Abstract
Protective protein/cathepsin A (PPCA), a lysosomal carboxypeptidase, is deficient in the neurodegenerative lysosomal disorder galactosialidosis (GS). PPCA−/− mice display a disease course similar to that of severe human GS, resulting in nephropathy, ataxia, and premature death. Bone marrow transplantation (BMT) in mutant animals using transgenic BM overexpressing the corrective enzyme in either erythroid cells or monocytes/macrophages has proven effective for the improvement of the phenotype, and encouraged the use of genetically modified BM cells for ex vivo gene therapy of GS. Here, we established stable donor hematopoiesis in PPCA−/− mice that received hematopoietic progenitors transduced with a murine stem cell virus (MSCV)–based, bicistronic retroviral vector overexpressing PPCA and the green fluorescent protein (GFP) marker. We observed complete correction of the disease phenotype in the systemic organs up to 10 months after transplantation. PPCA+ BM-derived cells were detected in all tissues, with the highest expression in liver, spleen, BM, thymus, and lung. In addition, a lysosomal immunostaining was seen in nonhematopoietic cells, indicating efficient uptake of the corrective protein by these cells and cross-correction. Expression in the brain occurred throughout the parenchyma but was mainly localized on perivascular areas. However, PPCA expression in the central nervous system was apparently sufficient to delay the onset of Purkinje cell degeneration and to correct the ataxia. The long-term expression and internalization of the PPCA by cells of systemic organs and the clear improvement of the neurologic phenotype support the use of this approach for the treatment of GS in humans.
Introduction
Defective genes encoding specific lysosomal hydrolases are responsible for more than 40 disorders of the metabolism, known as lysosomal storage diseases (LSDs).1One of the glycoproteinoses, the autosomal recessive galactosialidosis (GS),2,3 results from mutations in the PPCAgene,4 causing a secondary deficiency of β-galactosidase and N-acetyl-α-neuraminidase.3 Intracellular accumulation of sialyloligosaccharides and glycopeptides leads to the features characteristic of an LSD, including coarse facies, ocular cherry-red spots, vertebral changes, foamy bone marrow (BM) cells, and vacuolated peripheral blood lymphocytes.2,5 In addition, most patients with GS experience severe neurologic damage characterized by ataxia, diffuse leukoencephalopathy, and mental retardation; death usually occurs within the first 2 years of life.2
Enzyme replacement that ameliorates or reverses systemic and neurologic defects is the goal of curative treatment for LSDs. This strategy is based on the observation that soluble enzyme precursors secreted by one cell type can be internalized via receptor-mediated endocytosis by deficient cells with consequent resolution of toxic catabolite accumulation, that is, correction “in trans.” BM progenitor cells are an attractive source of corrective enzyme because of their potential to repopulate the recipient and to supply functional enzyme to cells in affected organs, including the central nervous system (CNS).6 Allogeneic bone marrow transplantation (BMT) and syngeneic BMTs in affected patients and animal models effectively ameliorate visceral and bony lesions7-13; however, diseases with early, predominantly CNS involvement respond poorly.8 14 Allogeneic BMT is still limited by difficulties in finding suitable HLA-compatible donors, high rates of nonengraftment, severe graft-versus-host disease, and other causes of transplantation-related morbidity and mortality.
The use of autologous hematopoietic progenitor cells (HPCs) that are genetically engineered to express a therapeutic gene could, in principle, circumvent some transplantation-associated obstacles. We have recently proven the feasibility of a BMT approach in our murine GS model. Early in life, PPCA−/− animals experience systemic and CNS abnormalities that closely mimic the severe form of GS in humans.13,15 The animals develop severe nephropathy as well as the ataxia and neuronal degeneration characteristic of this disorder, and die prematurely.13,15Transplantation of BM cells that overexpress a PPCAtransgene in either the erythroid or monocyte/macrophage lineage into lethally irradiated PPCA−/− animals resulted in complete correction of systemic pathology of GS.13,16Partial amelioration of the disease phenotype in the CNS was also observed, but a clear delay in the onset of the cerebellar phenotype was only achieved when transgenic mice were crossed into thePPCA−/− background.16 Overall these results reinforced the feasibility of using ex vivo gene therapy for the treatment of GS.
We have now tested whether genetically modified PPCA−/−BM cells could afford long-term expression of the therapeutic enzyme in transplanted PPCA−/− recipients and correct the disease phenotype. We report thatPPCA−/− HPCs transduced with a retrovirus that expresses PPCA leads to complete correction of systemic organ damage, amelioration of CNS pathology, and functional correction of motor coordination defects in GS mice.
Materials and methods
Cell lines and vector construction
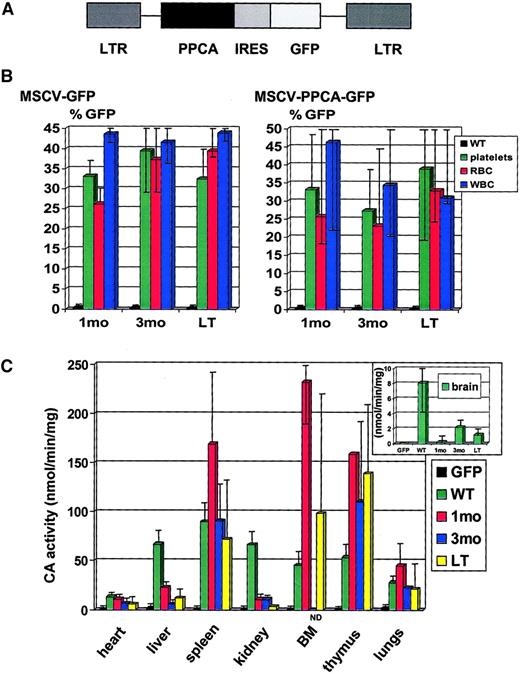
GP+E86, ecotropic packaging cell line,17 293T, and NIH3T3 cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), and antibiotics. We created the murine stem cell virus (MSCV)–PPCA retroviral plasmid by inserting human PPCA complementary DNA (cDNA) into a site that is 5′ of the internal ribosomal entry sequence18,19 in the MSCV-GFP vector previously described20 21 (Figure1A).
Retrovirally transduced PPCA−/− BM cells restore cathepsin A activity in transplanted PPCA−/−recipients.
(A) Schematic diagram of the retroviral bicistronic construct encoding the human PPCA cDNA. In this vector, expression of both the human PPCA and the GFP marker is driven by the viral LTR. Translation of GFP is initiated at the internal ribosomal entry site (IRES). (B) Platelets, white blood cells (WBCs), and erythrocytes (RBCs), obtained from PPCA−/−mice transplanted with either MSCV-GFP– or MSCV-PPCA-GFP–transduced PPCA−/−BM, at different time points after treatment, were FACS sorted and analyzed for GFP expression; 1 month (1 month, 7 mice total); 3 months (3 months, 6 mice total); LT (long-term, ages 6, 8, 9, and 10 months, 5 mice total). (C) Cathepsin A (CA) activity was assayed in tissue-homogenates of MSCV-PPCA-GFP–treated mice, at different time points after treatment. Wild-type (WT, 4 mice total) and MSCV-GFP–treated PPCA−/−mice (GFP, 4 mice total), ranging in age between 3 and 8 months, were used as positive and negative controls, respectively. The level of CA activity was independent from the age of the wild-type or MSCV-GFP–treated mice. The inset shows the CA activity in brain homogenates ofPPCA−/−treated and untreated mice, as well as controls. For the CA activity in the thymus of 1-month–treated mice, only one tissue sample was collected and measured; 1 month (1 month, 4 mice total); 3 months (3 months, 3 mice total); LT (long-term, ages 6, 8, 9, and 10 months, 5 mice total). We observed considerable variation in the measured CA activity between mice of the same age group, likely due to differences in engraftment of the transplanted BM cells. The presented data are average values with a typical high and low limit of ± 25% to 50%, which is significantly above the CA activity of the GFP controls. The bars represent SDs.
Retrovirally transduced PPCA−/− BM cells restore cathepsin A activity in transplanted PPCA−/−recipients.
(A) Schematic diagram of the retroviral bicistronic construct encoding the human PPCA cDNA. In this vector, expression of both the human PPCA and the GFP marker is driven by the viral LTR. Translation of GFP is initiated at the internal ribosomal entry site (IRES). (B) Platelets, white blood cells (WBCs), and erythrocytes (RBCs), obtained from PPCA−/−mice transplanted with either MSCV-GFP– or MSCV-PPCA-GFP–transduced PPCA−/−BM, at different time points after treatment, were FACS sorted and analyzed for GFP expression; 1 month (1 month, 7 mice total); 3 months (3 months, 6 mice total); LT (long-term, ages 6, 8, 9, and 10 months, 5 mice total). (C) Cathepsin A (CA) activity was assayed in tissue-homogenates of MSCV-PPCA-GFP–treated mice, at different time points after treatment. Wild-type (WT, 4 mice total) and MSCV-GFP–treated PPCA−/−mice (GFP, 4 mice total), ranging in age between 3 and 8 months, were used as positive and negative controls, respectively. The level of CA activity was independent from the age of the wild-type or MSCV-GFP–treated mice. The inset shows the CA activity in brain homogenates ofPPCA−/−treated and untreated mice, as well as controls. For the CA activity in the thymus of 1-month–treated mice, only one tissue sample was collected and measured; 1 month (1 month, 4 mice total); 3 months (3 months, 3 mice total); LT (long-term, ages 6, 8, 9, and 10 months, 5 mice total). We observed considerable variation in the measured CA activity between mice of the same age group, likely due to differences in engraftment of the transplanted BM cells. The presented data are average values with a typical high and low limit of ± 25% to 50%, which is significantly above the CA activity of the GFP controls. The bars represent SDs.
Generation of an ecotropic virus producer line
Conditioned medium containing high-titer, amphotropic MSCV-PPCA particles was derived by cotransfection of 293T cells with the retroviral vector plasmid and helper plasmid containing gag, pol, and env genes driven by a Moloney leukemia virus long terminal repeat (LTR).22 The conditioned medium was used to transduce the GP+E86 line, and viral producer cells were derived as previously described.23 The viral titer was determined by serial dilutions of the conditioned medium in NIH3T3, followed by analysis of green fluorescent protein (GFP) expression. The titer was 5 × 106 particles/mL.
BMT
PPCA−/− donors were given intraperitoneal injections of 150 mg/kg 5-fluorouracil (Sigma Chemical, St Louis, MO) 48 hours before marrow harvest to mobilize the HPCs. BM cells were prestimulated with 20 ng/mL mouse interleukin (IL)–3, 50 ng/mL human IL-6, and 50 ng/mL mouse stem cell factor (R D Systems, Minneapolis, MN) in DMEM plus 15% heat-inactivated FBS. The hematopoietic cells were cocultured with irradiated (1200 cGy) viral producer cells in the same medium supplemented with 6 μg/mL polybrene for 48 hours. PPCA−/− recipients were lethally irradiated (850 cGy) 24 hours prior to tail-vein injection of the genetically modified BM cells. A ratio of 2 to 4 GS donors to 1 recipient was used. Samples of the GS BM cells, transduced with either MSCV-GFP or MSCV-PPCA, were analyzed by fluorescence-activated cell sorting (FACS; Becton Dickinson, Mountain View, CA) and the percentage of GFP+ cells was determined (see “Results”). The initial GFP analysis of the second BM transduction showed an efficiency of only about 0.5%, similar to that of untransduced cells. This value did not correlate with the GFP expression levels detected subsequently in peripheral blood cells (PBCs) of the mice receiving transplants with this transduced marrow and with their cathepsin A activity in tissues (Figure 1B,C), which was in the same range of the activity in the other 4 groups of mice receiving transplants. Therefore, we assumed that the initial FACS analysis was erroneous.
Determination of GFP expression in PBCs
Recipients were bled by orbital sinus puncture at 1, 3, 6, and 8 to 10 months after BMT. Blood (20 μL) was collected in 1 mL cold phosphate-buffered saline (PBS) for FACS analysis of erythrocytes and platelets. For analysis of lymphocytes, erythrocytes were lysed in Gay solution and propidium iodide was added.23
Enzyme activity assay
Tissues were homogenized in water. Cathepsin A activity was measured with the synthetic dipeptide substrate Z-Phe-Ala as described earlier.24 Total protein concentration was determined with the bicinchoninic acid reagent (Pierce Chemical, Rockford, IL).
Histochemical analysis
Antibodies against the 32-kd subunit of human PPCA (α-32) were raised in rabbit and affinity purified against the human protein.25 This antibody was shown to selectively recognize the human PPCA protein and does not cross-react with the endogenous murine PPCA.16 Paraffin-embedded tissue sections were deparaffinized and hydrated; antigen retrieval was accomplished by boiling the sections in 0.1 M citrate, pH 6.0. After a 20-minute blocking process, the sections were incubated overnight with either α-32 or anti-GFP (α-GFP, Clontech Laboratories, Palo Alto, CA) antibodies followed by washing and incubation with goat-antirabbit IgG secondary antibodies (Pharmingen, San Diego, CA) for 2 hours at room temperature. Antigen-antibody complexes were detected with the ABC horseradish peroxidase system, which uses a VIP (purple) or diaminobenzidine (brown) substrate (Vector, Burlingame, CA). For PEP19 staining, serially sectioned, cerebella were processed and incubated with anti-PEP19 antibodies26 (a kind gift of Dr James Morgan, Developmental Neurobiology, St Jude Children's Research Hospital) as above.
Purkinje cell count
Counting of Purkinje cells was performed following the method described in Smeyne and Goldowitz.27
Results
Expression of retrovirally encoded PPCA in GS BM cells
To investigate whether genetically modified HPCs can correct the murine GS phenotype, we constructed a MSCV-based bicistronic vector containing PPCA cDNA that was linked by an internal ribosomal entry site to the gene encoding the GFP marker (MSCV-PPCA, Figure 1A). An identical vector carrying only the GFP gene was used as a control (MSCV-GFP). We first determined that the total number of BM cells harvested from PPCA−/−donors, aged 2 to 6 months, was similar to that of cells from wild-type, age-matched mice and that the different lineages were correctly represented. Total PPCA−/− BM was then transduced with either MSCV-PPCA or MSCV-GFP ex vivo,to assess the transducibility of deficient cells versus normal BM. In 2 pilot experiments performed before BMT, the transduction efficiency of PPCA−/− BM cells, calculated on the basis of GFP expression, was 15% and 20% with the MSCV-PPCA vector, and 19% and 39% with the MSCV-GFP vector. In parallel, a 15-fold increase in cathepsin A activity was measured in transduced PPCA−/− BM cells compared to untreated cells or cells transduced with the MSCV-GFP vector. We also assessed the level of cathepsin A activity in lysates of clonogenic progenitor colonies that were positive for GFP as visualized by fluorescence microscopy. Cathepsin A activity was more than 100-fold higher in MSCV-PPCA+ colonies than in MSCV-GFP–transduced colonies.
Correction of the murine GS phenotype by genetically modifiedPPCA−/−BM cells
To evaluate the effects of enforced PPCA expression in vivo, we transplanted PPCA−/− BM cells transduced with either MSCV-PPCA or MSCV-GFP into lethally irradiated, 3- to 6-week-old GS mice. In 5 independent transplantation experiments, the transduction efficiency of either the MSCV-PPCA or the MSCV-GFP retrovirus was calculated on the basis of GFP expression in FACS-sorted cells, immediately before transplantation. With the exception of the second BM transduction experiment (see “Materials and methods”), the transduction efficiency of the MSCV-PPCA virus was 28% (experiment [exp] 1); 23% (exp 3); 19% (exp 4); and 17% (exp 5). The transduction efficiency of the MSCV-GFP virus ranged from 19% to 44%. GFP-expressing cells of the erythroid, myeloid, or lymphoid lineage were detected by FACS analysis of peripheral blood samples, collected at different time points after transplantation. Regardless of the vector used, the percentage of gated cells expressing GFP varied between 18% and 40% in erythrocytes, 20% and 61% in platelets, and 20% and 56% in lymphocytes (Figure 1B). To estimate the levels of the therapeutic enzyme in different transplanted mice, cathepsin A activity was assayed in tissue homogenates from organs of recipients at various time points after BMT. For as long as 10 months after treatment, increased cathepsin A activity was detected in most tissues; the highest levels were measured in spleen, BM, and thymus, but also liver, kidney, and heart had persistent and increased activity compared to the knockout or BMT-GFP–treated mice (Figure 1C). Cathepsin A activity in total brain lysates, which is usually low also in wild-type samples, was only marginally increased and varied among animals receiving transplants, probably because of the uneven distribution of engrafted cells that expressed the corrective enzyme. Although transgene expression differed among recipients, the level of enzyme was apparently sufficient to correct or ameliorate the histologic changes consistent with PPCA deficiency (Figures 2-6).
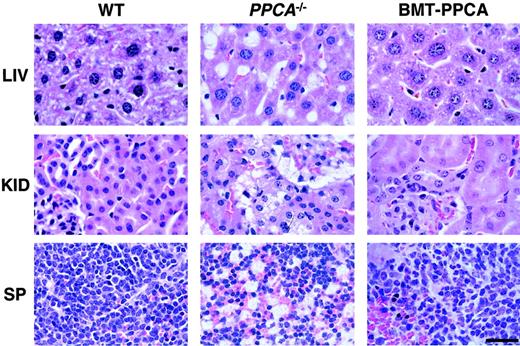
Histology of systemic organs from BM-transplanted GS mice.
Organs from PPCA−/−mice transplanted with total −/−BM transduced with the MSCV-PPCA (BMT-PPCA) retrovirus were isolated at different time points after treatment. Hematoxylin and eosin-stained tissue sections of the liver (LIV), kidney (KID), and spleen (SP) from a BMT-PPCA–treatedPPCA−/−mouse killed at 9 months after treatment, and from age-matched wild-type andPPCA−/−mice revealed the complete restoration of normal tissue morphology with BM expressing PPCA, compared to the extensive vacuolation present in thePPCA−/−control mouse. Size bar corresponds to 30 μm.
Histology of systemic organs from BM-transplanted GS mice.
Organs from PPCA−/−mice transplanted with total −/−BM transduced with the MSCV-PPCA (BMT-PPCA) retrovirus were isolated at different time points after treatment. Hematoxylin and eosin-stained tissue sections of the liver (LIV), kidney (KID), and spleen (SP) from a BMT-PPCA–treatedPPCA−/−mouse killed at 9 months after treatment, and from age-matched wild-type andPPCA−/−mice revealed the complete restoration of normal tissue morphology with BM expressing PPCA, compared to the extensive vacuolation present in thePPCA−/−control mouse. Size bar corresponds to 30 μm.
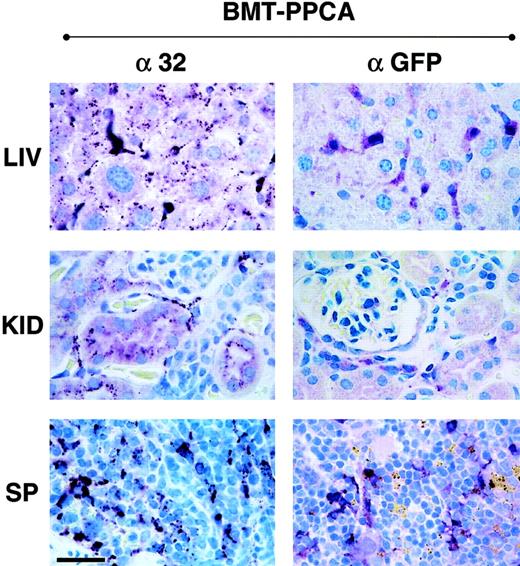
Immunostaining of tissue sections from BM-transplanted GS mice with α-32 and α-GFP antibodies.
Numerous human PPCA-expressing cells were detected by immunostaining with α-32 antibody, monospecific for the human protein (left panels). In the liver of a BMT-treated mouse killed at 9 months after treatment, strong immunostaining was detected in Kupffer cells, as confirmed by staining of adjacent sections with the macrophage-specific anti–Mac-1 antibody. The clear punctate staining of the hepatocytes demonstrated internalization of the corrective enzyme by these cells. In the kidney the presence of the human PPCA was detected in the proximal convoluted tubules and Bowman capsule. Numerous macrophages and splenocytes in the spleen of transplanted mice were positive for the human protein. Staining of the same tissues with α-GFP antibody (right panels) was restricted to cells in locations consistent with their being of hematopoietic origin. Size bar corresponds to 30 μm.
Immunostaining of tissue sections from BM-transplanted GS mice with α-32 and α-GFP antibodies.
Numerous human PPCA-expressing cells were detected by immunostaining with α-32 antibody, monospecific for the human protein (left panels). In the liver of a BMT-treated mouse killed at 9 months after treatment, strong immunostaining was detected in Kupffer cells, as confirmed by staining of adjacent sections with the macrophage-specific anti–Mac-1 antibody. The clear punctate staining of the hepatocytes demonstrated internalization of the corrective enzyme by these cells. In the kidney the presence of the human PPCA was detected in the proximal convoluted tubules and Bowman capsule. Numerous macrophages and splenocytes in the spleen of transplanted mice were positive for the human protein. Staining of the same tissues with α-GFP antibody (right panels) was restricted to cells in locations consistent with their being of hematopoietic origin. Size bar corresponds to 30 μm.
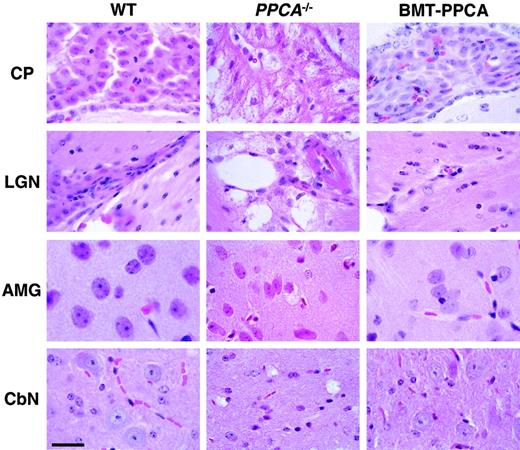
Histology of 4 regions of the brain from BM-transplanted GS mice.
Hematoxylin and eosin staining of the choroid plexus (CP), the lateral geniculate nucleus (LGN), the amygdala (AMG), and the deep cerebellar nucleus (CbN) isolated from BMT-PPCA–treated GS mouse at 9 months after transplantation showed clear reduction of storage in neural cells compared to an age-matched GS animal. The overall brain architecture was clearly improved due to the clearance of storage material in endothelial cells and perivascular and leptomeningeal macrophages. Storage in some of the neurons in the amygdala and cerebellar nucleus was also cleared. Size bar corresponds to 30 μm.
Histology of 4 regions of the brain from BM-transplanted GS mice.
Hematoxylin and eosin staining of the choroid plexus (CP), the lateral geniculate nucleus (LGN), the amygdala (AMG), and the deep cerebellar nucleus (CbN) isolated from BMT-PPCA–treated GS mouse at 9 months after transplantation showed clear reduction of storage in neural cells compared to an age-matched GS animal. The overall brain architecture was clearly improved due to the clearance of storage material in endothelial cells and perivascular and leptomeningeal macrophages. Storage in some of the neurons in the amygdala and cerebellar nucleus was also cleared. Size bar corresponds to 30 μm.
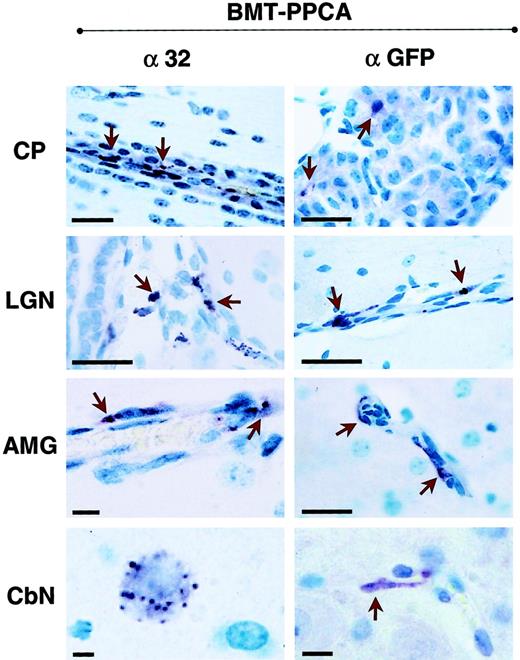
Immunostaining of 4 brain regions from BM-transplanted GS mice with α-32 and α-GFP antibodies.
α-32 immunostaining of brain sections derived from BMT-PPCA–transplanted mice at 9 months after treatment revealed numerous PPCA+ endothelial cells, perivascular macrophages, sparse neurons as well as the cuboidal epithelium of the choroid plexus and its macrophages (indicated by arrowheads). Similar but more restricted immunostaining was detected in sections of the same regions stained with α-GFP antibody. Size bars correspond to 40 μm.
Immunostaining of 4 brain regions from BM-transplanted GS mice with α-32 and α-GFP antibodies.
α-32 immunostaining of brain sections derived from BMT-PPCA–transplanted mice at 9 months after treatment revealed numerous PPCA+ endothelial cells, perivascular macrophages, sparse neurons as well as the cuboidal epithelium of the choroid plexus and its macrophages (indicated by arrowheads). Similar but more restricted immunostaining was detected in sections of the same regions stained with α-GFP antibody. Size bars correspond to 40 μm.
PEP19 staining of cerebellar sections from BM-transplanted GS mice.
Serial sections of the cerebellum from a 9-month-old GS mouse transplanted with MSCV-PPCA–marked BM cells were stained with anti-PEP19 antibody. Note the dramatic loss of Purkinje cells in an age-matched GS mouse and the significant number of these cells that are retained in the treated animal. Size bars correspond to 60 μm for the upper panels and 30 μm for the lower panels.
PEP19 staining of cerebellar sections from BM-transplanted GS mice.
Serial sections of the cerebellum from a 9-month-old GS mouse transplanted with MSCV-PPCA–marked BM cells were stained with anti-PEP19 antibody. Note the dramatic loss of Purkinje cells in an age-matched GS mouse and the significant number of these cells that are retained in the treated animal. Size bars correspond to 60 μm for the upper panels and 30 μm for the lower panels.
Correction of systemic pathology in PPCA−/− mice after transplantation of MSCV-PPCA–transduced BM cells
In contrast to the PPCA−/− untreated mice, mice transplanted with the MSCV-PPCA–marked BM had no systemic manifestations of disease; they had a normal gross appearance, a shiny fur, lack of diffuse edema, and inflammation of the eyelids, no tremor, or ataxic movements up to 10 months after BMT. These features become evident in PPCA−/− mice starting at the age of 3 to 4 months.13,15,16 To assess the effect of PPCA-expressing BM cells on organ morphology, we performed histologic and immunohistochemical analyses of tissue sections. The combined use of an anti-PPCA antibody, specific for the human PPCA, and an anti-GFP antibody enabled us to discriminate between cells expressing the transgene and cells that have internalized the corrective enzyme (Figure 3). Transplanted mice were analyzed at 1 to 10 months after treatment. Systemic correction was observed in all MSCV-PPCA–transplanted animals, although the number of PPCA+ cells varied slightly in different mice, according to the transduction efficiency and the repopulating capacity of retrovirally marked BM cells. PPCA expression persisted long term, indicating that sufficient numbers of HPCs were transduced. As predicted by the levels of cathepsin A activity in various organs, we detected high expression of PPCA in tissues of hematopoietic origin; in the spleen the distribution of PPCA-expressing cells was similar to that observed in previous studies13 16 (Figure 3, α-32 panel). This resulted in full reversal of the morphologic changes that remained apparent in untreated GS mice (Figure 2, BMT-PPCA andPPCA−/−). Clearance of storage material occurred in the liver, both in Kupffer cells and in the hepatic parenchyma (Figure 2, BMT-PPCA). Staining of adjacent sections with the macrophage-specific anti–Mac-1 antibody (not shown) confirmed that the BM-derived Kupffer cells were highly positive for PPCA (Figure 3, α-32 panel). In addition, hepatocytes displayed a PPCA-specific punctate staining characteristic of lysosomes; this finding indicated that PPCA was actively internalized (Figure 3, α-32 panel). Foamy histiocytes and vacuolated endothelial cells and hepatocytes persisted in the untreated mice of similar age (Figure 2,PPCA−/−). In the kidney, one of the most severely affected organs in GS, PPCA-specific immunostaining was observed throughout the renal parenchyma (Figure 3, α-32 panel). This feature was associated with complete resolution of lysosomal storage in the proximal tubular and glomerular epithelia that instead was still evident in PPCA−/− mice (Figure 2, BMT-PPCA). Strong immunostaining was also seen in the pulmonary alveolar macrophages, the heart, the thymus, and the salivary glands (data not shown). In all examined organs, the number of PPCA-expressing cells exceeded that of GFP+ cells (Figure 3, α-GFP panels) that represented the population of transduced BM-derived cells that repopulated the organs. These observations implied that efficient cell-to-cell transfer of PPCA had occurred, resulting in the clearance of lysosomal storage and correction of the systemic phenotype.
Amelioration of the pathologic changes in the CNS of recipient mice
Regional distribution of CNS abnormalities in murine GS13 15 makes it difficult to accurately estimate whether isolated neuronal cells have been cleared of storage material. To ascertain the effects of transplanted, genetically corrected cells on the CNS phenotype, we performed histologic, immunochemical, and enzymatic analyses of the CNS at various time points after transplantation. Comparison of brains from mice that received MSCV-PPCA–transduced BM cells with those fromPPCA−/− mice revealed a significant amelioration of the pathologic phenotype (Figure4, BMT-PPCA andPPCA−/−). In the regions most affected by PPCA deficiency, including the cerebellar nuclei, the lateral geniculate nuclei, and the amygdala, the amount of storage material appeared reduced in recipients of MSCV-PPCA–transduced BM cells (Figure 4, BMT-PPCA). The overall brain architecture was overtly improved in these mice, likely because the endothelial cells and perivascular macrophages were largely corrected. In accordance with this finding, immunoreactive PPCA in the brain was primarily restricted to leptomeningial, perivascular macrophages, and the vascular structure of the choroid plexus (Figure 5, α-32). This expression pattern coincided with that observed with α-GFP antibody (Figure 5, α-GFP), although PPCA expression was more widely distributed than GFP expression, and occasional neurons displaying a clear punctate staining were observed only with the α-32 antibody (Figure 5).
The relatively small number of PPCA-expressing cells detected in neural tissues was in agreement with the low levels of cathepsin A activity measured in total brain lysates. However, given the overall improvement of brain morphology, it is apparent that only small amounts of enzyme are required for amelioration of brain pathology.
Correction of the cerebellar defect in BM recipient mice
A dramatic and progressive death of Purkinje cells occurs in the cerebellum of the GS mice, starting at the age of 3 to 4 months, and is one of the most overt consequences of this disease in the mice. Purkinje cells are lost in an anteroposterior and mediolateral fashion, the anterior lobes being the ones that are affected most and sooner. We have used this feature as a marker to monitor reduction in the neurologic damage after BMT. Serial sections of cerebella from mice that received MSCV-PPCA–transduced BM cells were compared with sections from wild-type and PPCA−/− mice. The appearance of Purkinje cells in PPCA-corrected mice was determined at 9 months after BMT by staining serial sections of the cerebella with an antibody against PEP19.25 Purkinje cells were clearly more numerous in treated mice than in age-matched PPCA mutant animals (Figure 6). To quantify our observations, Purkinje cells were counted in these transplanted mice as well as in one of the 3-month–treated group, and compared to 3- and 9-month-oldPPCA−/− mice and age-matched controls. Purkinje cells were counted at 2 levels: (1) in the paravermis at the point where the lateral cerebellar nuclei first become obvious (medial) and (2) in the hemisphere at the level of the dorsal cochlear nucleus (hemisphere). In the medial region wild-type mice averaged 392 ± 19 Purkinje cells/section, whereas in the hemisphere the number was 362 ± 16. As expected, in the 3-month-oldPPCA−/− animal only a small number of Purkinje cells were lost: 24% in the medial and 21% in the hemisphere sections (Figure 7). The total numbers were practically identical in the 3-month-old–treated mouse, because the variations in the different cerebellar regions were too small to be detected. At this time point, there was also little variation in Purkinje cell number between the anterior and posterior lobes of the cerebellum. In contrast at 9 months, we observed a dramatic loss of Purkinje cells in the PPCA−/− mouse. In the midline the total loss was 79%, but it was clearly more dramatic in the anterior lobes of the cerebellum than in the posterior lobes, with a loss of 93% and 61% of the cells, respectively. After BMT, the rescue of Purkinje cells in the 9-month-old mice varied in different cerebellar lobes, but the total number of cells was substantially greater than that of age-matched PPCA−/− mice (Figure 7). In the medial cerebellum, the overall loss of Purkinje cells in BMT recipient mice was 44% of controls. In the anterior lobes of the BMT-treated medial cerebellum, 55% of the cells were missing, whereas in the posterior lobes only 30% were. In the cerebellar hemisphere, the overall loss of Purkinje cells in the BMT-treated mice compared to the wild-type mice was 60%, with the anterior lobes showing a 66% loss and the posterior lobes 51% loss of Purkinje cells. These results support the notion that BMT of genetically modified cells in GS mice delays the progressive loss of Purkinje cells characteristic of the GS mice.
Purkinje cell counts in BM-transplanted GS mice.
Purkinje cells were counted in recipient mice at 3 and 9 months after treatment and compared to 3- and 9-month-oldPPCA−/−mice and age-matched controls. Purkinje cells were counted at 2 levels: (1) in the paravermis at the point where the lateral cerebellar nuclei first become obvious (M, medial), and (2) in the hemisphere at the level of the dorsal cochlear nucleus (H, hemisphere). The values are expressed as percentage of Purkinje cells counted in the control group.
Purkinje cell counts in BM-transplanted GS mice.
Purkinje cells were counted in recipient mice at 3 and 9 months after treatment and compared to 3- and 9-month-oldPPCA−/−mice and age-matched controls. Purkinje cells were counted at 2 levels: (1) in the paravermis at the point where the lateral cerebellar nuclei first become obvious (M, medial), and (2) in the hemisphere at the level of the dorsal cochlear nucleus (H, hemisphere). The values are expressed as percentage of Purkinje cells counted in the control group.
Discussion
Transplantation of normal HPCs has been exploited for the treatment of LSDs because BM progenitor cells can differentiate and repopulate target organs, including the CNS, providing a permanent source of normal enzyme. The overall outcome of allogeneic and syngeneic BMT in patients and animal models has indicated that this procedure is relatively effective in alleviating the systemic manifestations of the disease and in stabilizing bone lesions, especially if BMT is performed early in life.10,28-32Correction, however, is often incomplete, suggesting that higher local levels of gene expression may be required in some organs. Moreover, diseases that have an early onset and involve predominantly the CNS respond poorly to BMT, albeit that some variation in outcome has been observed among disease subtypes.10 The difficulty to correct the CNS is attributed to the slow and incomplete engraftment of BM-derived cells into the adult brain33; it may also depend on the amount of enzyme secreted by normal cells, the extracellular stability of the enzyme,10 and the extent of uptake by target cells. This conclusion is supported by our previous finding that complete systemic correction and partial amelioration of the brain pathology occur in GS mice that received transgenic BM in which cells of the monocyte/macrophage lineage were modified to overexpress a PPCA transgene.16 In these studies, neurologic abnormalities, including the loss of cerebellar function, were dramatically delayed when the transgenic mice were crossed into thePPCA−/− background.16
Building on these observations, we have now tested the hypothesis that a similar or better outcome could be obtained in a gene therapy setting, if sustained and long-term expression of the transgene could be achieved. These studies allowed us to examine the feasibility of such an approach for treatment of GS patients. Somatic gene therapy of neurologic LSDs could be, in fact, the preferred treatment if autologous HPCs could be engineered in vitro to constitutively express and secrete high levels of the correcting enzyme. Early studies in animal models have been disappointing with persistence of the lysosomal defect and only negligible amelioration of the disease phenotype.34 These results have been attributed to ineffective transduction of HPCs, insufficient level or silencing of transgene expression, immune depletion of the enzyme, or a combination of these factors.35-37 However, some of these difficulties can now be circumvented by the use of improved viral vectors like the one used in the treatment of α-galA-deficient mice.38 In addition, MSCV-based retroviral vectors have been shown to selectively and efficiently infect HPCs.21-23,39 This vector system has been recently applied successfully for the treatment of arylsulfatase A–null mice.40 Interesting and encouraging studies have recently shown delayed onset of clinical signs and amelioration of the functional and physical defects in the mucopolysaccharidosis (MPS) VII mouse model, using in utero transplantation of fetal liver cells and nonablative neonatal BMT, respectively.41 42
We have established stable hematopoiesis in GS mice usingPPCA−/− BM transduced with an MSCV-based bicistronic retrovirus expressing both PPCA and the GFP marker. One of the main findings is the capacity of MSCV to mediate long-term high transgene expression, which is indicated by the analysis of PPCA and GFP levels in tissues and PBMCs of the reconstituted animals. It is noteworthy that the level of cathepsin A activity varied considerably between recipients. These differences can be attributed to varying ratios of human PPCA expressing HPCs, which dictate the relative number of differentiated hematopoietic cells secreting the enzyme. Heterogeneity of retroviral LTR-mediated expression can be a function of the site of integration into the genome within heterochromatin or euchromatin,43 position effect variegation,44and progressive silencing of retroviral-mediated expression.43 Our MSCV vector, although modified to enhance expression,20 may still be subjected to silencing.45 Further modifications in the LTR regulatory elements45 or inclusion of additional regulatory elements into the vector genome46 or both may be necessary to ensure persistent, high-level, retroviral-mediated gene expression in large animal models and in patients with LSDs.
Despite the variability in levels of enzyme among recipients we can conclude that sustained and long-term expression of PPCA, generated by the MSCV retroviral cassette, undoubtedly contributed to the prevention/correction of storage in the GS organs, including the CNS. Most importantly, the extent of correction of the phenotype, including the cerebellar defect, observed in BMT-treated GS mice is comparable to that observed in crosses between PPCA−/− and transgenic mice overexpressing the corrective enzyme in the monocyte/macrophage lineage.16 In a mouse model of Niemann-Pick disease, although neurologic dysfunction was corrected in part after transplantation, no histologic resolution of storage was observed and the mice died of the disease.47 The quantitative analysis of Purkinje cell present in BMT-treated GS mice correlates well with their motor coordination skills.PPCA−/− animals demonstrate a progressive deterioration in motor coordination skills as measured by a standardized rota-rod treadmill assay, whereas PPCA-corrected mice, at all tested time points after transplantation, perform better on the rota-rod treadmill, albeit that the statistical analysis failed to detect a significant effect in these results due to a small sample population. The functional amelioration of the cerebellar deficit in GS recipients of retrovirus-transduced BM cells was associated with significant amelioration of the histopathology observed inPPCA-deficient animals, although the persistence of neuronal cells with storage throughout the CNS in these mice suggests that further steps are needed to achieve complete correction.
Like the BMT approach, gene transfer into animal models of MPS I, MPS VII, Niemann-Pick disease, and metachromatic leukodystrophy has resulted in only partial correction of the enzyme deficiency in the brain although improvement in neurologic function could not be documented.48 Several features have been implicated in the poor response, including differences in disease type, the age of the animals at the time of transplantation, and the use of irradiation. To simulate a potential clinical intervention, we used total body irradiation (TBI), a conditioning modality important for engraftment in patients undergoing allogeneic HPC transplantation. TBI negatively affects neuronal development in infants. However, an unfavorable outcome due to TBI must be balanced with the ability of this procedure to disrupt transiently the integrity of the blood-brain barrier, and allow the entry of corrected cells into the CNS. Thus, it is possible that the high initial levels of enzyme that we achieved in this population and the early age at the time of treatment permitted the correction of a significant proportion of affected cells. Further studies in our model will be required to determine if sublethal doses of radiation allow a similar outcome.
The combination of the high dose of cells, levels of HPC transduction, and appropriate cellular expression of the corrective enzyme might have played a crucial role in the histologic and functional correction of the CNS pathology in treated mice. Given the devastation of the cerebellar cortex in untreated PPCA−/−animals,13 and the vulnerability of Purkinje cells to storage-mediated damage, it was surprising to observe the preservation of cerebellar architecture in our treated mice. Although estimates of gross cathepsin A activity are not significantly different from those observed in the transgenic BMT model, our results suggest that a crucially higher threshold of protein expression was reached by using the genetic modification approach than by using the transgenic BMT method. Additional studies are required to determine the exact mechanism of enzyme delivery by genetically modified HPCs. In addition, substrate mobility, accessibility, and accumulation rate must be determined before a more comprehensive picture evolves.
We are grateful to Gerard Grosveld for his continuous support, Tommaso Nastasi for his expert assistance in the preparation of the histology figures, Hongjun Wang for assistance in the preparation of the revised manuscript, and Charlette Hill for help in typing and editing the manuscript.
Supported by the Assisi Foundation of Memphis, National Institutes of Health grant RO1-DK 52025, the International Outreach Program at St Jude Children's Research Hospital (T.L.), the Cancer Center Support (CORE) grant CA 21765 from the National Cancer Institute, Phillip and Elizabeth Gross, and the American Lebanese Syrian Associated Charities (ALSAC). T.L., L.M., and M.d.P.M. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alessandra d'Azzo, Department of Genetics, St Jude Children's Research Hospital, 332 N Lauderdale, Memphis, TN 38105; e-mail: sandra.dazzo@stjude.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal