Abstract
Relapsed mantle cell lymphoma is a radiation-sensitive malignancy that is unlikely to be cured by treatment with conventional high-dose therapy and autologous stem cell transplantation. We tested the safety and efficacy of using a CD20-specific monoclonal antibody conjugated with 131I to deliver high-dose radiation selectively to all lymphoma sites. Patients with relapsed or refractory mantle cell lymphoma received infusions of 131I-labeled CD20-specific monoclonal antibody (Tositumomab). The antibody dose was 1.7 mg/kg body weight, and the amount of 131I was calibrated to deliver 20 to 25 Gy to vital normal organs. This treatment was followed 10 days later by administration of high-dose etoposide (30-60 mg/kg), cyclophosphamide (60-100 mg/kg), and infusion of cryopreserved autologous stem cells. The 16 patients in this study had received a median of 3 prior treatments, and 7 had chemotherapy-resistant disease. The median dose of 131I was 510 mCi (18.87 GBq). There were no therapy-related deaths. Among the 11 patients with conventionally measurable disease at the time of treatment, the respective complete and overall response rates were 91% and 100%. Fifteen patients remain alive, and 12 have had no progression of lymphoma at 6 to 57 months from transplantation and 16 to 97 months from diagnosis. Overall survival at 3 years from transplantation is estimated at 93%, and progression-free survival is estimated at 61%. High-dose treatment with 131I-Tositumomab, etoposide, and cyclophosphamide results in a high remission rate and may provide long-term disease-free survival for patients with relapsed or refractory mantle cell lymphoma.
Introduction
Mantle cell lymphoma (MCL) represents the second most common aggressive non-Hodgkin lymphoma and affords one of the greatest therapeutic challenges in hematologic oncology. This disease characteristically displays adverse features of both indolent and aggressive lymphomas, with short remission durations and few patients experiencing long-term disease-free survival. From the time of diagnosis, the duration of initial response is approximately 12 months, and the median survival is approximately 32 months.1-4Unlike other aggressive lymphomas, high-dose therapy with allogeneic or autologous stem cell transplantation for relapsed or refractory disease does not appear to provide durable remissions.5-10
The efficacy of standard high-dose therapy and autologous stem cell transplantation is limited, in part, by the ability of normal nonhematopoietic tissues to tolerate further dose escalation of cytotoxic therapy. One approach to overcome this dose limitation is by specifically targeting therapy to disease sites by means of B-cell–specific (anti-CD20) monoclonal antibodies. We hypothesized that, based on the exquisite radiosensitivity of non-Hodgkin lymphoma, dose-escalated radiation to all tumor sites with the use of CD20-targeted radioimmunotherapy may improve the remission durations and survival of patients with relapsed MCL.11-13 Prior studies have established the feasibility of delivering high-dose CD20-targeted radioimmunotherapy and autologous stem cell transplantation in other CD20-bearing B-cell lymphomas.14-16 In this report we describe the efficacy of high-dose chemo-radioimmunotherapy and autologous stem cell transplantation in the initial cohort of poor-risk, relapsed, or chemotherapy refractory MCL patients.
Patients, materials, and methods
Patient selection
Consecutive patients with the confirmed diagnosis of MCL treated with myeloablative anti-CD20 chemo-radioimmunotherapy on 2 sequential trials for relapsed B-cell lymphomas (a phase I dose escalation study16 and a subsequent phase II trial at the maximally tolerated dose) were evaluated. Patients were eligible if they were between the ages of 18 and 60 years, had a pathologically confirmed diagnosis of MCL expressing CD20, had progressive or persistent disease after at least one prior chemotherapy, had evidence of disease at the time of treatment, had acceptable organ function, and were otherwise considered transplantation candidates by the primary treating physician. Autologous peripheral blood stem cells had been previously collected and cryopreserved from all patients. All patients having evidence of circulating lymphoma in the peripheral blood at the time of stem cell collection underwent CD34 selection either with or without B-cell depletion of stem cell products based on the selection regimen available at the time of stem cell collection. Patients without evidence of circulating lymphoma cells had unmanipulated stem cell products stored. Patients were excluded if they had evidence of human antimouse antibody, had received more than 20 Gy of radiation therapy to a critical normal organ, had received antilymphoma therapy within 30 days, or had evidence of central nervous system lymphoma. Patients with more than 500 cc tumor bulk or splenomegaly at the time of treatment were required to have further cytoreduction because of prior observations that these patients would not have a favorable biodistribution.14 All patients provided written informed consent for participation in these sequential phase I and II trials for evaluating high-dose chemo-radioimmunotherapy in relapsed B-cell non-Hodgkin lymphoma. Both studies were approved by the Institutional Review Committees of The University of Washington and The Fred Hutchinson Cancer Research Center.
Biodistribution studies
The anti CD20 antibody Tositumomab (Corixa Pharmaceuticals, Seattle, WA) was radioiodinated with 131I as previously described.14,17 Patients were infused with antibody at 1.7 mg/kg body weight trace labeled with 131I (5-10 mCi [185-370 MBq]) to evaluate its biodistribution. Thyroid uptake was blocked with potassium iodide orally 24 hours before antibody infusion and was continued for 30 days. Patients underwent serial gamma camera imaging immediately after trace-labeled infusion and 48, 120, and 144 hours afterward to calculate the absorbed radiation dose to normal organs and whole body as previously described.15 16
Therapeutic antibody and chemotherapy infusions
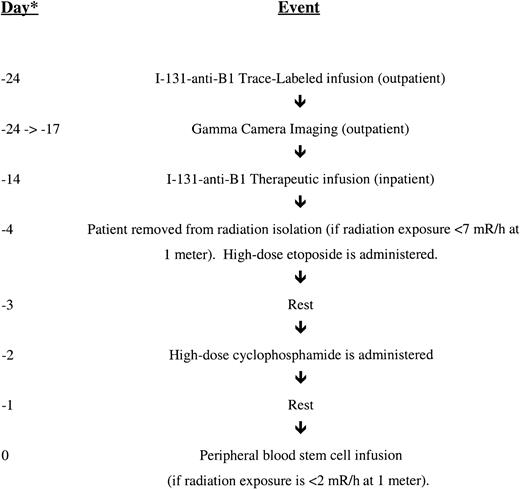
Ten to 14 days after biodistribution studies, patients received therapeutic 131I-Tositumomab at a protein dose of 1.7 mg/kg body weight to deliver the calculated target dose to the normal organ receiving the highest dose. Patients remained in radiation isolation until radiation exposure was less than or equal to 0.07 mSv/hour (7 mR/hour) at 1 m (median, 10 days; range, 8-13 days). Patients were then treated with high-dose etoposide, followed 2 days later by high-dose cyclophosphamide. Patients were infused with autologous peripheral blood stem cells when radiation exposure was less than 0.02 mSv/hour (2 mR/hour) at 1 m. The overall treatment schema is summarized in Figure 1.
Treatment schema for high-dose chemo-radioimmunotherapy.
PBSC indicates peripheral blood stem cells. *The day of treatment is listed only to give a loose time frame. The absolute determinant of the actual treatment day was the radiation exposure, which was measured daily.
Treatment schema for high-dose chemo-radioimmunotherapy.
PBSC indicates peripheral blood stem cells. *The day of treatment is listed only to give a loose time frame. The absolute determinant of the actual treatment day was the radiation exposure, which was measured daily.
Toxicity evaluation
Toxicity evaluation was classified according to the scale devised by Bearman et al.18 Toxicities on this scale were graded at grade III (life threatening) or grade IV (fatal). Toxicities not included in the Bearman Scale were evaluated by the National Cancer Institute Toxicity Scale.
Evaluation of response and follow-up
Response was determined in comparison to disease status after cytoreductive chemotherapy and stem cell collection. Complete response was defined as a resolution of all physical examination or radiographic evidence of tumor, including normalization of bone marrow by morphologic and flow cytometric criteria. Partial response was defined as a 50% or greater decrease in tumor size. The remaining patients were categorized as progression free if there was no evidence of progression (> 25% increase in size of tumor) at the 1-month posttreatment evaluation point. Patients with persistent evaluable but not bidimensional measurable disease and without evidence of blood or bone marrow involvement after cytoreductive therapy that remained unchanged after radioimmunotherapy were considered progression free. Computerized tomography evaluations were planned at 1, 3, 6, and 12 months after transplantation and annually thereafter. Bone marrow studies were performed at 1 month after therapy and annually thereafter whenever possible. Patients were clinically assessed more frequently at the discretion of the primary physician. Progression was defined as clinical, radiographic, bone marrow, flow cytometric, or molecular evidence of progressive disease, or administration of any antilymphoma therapy for suspected progression. Survival was estimated by the method of Kaplan and Meier.
Results
Baseline patient information
Sixteen consecutive patients with confirmed MCL were treated on sequential phase I and phase II trials designed for relapsed B-cell non-Hodgkin lymphomas. The clinical characteristics of these patients are detailed in Table 1. Thirteen patients (81%) were men, 4 (25%) had known gastrointestinal involvement, 2 (13%) had involvement of the orbit, and 1 patient had lymphomatous pulmonary infiltration. Thirteen patients had sufficient histologic specimens available to reconfirm the diagnosis of MCL in our own laboratories by characteristic cyclin D1 expression and/or translocation between chromosomes 11 and 14 by polymerase chain reaction (PCR). Patients underwent a median of 3 prior therapies (range, 2-7) and 9 prior cycles (range, 5-13) of chemotherapy with 88% and 31% receiving prior anthracycline-based and platinum-based regimens, respectively. The details of the prior therapy and responses are summarized in Table 2. In addition, 7 patients (44%) were refused for treatment by other major tertiary transplantation referral centers because of the perceived lack of efficacy of standard high-dose therapy and autologous stem cell transplantation for relapsed MCL, presence of chemotherapy refractory disease, or other high-risk features.
Baseline characteristics of 16 patients with relapsed mantle cell lymphoma treated with I-131-Tositumomab, etoposide, cyclophosphamide, and autologous stem cell transplantation
| Characteristic . | Value* . |
|---|---|
| Median age, y (range) | 54 (35-59) |
| Stage IV | 16 (100) |
| > 1 Extranodal site | 8 (50) |
| Elevated lactate dehydrogenase (at transplantation) | 8 (50) |
| B symptoms | 5 (31) |
| Age-adjusted International Prognostic Index score (at transplantation) | |
| Low-intermediate risk | 8 (50) |
| High-intermediate risk | 8 (50) |
| Chemosensitive disease | 8 (50) |
| Characteristic . | Value* . |
|---|---|
| Median age, y (range) | 54 (35-59) |
| Stage IV | 16 (100) |
| > 1 Extranodal site | 8 (50) |
| Elevated lactate dehydrogenase (at transplantation) | 8 (50) |
| B symptoms | 5 (31) |
| Age-adjusted International Prognostic Index score (at transplantation) | |
| Low-intermediate risk | 8 (50) |
| High-intermediate risk | 8 (50) |
| Chemosensitive disease | 8 (50) |
Unless otherwise stated, values are given as the number of patients with percentage in parentheses.
Prior therapies and response of 16 patients with relapsed mantle cell lymphoma treated with I-131-Tositumomab, etoposide, cyclophosphamide, and autologous stem cell transplantation
| Patient no. . | Prior therapies, no. of cycles, and response . |
|---|---|
| 1 | CHOP × 4→PR; CED × 1→NR |
| 2 | CVP × 5→UR; splenectomy; Flu × 5→NR; CHOP × 2→PR |
| 3 | CY × 6→PR; CED × 1→NR |
| 4 | COP × 6→NR; CY/Pred × 1→NR |
| 5 | CHOP + R × 6→CR; ESHAP × 3→PR |
| 6 | CHOP × 6→PR; ESHAP × 1→UR; DHAP × 1/CY × 1→PR; splenectomy |
| 7 | CHOP × 8→PR, CVP × 1→PR, R × 8→PR |
| 8 | CHOP × 6→CR, CY × 1→PR |
| 9 | CHOP × 6→PR, CVP × 5→NR, R→NR |
| 10 | CHOP × 6/R × 4→CR, R × 6→CR, R × 6→NR, CED × 1→PR |
| 11 | CHOP × 6→PR, CED→NR |
| 12 | CHOP+ R × 6→CR; Dex→NR; DHAP × 2→PD; R × 4/XRT→PR; CED→PR |
| 13 | CHOP × 6→PR; R × 4→PR; CED × 1→NR |
| 14 | CHOP+ R→PR; R→NR; CVP × 3→PR; ESHAP × 2→NR; CED × 1→PR |
| 15 | CHOP+ R→CR; DHAP × 1→PR; CY/Dex→PR |
| 16 | CHOP × 6→PR; CED/R→NR |
| Patient no. . | Prior therapies, no. of cycles, and response . |
|---|---|
| 1 | CHOP × 4→PR; CED × 1→NR |
| 2 | CVP × 5→UR; splenectomy; Flu × 5→NR; CHOP × 2→PR |
| 3 | CY × 6→PR; CED × 1→NR |
| 4 | COP × 6→NR; CY/Pred × 1→NR |
| 5 | CHOP + R × 6→CR; ESHAP × 3→PR |
| 6 | CHOP × 6→PR; ESHAP × 1→UR; DHAP × 1/CY × 1→PR; splenectomy |
| 7 | CHOP × 8→PR, CVP × 1→PR, R × 8→PR |
| 8 | CHOP × 6→CR, CY × 1→PR |
| 9 | CHOP × 6→PR, CVP × 5→NR, R→NR |
| 10 | CHOP × 6/R × 4→CR, R × 6→CR, R × 6→NR, CED × 1→PR |
| 11 | CHOP × 6→PR, CED→NR |
| 12 | CHOP+ R × 6→CR; Dex→NR; DHAP × 2→PD; R × 4/XRT→PR; CED→PR |
| 13 | CHOP × 6→PR; R × 4→PR; CED × 1→NR |
| 14 | CHOP+ R→PR; R→NR; CVP × 3→PR; ESHAP × 2→NR; CED × 1→PR |
| 15 | CHOP+ R→CR; DHAP × 1→PR; CY/Dex→PR |
| 16 | CHOP × 6→PR; CED/R→NR |
CHOP indicates cyclophosphamide, doxorubicin, vincristine, prednisone; PR, partial response; CED, cyclophosphamide, etoposide, dexamethasone; NR, no response; CVP, cyclophosphamide, vincristine, prednisone; UR, unknown response; Flu, fludarabine; CY, cyclophosphamide; COP, cyclophosphamide, vincristine, prednisone; Pred, prednisone; R, rituximab; CR, complete response; ESHAP, etoposide, methylprednisolone, cytarabine, cisplatin; DHAP, dexamethasone, cytarabine, cisplatin; Dex, dexamethasone; and XRT, radiation therapy.
Treatment received
Because patients were treated according to both phase I (dose-finding) and phase II trials, which also included patients with other types of B-cell lymphomas, not all MCL patients received identical doses of 131I-Tositumomab or chemotherapy. MCL patients received a median of 510 mCi (18.87 GBq) of 131I (range, 324-782 mCi, [11.99-29.94 GBq]). Although the physical half-life of 131I is 8 days, the median effective biologic half-time of 131I-Tositumomab was 73 hours (range, 45-86 hours) in these patients. Two patients received estimated absorbed radiation doses of 20 Gy and 23 Gy to the dose-limiting critical normal organ, and the remaining 14 patients received the maximally tolerated dose of 25 Gy. Likewise, 12 patients received 60 mg/kg etoposide along with 100 mg/kg cyclophosphamide, and 4 patients were treated with 30 mg/kg etoposide and 60 mg/kg cyclophosphamide. All patients were reinfused with autologous peripheral blood stem cells with 3 of the patients (19%) receiving CD34-selected stem cell products and 8 (50%) receiving products that were both CD34 selected and B-cell depleted with anti-CD20 and anti-CD19 antibodies. Three patients had molecular evidence of residual lymphomatous contamination of peripheral blood stem cells at the time of reinfusion with 2 of these 3 patients receiving CD34-selected and CD19/CD20-depleted stem cells and 1 receiving unmanipulated stem cells.
Toxicity
Overall, toxicities were comparable to standard autologous transplantation conditioning regimens. There was no treatment-related mortality. Toxicity experienced during radiation isolation was all less than grade 1, generally consisted of mild anorexia, and did not require entry of medical staff into the patient room. After transplantation one patient experienced reversible interstitial pneumonitis requiring temporary mechanical ventilation. Another patient who had received prior radiotherapy to lung fields also experienced delayed pneumonitis of grade II severity that responded to corticosteroid administration. All patients demonstrated expected myeloablation with a median time to neutrophil recovery (≥ 0.5 × 109/L) of 12 days (range, 9-19 days) after transplantation and a median time to unsupported platelet count (20 × 109/L) of 12 days (range, 8-24 days) after transplantation. One patient who remains in remission has developed delayed neutropenia and thrombocytopenia persisting for more than 1 year after transplantation without evidence of myelodysplasia.
Response to therapy
Eleven patients had disease that could be fully evaluated for response to the conditioning regimen, whereas 5 patients had minimal radiographically defined residual disease or only molecular evidence of disease at the time of transplantation. All 5 of these patients with minimal disease remained progression free at 1 month after transplantation and have remained progression free to date. Of the 11 patients with fully evaluable disease, 8 (73%) attained a complete response and 1 (9%) achieved a partial response at the initial 1-month posttransplantation evaluation, with the 2 nonresponding patients remaining progression free. One of these 2 evaluable patients who did not attain a remission had residual flow-cytometrically identified lymphoma involving the bone marrow at the 1-month point that became flow and morphologically negative at 3 months after transplantation. The second patient who had radiographically stable disease at the 1-month posttransplantation time point was radiographically in a complete remission at the 1-year follow-up. If these 2 initially progression-free patients are considered complete remissions, which they eventually achieved, the overall response rate among the 11 evaluable patients was 100%, with 91% attaining a complete response.
Ten of 16 patients had molecular evidence of disease either by PCR detection of a characteristic translocation between chromosomes 11 and 14 (BCL-1) or immunoglobulin heavy chain clonality in blood or bone marrow immediately pretreatment after cytoreduction and peripheral blood stem cell collection. Five of these 10 patients attained molecular remission in both blood and bone marrow at the 1-month time point (50%). In addition, 1 of these 5 remaining PCR-positive patients had a molecular evaluation of blood and bone marrow at 1 year and has converted to PCR negativity. The overall molecular response to date is 60% in the 10 evaluable patients.
Overall and progression-free survival
Fifteen (94%) of 16 patients remain alive at the time of data analysis (July 6, 2001) with a median follow-up of 19 months (range, 6-57 months) from the time of transplantation and 49 months (range, 16-97 months) from time of diagnosis. In addition, 12 patients (75%) remain alive and progression free with 3 patients remaining alive and in remission 4 or more years after treatment. These data result in an estimated 3-year overall survival of 93% and an estimated 3-year progression-free survival of 61% (Figure2). By univariate analysis, the chemotherapy dose; radiation dose; number of prior regimens; chemosensitivity at the time of transplantation; PCR status of patients' peripheral blood stem cells, blood, or bone marrow; elevated lactate dehydrogenase; or bulk of disease were not correlated with overall or progression-free survival, although the power of such an analysis is limited by sample size and low number of events (progression or death).
Overall and progression-free survival from the time of transplantation of 16 patients with relapsed mantle cell lymphoma treated with 131I-Tositumomab, etoposide, cyclophosphamide, and autologous stem cell transplantation.
Overall and progression-free survival from the time of transplantation of 16 patients with relapsed mantle cell lymphoma treated with 131I-Tositumomab, etoposide, cyclophosphamide, and autologous stem cell transplantation.
Discussion
Our study is the first to demonstrate the potential clinical efficacy of supplanting total body radiation with high-dose radioimmunotherapy in an autologous transplantation conditioning regimen for patients with relapsed or refractory MCL. This favorable outcome is particularly unexpected in these patients who were heavily pretreated (9 of 16 received 3 or more prior therapies), commonly had an elevated lactate dehydrogenase at the time of transplantation (50%), and whose disease frequently was not responsive to their last regimen before transplantation (44%). These negative pretransplantation features have been associated with poor outcomes in other non-Hodgkin lymphoma subtypes in which the use of high-dose therapy and autologous stem cell transplantation has been more extensively evaluated.19-22
The apparent efficacy of this approach in MCL may be attributed to several potential mechanisms. In prior studies we evaluated radiation doses to tumor by biopsy and gamma counting of involved nodes after trace infusions. By using this methodology, we have been able to demonstrate on average 10:1 and 2 to 3:1 tumor-to-whole body and tumor-to-highest critical normal organ ratios of delivered radiation, respectively. This approach resulted in doses to tumor as high as 91.5 Gy.14 The persistent remissions of these high-risk patients correspond with the observation that lymphoma patients rarely relapse within sites previously treated with external beam radiation therapy, especially when doses above 30 to 40Gy are administered.11,12,23,24 The delayed remissions seen in 2 of 11 evaluable patients parallel our previous observations with high-dose radioimmunotherapy and may be related to the low radiation dose rates (centi-Gray per minute) of 131I-Tositumomab relative to total body irradiation or the effect of the antibody alone.25 The potential efficacy of this approach may be further augmented by the high surface density of the CD20 target antigen on nearly all cases of MCL.26 This increased antigen density could allow for a greater number of sites for antibody binding, further optimizing the targeted radioimmunotherapy. Finally, the observation that 9 of the 16 patients had progressive disease after prior unlabeled anti-CD20 therapy (rituximab) suggests that the major therapeutic activity of radioimmunotherapy is derived from the targeted radiation, rather than the effect of the antibody itself.
In addition to the high response rate, we also observed molecular remissions in most of the patients remaining PCR positive despite pretransplantation cytoreductive therapy. Notably, the lack of correlation of progression-free survival with ability to attain PCR negativity may reflect the imprecision of the assay, the inability to fully assess the molecular status of nodal disease, or the small number of events on which to base an analysis. Longer follow-up, including repeat molecular analyses of the remaining 4 PCR-positive patients, will be necessary to determine the predictive role of molecular remissions when using high-dose radioimmunotherapy for MCL.
Although the results of this trial are encouraging, the findings should not be extrapolated to subsets of patients who were excluded from this study. Patients with splenomegaly or tumor bulk more than 500 cc were excluded because of previous experience demonstrating that such patients would not have optimal biodistributions of antibody.14 Even though 2 individuals with splenomegaly had a splenectomy before enrollment, excluding patients with enlarged spleens or excessively bulky disease may have favorably skewed our results. However, patients with no detectable evidence of disease after their pretransplantation cytoreductive therapy were also excluded from this trial (because of the absence of a target for the radioimmunotherapy), thus excluding the patients most likely to have the best outcome.
Other approaches have also been investigated to improve the outcome for patients with MCL. Encouraging results have come from the use of an aggressive multiagent acute lymphoblastic leukemialike induction therapy (HyperCVAD/HD MTX HDAC) followed by high-dose therapy and autologous or allogeneic stem cell transplantation in patients with newly diagnosed MCL.8 Unfortunately, this approach yielded an estimated 17% of previously treated patients similar to those in our study being event free at 3 years. Other groups have also observed similar results with little evidence of long-term remission for patients with relapsed/refractory disease by using standard high-dose therapy and autologous stem cell transplantation.5,6,9,10 In one large series all patients who had received 3 or more prior regimens relapsed by 14 months after transplantation.7
Our data provide the first suggestion that escalation of radiation therapy dose by means of a high-dose radioimmunotherapy-based transplantation-conditioning regimen may overcome the inherently poor prognosis of patients with relapsed or refractory MCL, potentially affording long-term remissions. Additional patients, further follow-up, and randomized trials will be needed to confirm the efficacy of this approach.
The technical expertise of Carolyn Thostenson and Bhavesha Patel is gratefully acknowledged. We also thank Corixa Pharmaceutical for providing Tositumomab antibody for these trials.
Support by National Institutes of Health grants P01CA44991 and K23CA85479 and by American Society of Clinical Oncology Young Investigator Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ajay K. Gopal, Seattle Cancer Care Alliance, Mailstop G6-800 (Rm 6802), 825 Eastlake Ave E, Seattle, WA 98109; e-mail: agopal@u.washington.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal