Abstract
Primary familial erythrocytosis (familial polycythemia) is a rare myeloproliferative disorder with an autosomal dominant mode of inheritance. We studied a new kindred with autosomal dominantly inherited familial erythrocytosis. The molecular basis for the observed phenotype of isolated erythrocytosis is heterozygosity for a novel nonsense mutation affecting codon 399 in exon 8 of the erythropoietin receptor (EPOR) gene, encoding an EpoR peptide that is truncated by 110 amino acids at its C-terminus. The newEPOR gene mutation 5881G>T was found to segregate with isolated erythrocytosis in the affected family and this mutation represents the most extensive EpoR truncation reported to date, associated with familial erythrocytosis. Erythroid progenitors from an affected individual displayed Epo hypersensitivity in in vitro methylcellulose cultures, as indicated by more numerous erythroid burst-forming unit-derived colonies in low Epo concentrations compared to normal controls. Expression of mutant EpoR in interleukin 3–dependent hematopoietic cells was associated with Epo hyperresponsiveness compared to cells expressing wild-type EpoR.
Introduction
Familial erythrocytosis, also known as primary familial and congenital polycythemia (PFCP), is a rare disorder involving isolated proliferation of bone marrow progenitors of the erythroid lineage.1,2 This disorder is typically characterized by an autosomal dominant mode of inheritance, and less frequently, by the occurrence of sporadic cases.3,4The clinical features include the presence of isolated erythrocytosis without evolution into leukemia or other myeloproliferative disorders, absence of splenomegaly, normal white blood cell and platelet counts, low plasma erythropoietin (Epo) levels, normal hemoglobin-oxygen dissociation curve indicated by a normal P50, and hypersensitivity of erythroid progenitors to exogenous erythropoietin in vitro.5-7 Mutations in the gene encoding the erythropoietin receptor (EPOR) have been described in several families with isolated familial erythrocytosis.4 8-14 We report a new kindred with dominantly inherited familial erythrocytosis associated with heterozygosity for a novel point mutation in theEPOR gene.
Study design
Patients, erythroid colony formation assays, DNA and RNA analyses
Peripheral blood samples from affected and unaffected individuals were obtained under a protocol approved by the Institutional Review Board at Yale University School of Medicine. In vitro erythroid colony formation assays were performed as described.10 The genomic DNA structure of theEPOR gene and RNA analyses for cloning of mutant EpoR complementary DNA (cDNA) were performed as described.10Identity of subcloned polymerase chain reaction (PCR) products containing amplified EPOR genomic DNA and cDNA fragments were confirmed by nucleotide sequencing.
Cloning and expression of mutant EpoR and Epo dose-response assays
Wild-type (WT) and mutant human EPOR cDNAs were cloned in pBabe-puro retroviral vector and plasmids were stably transfected into amphotropic producer cell line PA317 using Lipofectamine reagent (Invitrogen, Carlsbad, CA).15Puromycin-resistant (3.5 μg/mL) PA317 cells were cocultured with murine interleukin 3 (IL-3)–dependent 32D cells in the presence of 4 μg/mL polybrene (Sigma, St Louis, MO) for 24 hours. 32D cells infected with retrovirus were selected in medium containing 1.0 μg/mL puromycin for 10 days and cultured in Epo 2 U/mL (Amgen, Thousand Oaks, CA) instead of IL-3. As negative control, 32D cells transduced with empty pBabe-puro vector were generated. Single-cell clones of 32D cells were isolated by limiting dilution. Expression of EpoR protein in single-cell clones was demonstrated by immunoprecipitation and Western analysis as described15 and surface expression of EpoR was confirmed by a flow cytometry assay (data not shown) using recombinant human Epo (Amgen) that was biotinylated as described.16Negative control 32D cells transduced with vector only do not express EpoR protein, do not proliferate in Epo, and do not demonstrate surface binding of biotin-Epo by flow cytometry assay (data not shown). Epo dose-response assays were performed using MTT reagent (dimethylthiazol-2-yl-2,5-diphenyltetrazolium) as described.17
Results and discussion
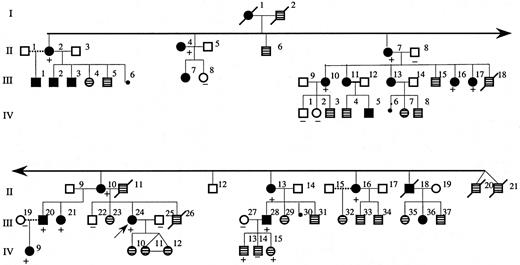
We studied a 4-generation white family from Maine (Figure1). The propositus (patient III:24 in Figure 1) was first evaluated at the age of 15 years because of headaches associated with a hemoglobin of 20.7 g/dL and a hematocrit of 62%. The white blood cell and platelet counts and morphology of the red blood cells in peripheral blood smear were normal. The plasma Epo level was less than 10 mU/mL and hemoglobin electrophoresis and P50 were normal. The persistent headaches were relieved by phlebotomy. Many affected individuals in this family underwent periodic phlebotomy to control hematocrit levels.
Pedigree of affected family.
The dark filled symbols represent affected individuals with erythrocytosis or history of periodic phlebotomy for polycythemia. The symbols with patterned fill represent individuals for whom clinical information was not available. The presence or absence of the 5881G>T mutation in tested individuals is indicated by + or − symbols, respectively. The propositus is indicated by the arrow (III:24).
Pedigree of affected family.
The dark filled symbols represent affected individuals with erythrocytosis or history of periodic phlebotomy for polycythemia. The symbols with patterned fill represent individuals for whom clinical information was not available. The presence or absence of the 5881G>T mutation in tested individuals is indicated by + or − symbols, respectively. The propositus is indicated by the arrow (III:24).
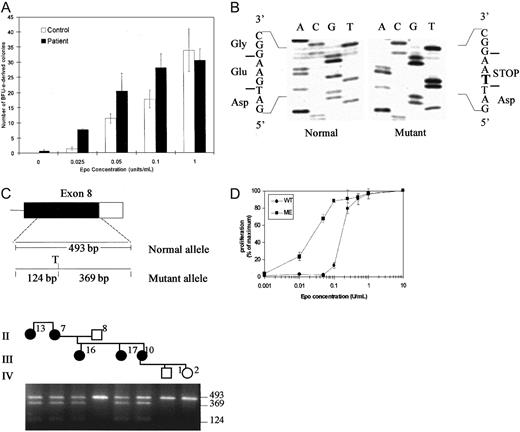
The phenotype of the propositus and other evaluated affected individuals in the pedigree were consistent with clinical features of PFCP.18 To determine whether erythrocytosis in this family was associated with Epo hypersensitivity of erythroid progenitors, we performed in vitro semisolid medium cultures using peripheral blood mononuclear cells. The results of scoring of day 14 erythroid burst-forming unit (BFU-E)–derived colonies in semisolid methylcellulose medium containing serum and various added Epo concentrations are illustrated in Figure2A. In the absence of added Epo in the culture medium, 0 to 1 BFU-E–derived colonies were detected in the samples from the affected individual and no erythroid colonies were present in control cultures. At highest added Epo concentration of 1 U/mL, the numbers of BFU-E–derived colonies were similar in cultures of the affected individual and those of the unaffected family member (30 ± 3.7 versus 33 ± 7, respectively). Notably, erythroid progenitors of the affected individual displayed hypersensitivity to Epo when compared to those of an unaffected family member in the presence of relatively low and intermediate range of Epo concentrations (25-100 mU/mL). A significant difference in the number of erythroid colonies was observed in 25 mU/mL, with 1.3 ± 0.5 colonies in the control plates compared to 8 ± 0.5 colonies in the patient cultures (P < .0002 by Student t test). In 50 mU/mL Epo, 20 ± 5.8 colonies were present in the cultures from the individual with erythrocytosis compared to 11 ± 1.5 in the unaffected family member (not significant). In 100 mU/mL Epo, the difference in colony numbers remained significant with 28 ± 0.5 in the patient versus 17 ± 2.8 in controls (P < .03). The differences in the total numbers of erythroid colonies was accompanied by a visible difference in the size of individual colonies when culture plates from the affected individual and control were examined (data not shown).
Erythroid colony assays, mutation analysis, and Epo-dose response.
(A) Erythroid colony formation assays in 2 family members. The vertical axis indicates the numbers of BFU-E–derived colonies per 2.5 × 105 peripheral blood mononuclear cells, expressed as the mean ± SD of assays performed in triplicate. The final Epo concentration added to the cultures is indicated on the horizontal axis. (B) DNA sequence of subcloned genomic PCR products from the EpoR gene of the proband. Analysis of individual clones shows the sequences for the normal allele (left) and the mutant allele (right). The bold letter T indicates 5881G>T substitution resulting in introduction of a STOP codon. (C) Upper panel shows diagram of exon 8 of EPORgene. The coding sequence is shown as solid box and the 3′ untranslated region as an open box. The position and size of genomic PCR amplification products and a Tru9I (T) restriction map of the 493-base pair (bp) PCR amplification product for the mutant allele are shown. Lower panel shows detection of the mutation by restriction endonuclease digestion of PCR-amplified genomic DNA. Part of the pedigree is shown at the top of the figure. Genomic DNA amplification products were digested with Tru9I and fractionated by electrophoresis in a 2% agarose gel. The mutation creates a uniqueTru9I site in mutant allele and yields fragments of 369 bp and 124 bp in addition to the 493-bp fragment from the normal allele in individuals heterozygous for the new mutation. None of the unaffected individuals (open symbols) have Tru9I site in their genomic DNA, whereas all affected individuals in the family (filled symbols) are heterozygous for the mutation. (D) Epo dose-response of 32D cells transfected with wild type (WT) or mutant (ME) EpoRs. The cells were cultured in the indicated concentrations of Epo (U/mL) and the results are expressed as a percentage of maximal proliferation in 10 U/mL Epo as determined by MTT assay. Each data point represents the mean of 4 determinations with SE bars shown. Similar results were obtained in several experiments using multiple independent single cell clones of transfected cells.
Erythroid colony assays, mutation analysis, and Epo-dose response.
(A) Erythroid colony formation assays in 2 family members. The vertical axis indicates the numbers of BFU-E–derived colonies per 2.5 × 105 peripheral blood mononuclear cells, expressed as the mean ± SD of assays performed in triplicate. The final Epo concentration added to the cultures is indicated on the horizontal axis. (B) DNA sequence of subcloned genomic PCR products from the EpoR gene of the proband. Analysis of individual clones shows the sequences for the normal allele (left) and the mutant allele (right). The bold letter T indicates 5881G>T substitution resulting in introduction of a STOP codon. (C) Upper panel shows diagram of exon 8 of EPORgene. The coding sequence is shown as solid box and the 3′ untranslated region as an open box. The position and size of genomic PCR amplification products and a Tru9I (T) restriction map of the 493-base pair (bp) PCR amplification product for the mutant allele are shown. Lower panel shows detection of the mutation by restriction endonuclease digestion of PCR-amplified genomic DNA. Part of the pedigree is shown at the top of the figure. Genomic DNA amplification products were digested with Tru9I and fractionated by electrophoresis in a 2% agarose gel. The mutation creates a uniqueTru9I site in mutant allele and yields fragments of 369 bp and 124 bp in addition to the 493-bp fragment from the normal allele in individuals heterozygous for the new mutation. None of the unaffected individuals (open symbols) have Tru9I site in their genomic DNA, whereas all affected individuals in the family (filled symbols) are heterozygous for the mutation. (D) Epo dose-response of 32D cells transfected with wild type (WT) or mutant (ME) EpoRs. The cells were cultured in the indicated concentrations of Epo (U/mL) and the results are expressed as a percentage of maximal proliferation in 10 U/mL Epo as determined by MTT assay. Each data point represents the mean of 4 determinations with SE bars shown. Similar results were obtained in several experiments using multiple independent single cell clones of transfected cells.
Mutations in EPOR have been found in about 12% of cases in a series of individuals with PFCP and it appears that mutation in a gene(s) other than EPOR may account for the polycythemic phenotype in the majority of PFCP cases.4 To determine whether the PFCP phenotype in the new family was associated with a mutation in the EPOR gene, we evaluated the structure of the gene with particular focus on the region encoding the C-terminal domain of the receptor that exerts a negative effect on receptor mitogenic function.19-21 Direct nucleotide sequencing as well as sequencing of subcloned genomic PCR amplification products from the propositus revealed heterozygosity for presence of G>T substitution at position 5881 of EpoR (Figure 2B), introducing a premature termination codon at position 399 that would result in deletion of 110 amino acid residues at the C-terminus of the receptor. This novel mutation introduces a new restriction endonuclease site (Tru9I) in exon 8 of EPOR gene, which allowed rapid, PCR-based detection of the mutation in each family member (Figure 2C). Restriction endonuclease digestion of genomic PCR amplification products with Tru9I was observed only in affected individuals all of whom were heterozygous for the 5881G>T mutation. The mutation was not present in any of the unaffected individuals tested.
The EpoR mutations associated with PFCP phenotype described to date are all located within exon 8 of the gene and result in truncation of 59 to 84 amino acids from the C-terminal region.4 The function of the new EpoR mutation (5881G>T) was evaluated in vitro in 32D cells engineered to express either wild-type or mutant EpoR cDNA isolated from the proband. Figure 2D illustrates an assay measuring the Epo dose-response of 32D cells transfected with wild-type EpoR or mutant EpoR. A significant percentage of mutant EpoR-expressing cells proliferate in relatively low concentrations of Epo and thus display increased sensitivity to Epo compared to wild-type EpoR-expressing cells within an Epo concentration range of 0.01 to 0.1 U/mL. This result is consistent with previous findings from our laboratory as well as others demonstrating increased Epo sensitivity of transfected hematopoietic cells expressing truncated human EpoRs compared to cells expressing wild-type EpoR.9,10 14
The distal cytoplasmic region of EpoR is required for down-regulation of Epo-mediated activation of JAK2, a cytoplasmic tyrosine kinase critical for Epo-induced mitogenesis as well as inhibition of apoptosis.14,17,22,23 Recently, a mouse model for congenital polycythemia has been generated by homologous replacement of murine EPOR gene by a mutant human EPOR gene described in a family with PFCP.24 This mutant human EpoR is truncated by 83 amino acids at its C-terminus13 and mice heterozygous for the mutant EpoR allele developed marked polycythemia, mimicking the dominantly inherited human disorder.24 In contrast, in a study by Zang et al,25 mice heterozygous for a targeted mutation in murine EpoR gene, with a more extensive truncation of 108 distal cytoplasmic amino acids, did not display increased hematocrits. The differences between the polycythemic phenotype obtained with in vivo expression of mutant Epo receptors with different cytoplasmic truncations suggested that the extent of the truncations may be important for generation of the PFCP phenotype.24,25 The new human EpoR mutant described in our study represents the most extensive human EpoR truncation reported to date that is associated with PFCP in heterozygous individuals and confers Epo hypersensitivity in erythroid progenitors and transfected hematopoietic cells. This human EpoR mutant lacks 110 distal amino acid residues, comparable with regard to the extent of the cytoplasmic truncation, to the truncated murine EpoR studied by Zang et al.25 It is possible that specific structural differences between truncated human and murine EpoRs other than the extent of the cytoplasmic truncation play a role in generation of the polycythemic phenotype. For instance, compared to the murine EpoR, a notable difference in the structure of truncated human EpoR mutants is the presence of an additional tyrosine residue in the membrane proximal region of human EpoR that could potentially contribute to the dominant, gain-of-function effect of truncated human receptors resulting in generation of PFCP phenotype in vivo.
The authors thank Amgen for providing recombinant human erythropoietin.
Supported in part by a grant from the National Institutes of Health DK-02566 (to M.O.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Murat O. Arcasoy, Division of Hematology-Oncology, Duke University Medical Center, DUMC Box 3912, Durham, NC 27710; e-mail: arcas001@mc.duke.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal