Abstract
Allogeneic bone marrow transplantation (BMT) may be curative for more patients than chemotherapy for the child with relapsed acute lymphoblastic leukemia. This study reviewed the outcomes of 363 children with acute lymphoblastic leukemia in second remission who received unrelated donor BMT from 1988 to 2000 in order to define prognostic factors that affect leukemia-free survival (LFS). Median patient age was 9 years (range, 0-19 years), and median follow-up 29 was months (range, 0-125 months). The median duration of first remission was 24 months (range, 0-109 months). Prognostic factors, including age, duration of first remission, HLA matching, and graft-versus-host (GVH) disease, were analyzed using both univariate and multivariate analyses. Overall survival was 38%, and LFS was 36% at 5 years. LFS was significantly worse for patients 15 years or older (log-rank, P = .009). HLA matching was associated with improved LFS. Acute GVH disease developed in 71%, with 29% having grades III-IV. The incidence of chronic GVH disease was 39% for patients who survived more than 80 days and was significantly higher for female patients receiving marrow from female donors (P = .0009). Transplantation-related mortality was 42% and was associated with HLA mismatches, age 15 years and older, and first remission less than 12 months. The 5-year estimate for relapse was 22%, with first remission at least 6 months associated with a lower risk. Results of unrelated donor BMT appear similar to multi-institutional studies of matched related donor BMT, and this approach appears to be curative for many patients. However, innovative approaches are needed for patients with initial remissions of less than 6 months and for older teenagers.
Introduction
Chemotherapy is curative for more than 75% of children with acute lymphoblastic leukemia (ALL). However, for children who relapse, the best therapeutic option is unclear. At least 75% of children may achieve a second remission (CR2), but the probability of leukemia-free survival (LFS) with chemotherapy alone has been reported as less than 10% to 40%.1-8 There are no randomized therapeutic trials that have defined the role of allogeneic stem cell transplantation for these children, and the decision to pursue this option may rest upon availability of a matched related donor (MRD), duration of first remission (CR1), and institutional bias. MRD transplantation may be curative for many patients with recurrent ALL, but only 25% to 30% of children will have an MRD. The benefits of alternative donor transplantation have not been established in children in CR2, and transplantation-related mortality (TRM) has been more than 50% in adult studies.9 The availability of alternative donors has increased with the establishment of programs such as the National Marrow Donor Program (NMDP), and more than 60% of patients are able to find well-matched unrelated donors (URDs). However, prognostic factors that determine outcome with URD bone marrow transplantation (BMT) for pediatric patients with ALL in CR2 have not been defined. The main purpose of this analysis was to determine prognostic factors that influence the outcome of unrelated BMT in recurrent ALL. This information may assist oncologists caring for these patients in making informed decisions.
Patients and methods
National Marrow Donor Program
The NMDP was established in 1986 by an act of the United States Congress. A registry of more than 4 million volunteer stem cell donors is maintained, with a network of cord blood banks and transplantation, donor, and collection centers. The NMDP is currently under contract with the Health Resources and Services Administration, and more than 11 000 stem cell transplantations have been facilitated through its services.
Patients
This study comprises 363 patients 19 years of age or younger with CR2 ALL who received URD marrow transplantations facilitated by the NMDP between 1988 and 2000. Patient characteristics and transplantation details are shown in Table1. Median patient age was 9 years (range, 0-19 years), and median follow-up was 29 months (range, 0-125 months). Most patients (91%) received total body irradiation containing conditioning regimens. The median duration of CR1 was 24 months (range, 0-109 months). The median time from relapse to BMT was 156 days (range, 39-1619 days).
Clinical features
| . | No. . | % . |
|---|---|---|
| Age (median 9 y, range 0-19 y) | ||
| Less than 15 y | 289 | 81.6 |
| More than 15 y | 65 | 18.4 |
| Diagnostic WBC count (× 109/L) | ||
| Less than 1 | 5 | 1.4 |
| 1 to 50 | 214 | 60.5 |
| 51 to 100 | 31 | 8.7 |
| More than 100 | 60 | 16.9 |
| NA | 44 | 12.4 |
| Immunophenotype | ||
| B-cell precursor CD10+ | 174 | 47.9 |
| B-cell precursor CD10− | 25 | 6.9 |
| B cell mature | 29 | 8.9 |
| T cell | 37 | 10.2 |
| Other/NA | 98 | 27.0 |
| Cytogenetics | ||
| Normal | 236 | 65.0 |
| Hyperdiploid or hypodiploid | 35 | 9.6 |
| t(9;22) or t(8;14) or t(14;18) | 22 | 6.1 |
| Other | 70 | 19.3 |
| Extramedullary disease | ||
| Present | 52 | 14.3 |
| Absent | 294 | 83.0 |
| NA | 17 | 4.7 |
| CR1 duration, mo | ||
| Less than 1 to 5 | 45 | 12.4 |
| 6 to 12 | 32 | 8.8 |
| 13 to 24 | 81 | 22.3 |
| 25 to 36 | 80 | 22.0 |
| More than 36 | 76 | 22.9 |
| NA | 49 | 13.5 |
| Donor/patient CMV status | ||
| Donor negative/patient negative | 154 | 42.4 |
| Donor negative/patient positive | 73 | 20.1 |
| Donor positive/patient negative | 77 | 21.2 |
| Donor positive/patient positive | 51 | 14.0 |
| NA | 8 | 2.2 |
| Conditioning | ||
| CPM + TBI ± other | 310 | 85.4 |
| Other + TBI | 21 | 5.8 |
| Chemotherapy only | 32 | 8.8 |
| GVH disease prophylaxis | ||
| CSA or FK506 + MTX ± CS ± ALS | 212 | 58.4 |
| CSA or FK506 ± CS ± ALS | 117 | 32.2 |
| CSA and/or ALS | 23 | 6.3 |
| MTX | 1 | 0.3 |
| NA | 10 | 2.8 |
| . | No. . | % . |
|---|---|---|
| Age (median 9 y, range 0-19 y) | ||
| Less than 15 y | 289 | 81.6 |
| More than 15 y | 65 | 18.4 |
| Diagnostic WBC count (× 109/L) | ||
| Less than 1 | 5 | 1.4 |
| 1 to 50 | 214 | 60.5 |
| 51 to 100 | 31 | 8.7 |
| More than 100 | 60 | 16.9 |
| NA | 44 | 12.4 |
| Immunophenotype | ||
| B-cell precursor CD10+ | 174 | 47.9 |
| B-cell precursor CD10− | 25 | 6.9 |
| B cell mature | 29 | 8.9 |
| T cell | 37 | 10.2 |
| Other/NA | 98 | 27.0 |
| Cytogenetics | ||
| Normal | 236 | 65.0 |
| Hyperdiploid or hypodiploid | 35 | 9.6 |
| t(9;22) or t(8;14) or t(14;18) | 22 | 6.1 |
| Other | 70 | 19.3 |
| Extramedullary disease | ||
| Present | 52 | 14.3 |
| Absent | 294 | 83.0 |
| NA | 17 | 4.7 |
| CR1 duration, mo | ||
| Less than 1 to 5 | 45 | 12.4 |
| 6 to 12 | 32 | 8.8 |
| 13 to 24 | 81 | 22.3 |
| 25 to 36 | 80 | 22.0 |
| More than 36 | 76 | 22.9 |
| NA | 49 | 13.5 |
| Donor/patient CMV status | ||
| Donor negative/patient negative | 154 | 42.4 |
| Donor negative/patient positive | 73 | 20.1 |
| Donor positive/patient negative | 77 | 21.2 |
| Donor positive/patient positive | 51 | 14.0 |
| NA | 8 | 2.2 |
| Conditioning | ||
| CPM + TBI ± other | 310 | 85.4 |
| Other + TBI | 21 | 5.8 |
| Chemotherapy only | 32 | 8.8 |
| GVH disease prophylaxis | ||
| CSA or FK506 + MTX ± CS ± ALS | 212 | 58.4 |
| CSA or FK506 ± CS ± ALS | 117 | 32.2 |
| CSA and/or ALS | 23 | 6.3 |
| MTX | 1 | 0.3 |
| NA | 10 | 2.8 |
NA indicates not available; CPM, cyclophosphamide; TBI, total body irradiation; CSA, cyclosporine; MTX, methotrexate; CS, corticosteroids; and ALS, antilymphocyte serum.
HLA matching and donors
URDs were identified through the NMDP. There were 216 male donors and 147 female donors, with a median age of 37 years (range, 19-59 years).
The technology used in the HLA typing of donors and recipients varied over the span of this study. Standard serologic testing for HLA-A and -B was used. Patient-donor matching was classified into 3 categories based upon level of typing and match, including (a) match at HLA-A, -B, and -DRB1 (65%); (b) match at -A, -B, and serologic match at -DR (11%); and (c) mismatch at -A or -B or -DR (24%). In vitro T-lymphocyte depletion was used in 146 (40%) patients and in 59 of 112 patients with serologic or molecular mismatched donors. This was accomplished with monoclonal antibody and complement (58.3%), elutriation (16.7%), lectin/sheep cell rosetting (12.5%), and other methods (12.5%).
Statistical analysis
End points analyzed in this study were overall survival (OS), LFS, TRM, acute graft-versus-host (GVH) disease, and chronic GVH disease. Acute GVH disease was analyzed by the classifications of mild (I-II) or severe (III-IV).10 Prognostic factors were examined for each end point using both univariate and multivariate analyses. Kaplan-Meier and log-rank methods were used in the univariate analysis of OS and LFS. Relapse and acute and chronic GVH disease were calculated using cumulative incidence, with death treated as a competing risk. TRM was also calculated using cumulative incidence, with relapse treated as a competing risk.
The Cox proportional hazards model was used in the multivariate analysis of each end point. While each multivariate analysis examined more than 15 different prognostic factors, only those with aP value of .05 or less were included in the final models. Each model adjusted for transplantation center effects. These models were later modified to include acute and chronic GVH disease effects. Variables in the multivariate analysis included patient age, sex, presence of extramedullary disease, leukocyte count at diagnosis, and cytomegalovirus (CMV) status. Age was analyzed by subgroups 0 to 1 years, 2 to 4 years, 5 to 9 years, 10 to 14 years, and 15 to 19 years. Duration of CR1 was analyzed by subgroups 0 to 5, 6 to 12, 13 to 24, 25 to 36, and more than 36 months. Other variables included leukemia cytogenetics, including presence of hyperdiploidy or hypodiploidy, translocations, or other abnormalities; donor age; sex; CMV status; HLA matching; Karnofsky/Lansky score prior to conditioning; cell dose; and T-cell depletion of marrow.
Results
Engraftment and GVH disease
Engraftment, as defined by the achievement of an absolute neutrophil count of at least 500/μL (0.5 × 109/L) for 3 consecutive days, occurred at a median of 18 days (range, 9-52 days). There were 347 patients who survived at least 21 days and had engraftment data. Seven patients (2%) failed to engraft. The cumulative incidence of engraftment for patients surviving at least 21 days is 98% ± 1%.
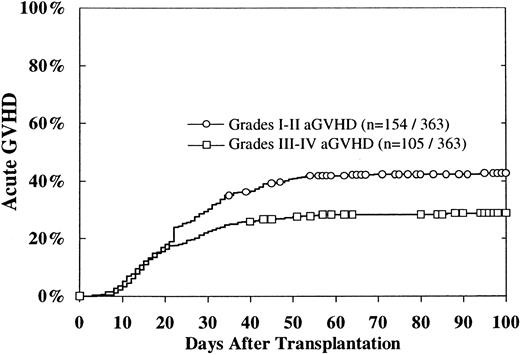
The cumulative incidence of grades II-IV acute GVH disease was 47% ± 5%. Grades I-II occurred in 154, with a 100-day cumulative incidence of 43%, and grades III-IV acute GVH disease occurred in 105, with a 100-day cumulative incidence estimate of 29% (Figure1). The 100-day cumulative incidence of grades III-IV acute GVH disease was 36% for patients with mismatched donors, 33% for patients with potentially matched donors, and 24% for patients with donors matched at -DRB1 (P = .07). CMV-negative patients had a cumulative incidence of grades III-IV acute GVH disease of 24%, in contrast to the 37% incidence in CMV-seropositive patients (P = .01). Multivariate analysis revealed that grades III-IV acute GVH disease were associated with HLA mismatches, CMV seropositivity in the recipient, and extramedullary disease (Table 2). However, on univariate analysis, extramedullary disease at diagnosis did not impact upon acute GVH disease. There was no difference in the incidence of acute GVH disease between patients younger or older than 15 years.
Cumulative incidence of grades I-II and III-IV acute GVH disease.
The cumulative incidence of grades II-IV acute GVH disease was 47% ± 5%.
Cumulative incidence of grades I-II and III-IV acute GVH disease.
The cumulative incidence of grades II-IV acute GVH disease was 47% ± 5%.
Multivariate analysis of grades III-IV acute GVH disease
| Variable . | RR . | 95% Confidence interval . | P . | Favorable . |
|---|---|---|---|---|
| Known HLA match | 1.00 | — | — | Baseline |
| Potential HLA match | 1.51 | 0.78, 2.92 | .22 | |
| HLA mismatch | 1.90 | 1.23, 2.94 | .004 | HLA match |
| Patient CMV-positive | 1.63 | 1.09, 2.45 | .02 | Patient CMV-negative |
| Extramedullary disease at diagnosis | 1.74 | 1.03, 2.95 | .04 | No extramedullary disease |
| Variable . | RR . | 95% Confidence interval . | P . | Favorable . |
|---|---|---|---|---|
| Known HLA match | 1.00 | — | — | Baseline |
| Potential HLA match | 1.51 | 0.78, 2.92 | .22 | |
| HLA mismatch | 1.90 | 1.23, 2.94 | .004 | HLA match |
| Patient CMV-positive | 1.63 | 1.09, 2.45 | .02 | Patient CMV-negative |
| Extramedullary disease at diagnosis | 1.74 | 1.03, 2.95 | .04 | No extramedullary disease |
T-cell depletion affected the development of severe GVH disease on univariate analysis. Patients who received T-depleted grafts had a 21% incidence of grades III-IV acute GVH disease, in contrast to 34% for patients who received unmodified grafts (P = .006). However, there was no difference in multivariate analysis.
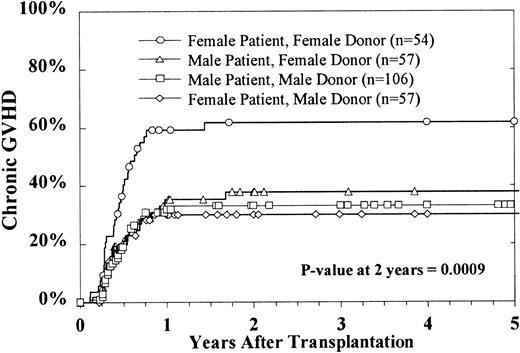
Of the 275 patients who survived 80 days after BMT, 101 (37%) developed chronic GVH disease. The 2-year cumulative incidence estimate among evaluable patients was 39% ± 6%. Multivariate analysis indicated that only donor and patient sex combinations significantly affected the rates of chronic GVH disease. Female patients who received marrow from female donors had a significantly higher incidence of chronic GVH disease (Figure 2).
Cumulative incidence of chronic GVH disease by sex combinations.
This excludes patients with a censoring time of less than 80 days.
Cumulative incidence of chronic GVH disease by sex combinations.
This excludes patients with a censoring time of less than 80 days.
Survival
The Kaplan-Meier estimate for OS at 5 years is 38% ± 6%, with 156 patients alive. LFS is 36% ± 6% (Figure3). Multivariate analysis showed HLA matching, Karnofsky score of 90 or more, lower white blood cell (WBC) count at diagnosis, age younger than 15 years, and duration of CR1 at least 6 months to be independently associated with improved LFS (Table 3). When included as a time-dependent covariate, grades III-IV acute GVH disease had an adverse effect upon survival (relative risk 2.70,P < .00001).
Multivariate regression: LFS, relapse, and TRM
| Variable . | LFS RR . | 95% Confidence interval (CI) . | P . | Relapse RR . | 95% CI . | P . | TRM RR . | 95% CI . | P . | Favorable . |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient age 15 y and older | 1.67 | 1.16, 2.39 | .006 | — | — | — | 2.00 | 1.33, 3.02 | .0009 | Younger patient |
| Duration of CR1 6 months and longer | 0.54 | 0.36, 0.80 | .002 | 0.37 | 0.18, 0.74 | .005 | — | — | — | Longer duration |
| Duration of CR1 12 months and longer | — | — | — | — | — | — | 0.62 | 0.41, 0.94 | .02 | Longer duration |
| Known HLA match | 1.0 | — | — | — | — | — | 1.0 | — | — | Baseline |
| Potential HLA match | 1.28 | 0.78, 2.12 | .33 | — | — | — | 1.53 | 0.84, 2.79 | .17 | — |
| HLA mismatch | 1.85 | 1.35, 2.52 | ≤ .0001 | — | — | — | 2.46 | 1.68, 3.61 | ≤ .0001 | HLA match |
| Donor age, decades | — | — | — | 0.95 | 0.92, 0.99 | .006 | — | — | — | Older donor |
| Karnofsky score 90 or more | 0.62 | 0.41, 0.95 | .03 | — | — | — | 0.47 | 0.36, 0.92 | .02 | Karnofsky score 90 or more |
| WBC count more than 17.7 × 109/L | 1.13 | 1.04, 1.23 | .002 | 1.27 | 1.13, 1.42 | < .0001 | — | — | — | Lower WBC count |
| Variable . | LFS RR . | 95% Confidence interval (CI) . | P . | Relapse RR . | 95% CI . | P . | TRM RR . | 95% CI . | P . | Favorable . |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient age 15 y and older | 1.67 | 1.16, 2.39 | .006 | — | — | — | 2.00 | 1.33, 3.02 | .0009 | Younger patient |
| Duration of CR1 6 months and longer | 0.54 | 0.36, 0.80 | .002 | 0.37 | 0.18, 0.74 | .005 | — | — | — | Longer duration |
| Duration of CR1 12 months and longer | — | — | — | — | — | — | 0.62 | 0.41, 0.94 | .02 | Longer duration |
| Known HLA match | 1.0 | — | — | — | — | — | 1.0 | — | — | Baseline |
| Potential HLA match | 1.28 | 0.78, 2.12 | .33 | — | — | — | 1.53 | 0.84, 2.79 | .17 | — |
| HLA mismatch | 1.85 | 1.35, 2.52 | ≤ .0001 | — | — | — | 2.46 | 1.68, 3.61 | ≤ .0001 | HLA match |
| Donor age, decades | — | — | — | 0.95 | 0.92, 0.99 | .006 | — | — | — | Older donor |
| Karnofsky score 90 or more | 0.62 | 0.41, 0.95 | .03 | — | — | — | 0.47 | 0.36, 0.92 | .02 | Karnofsky score 90 or more |
| WBC count more than 17.7 × 109/L | 1.13 | 1.04, 1.23 | .002 | 1.27 | 1.13, 1.42 | < .0001 | — | — | — | Lower WBC count |
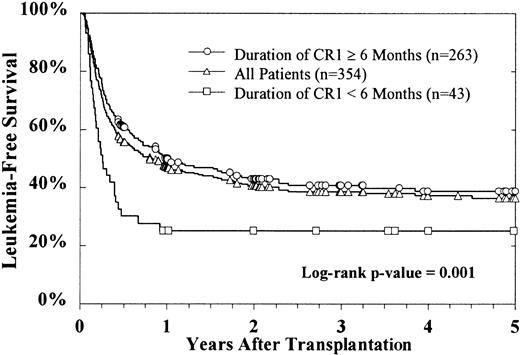
Duration of CR1, age at transplantation, and HLA matching were important prognostic factors. Only CR1 duration of less than 6 months was significant in predicting outcome, and there were no differences for patients with CR1 durations of 6 to more than 36 months. These patients had LFS of 39% versus 25% for patients who relapsed before 6 months of CR1 (P = .001). LFS was significantly worse for the 65 patients aged 15 or more years at the time of BMT, compared with 289 patients less than 15 years (Figure4). The LFS at 5 years for children with matched donors or for donors matched at -A, -B, and potentially -DR was 42% and 38%, respectively, in contrast to 26% for patients with mismatched donors (P = .0007).
LFS by patient age.
Age more than 15 years was associated with inferior outcome compared with younger patients. TRM was 38% for patients younger than 15 years and 60% for patients older than 15 years (P = .003).
LFS by patient age.
Age more than 15 years was associated with inferior outcome compared with younger patients. TRM was 38% for patients younger than 15 years and 60% for patients older than 15 years (P = .003).
There was no difference between males and females in LFS. For the 154 patients with an initial leukocyte count more than 17.7 × 109/L, LFS was 32%, in contrast to 42% for patients with lower leukocyte counts at diagnosis (P = .05). Leukemia cytogenetics had no effect, but immunophenotype did impact upon outcome. On univariate analysis, T-cell disease had a significantly worse LFS (23% ± 14%) compared with patients with non–T-cell disease, (38% ± 6%,P = .01). This effect was not found on multivariate analysis.
Time from relapse to BMT did not influence LFS. Median time from relapse to BMT was 5 months. The relative risk (RR) per month of time from relapse to BMT was 0.99, with 95% confidence interval (P = .56). The RR for relapse to BMT over 5 months was 0.86 compared with 5 months or less (P = .34). Cell dose for T-replete graft recipients did not affect LFS. Neither cell doses less than 3.6 × 108/kg nor more than 5.0 × 108/kg were associated with statistically significant differences. There was no difference between patients who received total body irradiation versus those who did not (40% vs 30%,P = .49), but this analysis was limited by the small number of non–total body irradiation patients. In vitro T-cell depletion also did not impact upon LFS by univariate or multivariate analysis.
Neither univariate nor multivariate analyses revealed any differences in OS and LFS by year of BMT over the 12-year span of this study.
Relapse
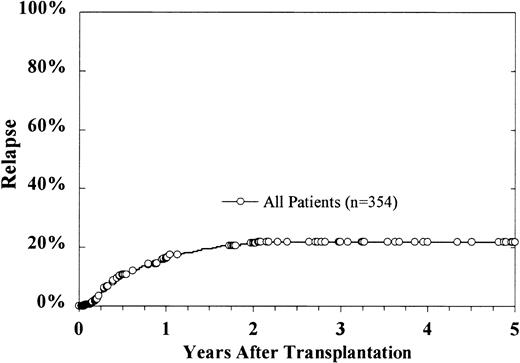
At the time of this analysis, 69 patients have relapsed, with a cumulative incidence estimate for relapse at 5 years of 22% ± 4% (Figure 5). In a multivariate analysis, lower diagnostic leukocyte count, older donor age, and duration of CR1 for more than 6 months were correlated with lower relapse rates (Table3). Grades I-II acute GVH disease predicted for a higher rate of relapse, though this effect was borderline (risk ratio 1.93,P = .03). There was no effect of grades III-IV acute GVH disease upon relapse. When added to the model as a time-dependent covariate, chronic GVH disease was significantly associated with lower relapse (risk ratio 0.35, P = .008). Grades I-II acute GVH disease negatively affected relapse (RR = 1.93,P = .02). Cell dose did not affect cumulative incidence of relapse.
Cumulative incidence of relapse.
At 5 years, the cumulative incidence estimate is 22% ± 4%.
Cumulative incidence of relapse.
At 5 years, the cumulative incidence estimate is 22% ± 4%.
Recurrent disease accounted for 24.6% of deaths. Of the 69 patients who have relapsed, 60 (87%) have died. The median time from relapse to death was less than 2 months.
Transplantation-related mortality
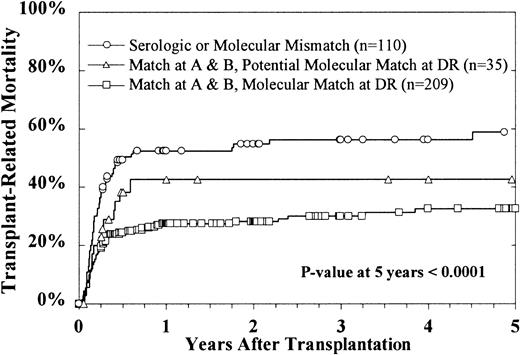
The overall cumulative incidence of TRM at 100 days is 27% ± 5%. The cumulative incidence estimate for TRM at 5 years is 42% ± 6%, with 147 patients dying of nonrelapse causes (Table4). Multivariate analysis revealed that HLA mismatches, patients aged more than 15 years, Karnofsky scores less than 90, and duration of CR1 less than 12 months were significantly associated with a higher incidence of TRM (Table 3). Increased TRM was responsible for the poorer outcome in older patients. At 5 years after BMT, TRM was 38% for 289 patients younger than 15 years, in contrast to 60% for 65 patients older than 15 years (P = .003). When included in the Cox model as a time-dependent covariate, grades III-IV acute GVH disease were found to be highly correlated with TRM (risk ratio 3.15, P < .0001). There was no effect from grades I-II acute GVH disease or chronic GVH disease. However, the cumulative incidence of TRM was significantly related to HLA disparity (Figure 6). Patients who received marrow from matched or serologic matched donors had a 5-year TRM of 33% and 43%, respectively, in contrast to the 58% TRM of patients receiving mismatched donor marrow (P = .0004). Both univariate and multivariate analysis failed to show a significant impact of T-cell depletion on TRM.
Primary causes of death
| 100 Days and less . | Overall . | ||
|---|---|---|---|
| Cause . | % . | Cause . | % . |
| Infection | 30.8 | Relapse | 24.6 |
| GVH disease | 19.2 | Infection | 24.2 |
| Toxicity | 19.2 | Toxicity | 15.5 |
| ARDS | 6.7 | GVH disease | 15.0 |
| Graft failure | 6.7 | ARDS | 7.7 |
| Relapse | 6.7 | Hemorrhage | 4.3 |
| Hemorrhage | 5.8 | Graft failure | 3.4 |
| Other | 4.8 | Other | 5.3 |
| 100 Days and less . | Overall . | ||
|---|---|---|---|
| Cause . | % . | Cause . | % . |
| Infection | 30.8 | Relapse | 24.6 |
| GVH disease | 19.2 | Infection | 24.2 |
| Toxicity | 19.2 | Toxicity | 15.5 |
| ARDS | 6.7 | GVH disease | 15.0 |
| Graft failure | 6.7 | ARDS | 7.7 |
| Relapse | 6.7 | Hemorrhage | 4.3 |
| Hemorrhage | 5.8 | Graft failure | 3.4 |
| Other | 4.8 | Other | 5.3 |
ARDS indicates acute respiratory distress syndrome.
Discussion
Therapy for children with recurrent ALL remains controversial. Analysis of studies is complicated by differences in disease status, differing intensities of initial therapies, and selection bias. Decisions regarding therapy for the relapsed patient are often based upon duration of CR1, availability of an MRD, intensity of initial therapy, and initial prognostic factors. Duration of CR1 has been an important predictor of outcome in many studies. A major difficulty with interpreting outcomes, however, has been the variable definition of what constitutes an early relapse. An early relapse has been defined as occurring as early as less than 18 months CR1 to within 12 months from discontinuation of initial therapy. Patients with variably defined early relapses have extremely poor survival with chemotherapy, with less than 15% survival.2,3,11-13 Most studies have demonstrated improved survival in patients with early relapses with MRD BMT.1,14-19 Barrett et al compared 76 patients treated with chemotherapy alone by the Pediatric Oncology Group to those who received MRD BMT. Patients were matched for age, sex, immunophenotype, diagnostic leukocyte count, and duration of CR1. LFS was 35% for patients who relapsed within 36 months of CR1 and who received MRD BMT, in contrast to 10% LFS for those treated with chemotherapy.15 Boulad et al reported a 5-year DFS of 48% with MRD BMT for patients with CR1 less than 24 months compared with 9% for those treated with chemotherapy.17 Uniformly poor results with any therapy have been the experience in other studies. In the UKALL X study, patients who had a bone marrow relapse within 2 years of diagnosis had an event-free survival of 0%, with no significant improvement with BMT.11 In that study, URD BMT after CR1 of less than 6 months resulted in a poorer LFS due to increased relapse and increased TRM. In our study, duration of CR1 beyond 6 months did not impact upon outcome, which contrasts with other studies, which show poorer outcome for patients who have relapsed before 36 months' CR1.
The role of BMT in the patient with “late” relapse is less clear, because patients treated with chemotherapy have LFS comparable to that reported for BMT.4,5,8,11,13,16,20-22 Our analysis showed no difference for patients who relapsed beyond 6 months compared with those who relapsed following discontinuation of therapy. This finding is consistent with studies that demonstrate an overall improvement with matched sibling BMT compared with chemotherapy alone, regardless of duration of CR1.1,15,17,21,23-25 The IBMTR study by Barrett et al demonstrated superior LFS with MRD BMT even for patients with late relapses.15 Patients undergoing transplantation who relapsed more than 30 months from CR1 had a 5-year LFS rate of 53%, compared with 32% for chemotherapy-only patients. In contrast, the prospective multicenter study of Uderzo et al did not demonstrate an advantage with MRD BMT for patients with CR1 more than 30 months.14 A prospective study by Torres et al confirmed a lack of survival advantage with BMT for patients with later relapses.21
The 38% LFS in our study is similar to reports for MRD BMT, with LFS of 21% to 58% reported for pediatric patients.14,15,17,19,25-30 Relapse accounted for almost 25% of deaths in this study, and this has accounted for 25% to 56% of deaths after MRD BMT.1,14-16,23,25,26,30-32 A lower incidence of relapse was noted in this study compared with some studies with MRDs, but a higher incidence of TRM countered this effect.33-35Relapse was increased by initial higher leukocyte count in this study, and this has been confirmed in studies of MRD BMT.27,31 36
Because initial treatment for ALL has become more intensive, as per BFM- (Berlin-Frankfurt-Munster) based therapy or intensive antimetabolite therapy, salvage may become more difficult regardless of therapy modality. There were no differences in outcome noted over the 12-year span of the study, but longer follow-up is needed to evaluate the outcome of the patients most recently undergoing transplantation. Many of these individuals would have received more intensive initial therapy than those undergoing transplantation a decade before. One recent study suggested that stratifying for time, the survival with MRD BMT has decreased due to increased relapse.32
If immunologic mechanisms such as graft-versus-leukemia effect are necessary to improve outcome, this could be better achieved with a URD. The role of graft-versus-leukemia in ALL is unclear, with some studies reporting no effect or a limited effect with acute GVH disease only.37-39 Other analyses have noted a correlation with either acute or chronic GVH disease.34,40 41 In our study, chronic GVH disease decreased relapse, whereas there was a negative effect from acute GVH disease upon survival.
Reducing TRM remains a major challenge. TRM in this study was 42% at 5 years and was influenced by patient age, duration of CR1, activity score, and HLA matching. Severe (grades III-IV) acute GVH disease occurred in twice as many URD recipients compared with MSD recipients, and infections, possibly linked to GVH disease, were responsible for almost one third of deaths. TRM in MRD BMT in pediatric patients with leukemias has been reported as 10% to 20% in recent studies,11,14,19,40,42,43 and relapse remains the main obstacle to cure. An IBMTR study, which compared unrelated to MRD BMT for patients with leukemia, reported a significantly higher TRM with unrelated BMT.44 There was no separate analysis of pediatric patients. More recent single-institution studies have shown no difference in TRM between MRD and URD BMT in pediatric patients.34,45-48 A disturbing observation in this study was the worse outcome for patients aged 15 years and older, with TRM of 60% compared with 38% for younger patients. This observation is more consistent with the adult literature, in which HLA mismatching is not tolerated, and TRM from GVH disease and organ toxicity is higher than seen in younger patients.9,33 49
Tissue typing became more sophisticated over the course of this study, with the development of molecular techniques, and studies have demonstrated benefits of matching at -DRB1 for both related and URDs.9,50-52 However, the impact of improved typing was not discernible in our study, but younger patients appear to better tolerate one-antigen mismatched donors.33,49 The incidence of severe (grades III-IV) acute GVH disease was similar among the 3 matching groups of patients, but there was a trend toward more frequent severe GVH disease in patients receiving mismatched marrow. In our study, the cumulative incidence of TRM with mismatched donors was 58%, in contrast to 42% for patients with serologic matches and 33% for patients with matched donors. The criteria for matching could be made more stringent to reduce the risk of severe GVH disease, but this may decrease the ability of patients to expeditiously locate an acceptable donor. Recipient CMV status was also important in the development of severe GVH disease, as has been noted in other studies.53,54 T-cell depletion did not impact upon survival, relapse, or TRM, in contrast to other studies that have shown increased rejection and relapse.40,55-57 T-cell depletion may prevent severe GVH disease, but this may be countered by the development of severe infections. There were various types of T-cell depletion used, however, and these differences can affect outcome.58
Chronic GVH disease was significantly increased in females receiving transplants from female donors. In previous studies, an increased risk of treatment failure was noted in females receiving transplants from male donors.40 The explanation for our finding is not apparent.
Our study indicates that many children with ALL in CR2 will achieve LFS with a URD BMT. Results appear similar to MSD BMT reported in retrospective and single-institution series. Duration of CR2 less than 6 months and age more than 15 years have emerged as important prognostic factors. A weakness of our study and other nonrandomized transplantation studies is selection bias. Patients who died early, had inadequate organ function, or had second relapse prior to URD BMT were obviously excluded, whereas chemotherapy series include patients from time of relapse. Although time from relapse to BMT was not an important prognostic factor, this does not take into account patients who relapse before BMT. There is a need for prospective, randomized trials for children who have relapsed following current intensive therapies to address this question. However, randomization is difficult, because side effects differ markedly between chemotherapy and a URD BMT. A recent study in which attempts were made to randomize patients without matched siblings to autologous transplantation or chemotherapy was unsuccessful. This was due not only to parental choice but physician bias.22 There may be a benefit to achieving minimal residual leukemia prior to transplantation,59 but there is also a risk that patients may not tolerate more than a few months of chemotherapy or may not maintain a CR2. Cord blood transplantation offers another option for these patients, with less acute and chronic GVH disease reported, even with mismatches.60-62 It is not yet clear whether a decreased risk of GVH disease improves LFS or can be counterbalanced by an increased risk of relapse.
In summary, URD BMT results in this group appear similar to those reported for multi-institutional studies for MRD transplantations and should be considered for a pediatric patient with recurrent ALL in whom the likelihood for LFS with chemotherapy is less than 40%.
The following members of the ALL Working Group were additional contributing authors to this work: Kevin Scott Baker, University of Minnesota Hospitals, Minneapolis, MN; Bruce Camitta, Medical College of Wisconsin, Milwaukee, WI; Ka Wah Chan, The University of Texas, MD Anderson Cancer Center, Houston, TX; Stephen Feig, University of California Los Angeles School of Medicine, Los Angeles, CA; Roger Giller, University of Colorado School of Medicine, Denver, CO; Gregory A. Hale, St Jude Children's Research Hospital, Memphis, TN; Cheryl Hardy, University of Mississippi School of Medicine, Jackson, MS; John Horan, University of Rochester School of Medicine, Rochester, NY; Ralph Quinones, Children's Hospital of Denver, Denver, CO; Norma Ramsay, University of Minnesota Hospital, Minneapolis, MN; Randy K. Wada, Cancer Research Center of Hawaii, Honolulu, HI; and Paul Woodard, St Jude Children's Research Hospital, Memphis, TN.
Supported by funding from the National Marrow Donor Program, the Health Resources and Services Administration no. 240-97-0036, and the Office of Naval Research N00014-93-0658 to the National Marrow Donor Program. The views expressed in this article do not reflect the official policy or position of the Department of the Navy, the Department of Defense, or the U.S. government.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nancy Bunin, Children's Hospital of Philadelphia, Division of Oncology, 34th and Civic Ctr Blvd, Philadelphia, PA 19104; e-mail: buninn@emailchop.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal