cells consisted of 5 mice. The sizes of J558-Neo and J558-B7 tumors in mice that received CD28−/− P1CTL were significantly different between day 12 and day 22 (2-sided P value between .05 and .0001 by Student t test).
B7-dependent rejection of J558 tumor: role of CD28 and CTLA4.
(A) Diagram of experimental design. J558-B7 and J558 tumor cells were injected at separate flanks of the RAG-2−/− BALB/c mice. These mice then either received no T cells or they received CD28+/− or CD28−/− T cells on the day of tumor injection. The tumor incidence (top panels) and growth kinetics were monitored. (B) Tumor rejection by 5 × 106 of CD28+/− or CD28−/− P1CTL. Groups that received no T cells or CD28+/− T cells had 3 mice per group; the group that received CD28−/− T cells consisted of 5 mice. The sizes of J558-Neo and J558-B7 tumors in mice that received CD28−/− P1CTL were significantly different between day 12 and day 22 (2-sided P value between .05 and .0001 by Student t test).
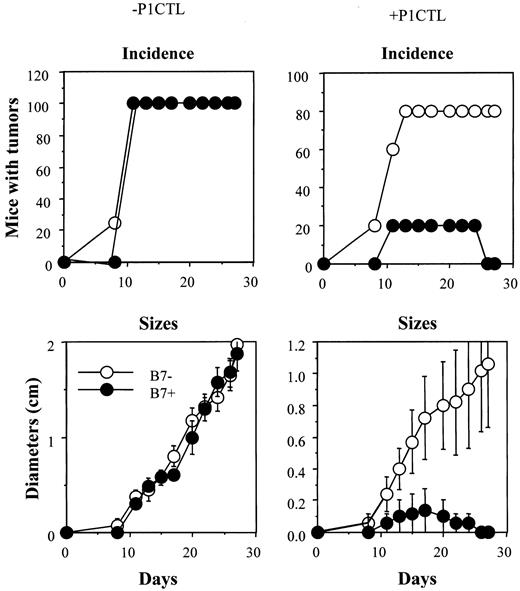
In mice that received 5 × 106 CD28−/− T cells, we observed a reduction of tumor incidence and growth kinetics, though B7+ tumors still developed in 60% of the mice (Figure 6B). Thus, the expression of B7-1 on the tumor is a significant growth disadvantage. To substantiate the function of B7-CTLA4 interaction, we increased the number of CD28−/− T cells 3-fold, to 15 × 106/mouse. As shown in Figure7, an increase in the amounts of CD28−/− P1CTL leads to a complete rejection of B7+ tumor cells, whereas growth of the B7−tumor cells is only slightly affected. Selective elimination of B7+ tumors by the CD28−/− T cells indicates that B7-CTLA4 interaction promotes tumor rejection.
Tumor rejection by 15 × 106 of CD28−/− P1CTL.
The group that received no T cells consisted of 4 mice, whereas the group that received CD28−/− T cells consisted of 5 mice. Differences in the sizes of J558-Neo and J558-B7 tumors were statistically significant from day 17 on (Welch t test, 2-sided P value between .05 and .03).
Tumor rejection by 15 × 106 of CD28−/− P1CTL.
The group that received no T cells consisted of 4 mice, whereas the group that received CD28−/− T cells consisted of 5 mice. Differences in the sizes of J558-Neo and J558-B7 tumors were statistically significant from day 17 on (Welch t test, 2-sided P value between .05 and .03).
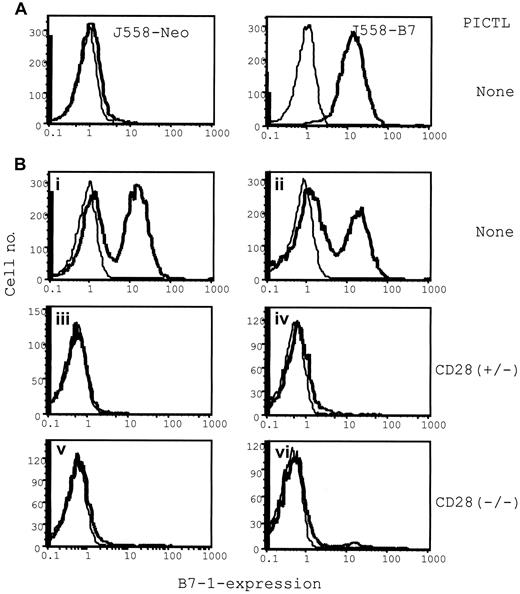
The failure to reject the J558-Neo tumors can be attributed to a lack of CTL maturation or to a requirement for B7-1 at the effector phase. To bypass the requirement for B7-1 at the inductive phase, we mixed the J558-B7 and J558-Neo cells before injection into RAG-2–deficient mice, which then received purified CD28−/− P1CTL intravenously. In RAG-2–deficient mice that received no T cells, all J558-Neo tumor cells were B7− and all J558-B7 tumor cells were B7+(Figure 8A), as expected. In mice that received a mixture of J558-Neo and J558-B7, both types of cells were presented at an approximate 1:1 ratio when T cells were not present (Figure 8Bi-ii, for 2 examples). In mice that received either CD28+/− or CD28−/− P1CTL, the overwhelming majority of the surviving tumor cells were devoid of B7 expression (Figure 8Biii-iv and 8Bv-vi, respectively; 2 cases presented for each group). The failure to eliminate B7− tumor cells that colocalized with the B7+ cells demonstrates a critical role for B7 in the cognate destruction of tumor cells by CTL. Because the T cells were devoid of CD28, B7 must have interacted with CTLA4 to promote the cognate destruction of tumor cells. The increased efficacy of CD28+ T cells could have resulted from a role for CD28 in effector function or from an increased number of T cells in the tumors (data not shown).
Selective elimination of B7+ tumor cells by P1CTL from a mixture of B7+ and B7− tumor cells: flow cytometric analysis of the composition of B7+ and B7− tumors in the presence or absence of P1CTL.
Single viable cell suspensions were prepared from freshly isolated tumors and were stained with anti–B7-1 mAb hybridoma supernatants (bold lines) or medium as control (thin lines). (A) J558-B7 or J558 tumor cells in separate mice. (B) J558-Neo and J558-B7 cells were mixed before injection. Tumors in panels i-ii were from mice that received no T cells; those in panels iii-iv received 5 × 106 of CD28+/− T cells, and those in panels v-vi received 5 × 106 of CD28−/− T cells.
Selective elimination of B7+ tumor cells by P1CTL from a mixture of B7+ and B7− tumor cells: flow cytometric analysis of the composition of B7+ and B7− tumors in the presence or absence of P1CTL.
Single viable cell suspensions were prepared from freshly isolated tumors and were stained with anti–B7-1 mAb hybridoma supernatants (bold lines) or medium as control (thin lines). (A) J558-B7 or J558 tumor cells in separate mice. (B) J558-Neo and J558-B7 cells were mixed before injection. Tumors in panels i-ii were from mice that received no T cells; those in panels iii-iv received 5 × 106 of CD28+/− T cells, and those in panels v-vi received 5 × 106 of CD28−/− T cells.
Mutant B7-1 that selectively binds CTLA4 promotes tumor rejection in vivo
Given the potential contribution of CD28 in T-cell development,48,49 the signaling machinery of T cells that develop in the absence of CD28 can be different from that of wild-type T cells. Because wild-type B7-1 binds to CD28 and CTLA4, a mutant B7-1 that binds to CTLA4 alone would help to bypass this difficulty. We recently produced a mutant of murine B7-1 that has a substitution of W to A at position 88 in the IgV domain. In semiquantitative assays, we showed that this mutant binds CTLA4, but not to CD28.33 34To quantitatively determine its binding to CD28 versus CTLA4, we tagged the wild-type and mutant B7-1 with green fluorescence protein (GFP) and compared their binding to the 2 receptors. As shown in Figure9A, based on the density of GFP, the levels of WT and mutant B7 cells were extremely heterogeneous, with almost 1000-fold variations in their expression levels. This gave us an opportunity to examine their receptor bindings over a large dose range. WT B7-1 bound to CD28 immunoglobulin and CTLA4 immunoglobulin, with the level of B7 expression correlating almost linearly with their binding. As expected, approximately 10-fold more B7-1 was needed to achieve a comparable binding to CD28 immunoglobulin. Based on the ratio of CTLA4 binding to GFP signal, WT B7 and B7W bound CTLA4 equally well over a 1000-fold dose variation. These results established that mutant B7W maintains full binding to CTLA4 while lacking any detectable binding to CD28 over a large range. As expected, when B7W was expressed in the J558 cells, it also failed to bind CD28 immunoglobulin, though its binding to CTLA4 immunoglobulin was unaffected (Figure 9A). To test whether B7-CTLA4 interaction is sufficient to costimulate tumor rejection, we adoptively transferred CD28+/− P1CTL into RAG-2−/− mice and challenged them at separate sites with J558-Neo and J558-B7W. As shown in Figure 9B, P1CTL rejected J558-B7W, but not J558-Neo. Thus, B7-CTLA4 interaction caused the rejection of J558 tumors.
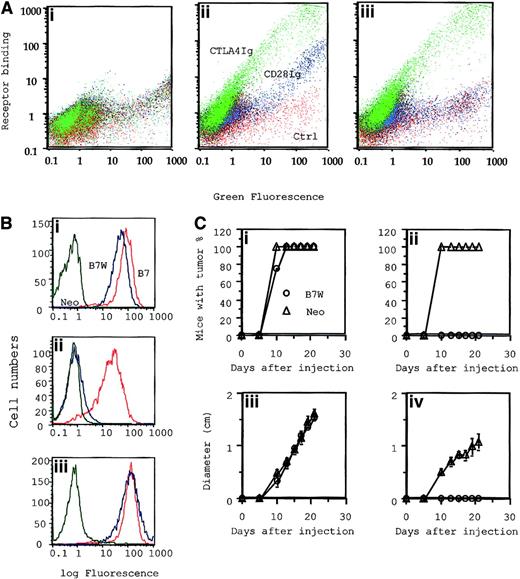
Mutant B7-1–transfected tumor cells that bind to CTLA4, but not to CD28, were selectively eliminated by P1CTL.
(A) Characterization of receptor binding of WT and mutant B7-1 by flow cytometry. COS cells were transiently transfected with plasmid expressing GFP (i), GFP-tagged wild-type B7 (B7-GFP) (ii), or mutant B7(W88→A)(iii) were stained with 100 μg/mL of either CD28 immunoglobulin or CTLA4 immunoglobulin mixed with PE-conjugated goat–antihuman IgG. Two-color flow cytometry was used to determine B7 expression versus receptor binding. Green indicates binding of CTLA4 immunoglobulin; blue, CD28 immunoglobulin; red, control. (B) Receptor binding and (C) tumorigenicity of J558-Neo and J558-B7W. (B) Binding to anti–B7-1 mAb (i), CD28 immunoglobulin (ii), and CTLA4 immunoglobulin (iii). (Ci, iii) (n = 4): tumor incidence (i) and growth kinetics (iii) of J558-Neo and J558-B7W tumors in RAG-2−/− mice that received no T cells. (Cii, iv) (n = 5): incidence (ii) and growth kinetics (iv) in the presence of 15 × 106CD28+/− P1CTL.
Mutant B7-1–transfected tumor cells that bind to CTLA4, but not to CD28, were selectively eliminated by P1CTL.
(A) Characterization of receptor binding of WT and mutant B7-1 by flow cytometry. COS cells were transiently transfected with plasmid expressing GFP (i), GFP-tagged wild-type B7 (B7-GFP) (ii), or mutant B7(W88→A)(iii) were stained with 100 μg/mL of either CD28 immunoglobulin or CTLA4 immunoglobulin mixed with PE-conjugated goat–antihuman IgG. Two-color flow cytometry was used to determine B7 expression versus receptor binding. Green indicates binding of CTLA4 immunoglobulin; blue, CD28 immunoglobulin; red, control. (B) Receptor binding and (C) tumorigenicity of J558-Neo and J558-B7W. (B) Binding to anti–B7-1 mAb (i), CD28 immunoglobulin (ii), and CTLA4 immunoglobulin (iii). (Ci, iii) (n = 4): tumor incidence (i) and growth kinetics (iii) of J558-Neo and J558-B7W tumors in RAG-2−/− mice that received no T cells. (Cii, iv) (n = 5): incidence (ii) and growth kinetics (iv) in the presence of 15 × 106CD28+/− P1CTL.
Discussion
The function of B7-CTLA4 interaction remains controversial.23 50 Here we used an adoptive transfer model to evaluate the function of B7-CTLA4 interaction during an in vivo antitumor CTL response.
A comparison between CD28+/− and CD28−/−P1CTL for their proliferative response to tumor antigens revealed a potent costimulatory function of CD28 in T-cell proliferation, which is consistent with previous results from this and other laboratories.33,45,51 In vitro, CD28+/− T cells require about 100-fold less antigenic peptide than do CD28−/− P1CTL in the proliferative response. This difference can be eliminated by anti–B7-1 and anti–B7-2 mAbs. In contrast, anti-B7 mAbs have no effect on the proliferative response of CD28−/− T cells. The results of in vivo analysis corresponded well to the in vitro observations. In the spleen, CD28+/− T cells divided substantially faster than did the CD28−/− T cells in tumor-bearing mice. Again, antibody-blocking studies indicate that B7-CTLA4 interaction does not contribute to T-cell clonal expansion in vitro and in vivo. Thus, although B7-1 overexpressed on fibroblasts promotes the proliferation of CD28−/− T cells,33 the amount of B7 expressed on host APCs appears insufficient to induce T-cell division without the participation of CD28.
Because we did not use CTLA4−/− T cells for the current study, it is unclear whether B7-CD28 interaction provided costimulation without the participation of CTLA4-B7 interaction. However, the profound B7-dependent lymphoproliferative disease in CTLA4-deficient mice52 53 indicated that B7-CD28 interaction is sufficient for T-cell clonal expansion.
The most important conclusion from the current study is that B7-CTLA4 interaction promotes CTL-mediated tumor destruction in vivo. The effector mechanism is not clearly understood at present. However, we do not believe CTL-activated NK cells are the direct effector because P1CTL select for major histocompatibility complex (MHC) class Ilow tumor cells in vivo.54 This is consistent with the direct function of that CTL because it would require tumor expression of MHC class I. In contrast, CTL could be cross-primed by host APCs and would not require MHC on tumor cells to produce cytokines for NK activation. Moreover, because NK cells prefer MHC class Ilow targets, MHC down-regulation would lead to a growth disadvantage if NK cells were the effectors. The role for CTLA4-B7 interaction is based on results from 2 experimental approaches. The first approach involved CD28+/− and CD28−/−P1CTL. We found that in the same mice challenged with both B7-1+ and B7-1− tumor cells, CD28−/− transgenic T cells rejected the B7-1+, but not the B7−, tumors. More strikingly, when the 2 types of tumor cells were injected as a mixture, CD28−/− T cells selectively eliminated B7-1+tumor cells while leaving B7-1− tumor cells in the same sites. Because the B7-1 binding to CD28−/− P1CTL can be completely blocked by anti-CTLA4 mAbs, it is likely that the enhanced effector function of CD28−/− is mediated by CTLA4. Nevertheless, given the suggestion that a yet unidentified B7 receptor may exist on T cells,44 this approach alone cannot formally rule out the possibility that other unidentified B7 receptors can be responsible. In the second approach, tumor cells expressing mutant B7-1 that bound to CTLA4, but not to CD28, were also preferentially rejected by P1CTL that expressed CD28 and CTLA4 and should have developed normally. This approach not only ruled out the possibility that P1CTL—developed in the absence of CD28—differ from those that develop in the presence of CD28 with regard to their CTLA4 function, it also demonstrated that specificity similar to that of B7-CTLA4 interaction (and unlike that of B7-CD28 interaction) is responsible for the effector function. Taken together, the data from these 2 approaches provided a compelling case that B7-CTLA4 interaction promotes cognate destruction of tumor cells in vivo.
The mechanism by which B7 promotes the effector function of tumor-specific CTL remains unclear. Based on our finding that B7-CTLA4 interaction promoted the production of IFN-γ but not cytotoxicity, it is possible that the function of CTLA4 is mediated through local production and effector function of IFN-γ, which has been shown to be important for T-cell–mediated tumor immunity.55
To dissect the function of B7 receptors in a defined system, we have chosen to use an adoptive transfer of transgenic T cells specific for a natural tumor antigen P1A into immune-deficient mice as our basic model. As such, our model differs from physiological conditions in which the frequency of T cells are lower and in which immunity is provided by interactions among different subset of T cells.56 However, it is important to emphasize that the conclusion that B7-CTLA4 interaction promotes tumor immunity was first reached in a nontransgenic mouse model.34 The simplicity of the current model helps to define the subset of T cells and the stage of immune response at which B7-CTLA4 interaction mediates this important function. Moreover, because the function of CTLA4 uncovered in the current study involves CD8 T cells in the absence of CD4 T cells, it is distinct from the hypothetical role of B7-CTLA4 interaction in the induction and function of CD25+CD4 T cells.57
In summary, our data revealed a novel function of CTLA4 during the effector phase of CTL responses. The relation between this function in the effector phase of CD8 T cells and the previously proposed negative regulation at the inductive phase of CD4 T cells14,15 is unclear. Understanding the mechanism of CTLA4-enhanced CTL effector function may lead to novel approaches to reinvigorate the effector function of the high number of tumor-specific CTL found in cancer patients58 59 for optimal antitumor effector function.
We thank Jing Wen for purification of the fusion protein and Jennifer Kiel for editorial assistance.
Supported by National Cancer Institute grants CA69091, CA58033, and CA82355.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yang Liu and Pan Zheng, Department of Pathology and Comprehensive Cancer Center, Ohio State University Medical Center, 129 Hamilton Hall, 1645 Neil Ave, Columbus, OH 43210; e-mail: liu-3@medctr.osu.edu and zheng-1@medctr.osu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal