Abstract
Retroviral transduction of primary hematopoietic cells with human oncogenes provides a powerful approach to investigating the molecular mechanisms controlling the normal proliferation and differentiation of these cells. Here we show that primitive human CD34+ cord blood cells, including multipotent as well as granulopoietic- and erythroid-restricted progenitors, can be efficiently transduced with a MSCV-BCR-ABL-IRES-GFP retrovirus, resulting in the sustained expression by their progeny of very high levels of tyrosine phosphorylated p210BCR-ABL. Interestingly, even in the presence of growth factors that supported the exclusive production of granulopoietic cells from green fluorescent protein (GFP)–transduced control cells, BCR-ABL–transduced progenitor subpopulations generated large numbers of erythropoietin-independent terminally differentiating erythroid cells and reduced numbers of granulopoietic cells. Analyses of individual clones generated by single transduced cells in both semisolid and liquid cultures showed this BCR-ABL–induced erythroid differentiation response to be elicited at a high frequency from all types of transduced CD34+ cells independent of their apparent prior lineage commitment status. Additional experiments showed that this erythroid differentiation response was largely prevented when the cells were transduced and maintained in the presence of the BCR-ABL–specific tyrosine kinase inhibitor, STI-571. These findings indicate that overexpression of BCR-ABL in primary human hematopoietic cells can activate an erythroid differentiation program in apparently granulopoietic-restricted cells through a BCR-ABL kinase-dependent mechanism, thus providing a new molecular tool for elucidating mechanisms underlying lineage fate determination in human hematopoietic cells and infidelity in human leukemia.
Introduction
The hematopoietic system is composed of a hierarchy of progenitors with the most primitive stem cells uniquely characterized by their combined capacity for lymphomyeloid lineage differentiation, extensive self-renewal, and, on transplantation, lifelong maintenance of blood cell production. The mechanisms regulating the initial differentiation steps these cells undergo are still poorly understood, although considerable evidence now indicates that this occurs in a consistently ordered fashion to give a relatively simple picture of progressive restriction in differentiation ability to specific lineages.1 Thus it has been possible to define a series of phenotypic changes that distinguish progenitors with different lineage options2-4 and to identify a number of transcription factors whose presence (or absence) is important to the ability of early hematopoietic cells to undertake specific differentiation steps.1,5 To date, dedifferentiation or transdifferentiation during normal hematopoiesis has not been described, although examples of such events have recently been reported to occur in neural cells6,7 or in occasional examples of transformed hematopoietic cells.8-13
The BCR-ABL oncogene is a fusion gene that characterizes the neoplastic clone of patients with chronic myeloid leukemia (CML).14,15 It encodes a 210-kd oncoprotein (p210BCR-ABL)16 with constitutively active tyrosine kinase activity17 and an abnormal cytoplasmic location.18 Both of these features are important to its transforming activity in model systems.19-24 The BCR-ABL oncoprotein is also known to perturb many intracellular signaling pathways, including the RAS, mitogen-activated protein (MAP) kinase, phosphatidylinositol-3′ kinase (PI3-K), janus-kinase/signal transducer and activator of transcription (JAK/STAT), and MYC pathways (for a review, see Deininger et al25). The development of packaging cells that allow production of high titer preparations of p210BCR-ABL complementary (cDNA)–containing retroviruses has greatly facilitated analysis of the effects of this gene in primary hematopoietic cells. These have shown that BCR-ABL–transduced mouse bone marrow cells produce a rapidly fatal CML-like myeloid leukemia on their transplantation into irradiated recipients.26-28However, the speed with which the leukemia develops is clearly not comparable to the approximately 7 year average duration of the latent phase of human CML.29 This discrepancy in disease kinetics might be caused by species-specific effects of p210BCR-ABLor, alternatively, by differences in BCR-ABL message levels determined by differences in the promoters active in the 2 systems (the endogenous BCR promoter in human CML cells and the long terminal repeat [LTR] in retrovirally transduced hematopoietic cells). To investigate this question we began experiments to examine the effects of BCR-ABL expression in primary human CD34+ cord blood (CB) cells after transduction with a p210BCR-ABLretrovirus. This resulted in the unexpected but consistent observation of a rapid outgrowth of an erythroid population. Additional experiments designed to investigate the basis of this response suggest that it resulted from the very high level of p210BCR-ABL kinase activity attained in the target cells independent of their apparent prior “commitment” status.
Materials and methods
Virus production
A full-length b3a2 p210 BCR-ABL cDNA (obtained from J. Griffin, Dana Farber Cancer Institute, Boston, MA) was cloned as anEcoRI fragment and then inserted into a MSCV-IRES-GFP vector (MIG, from K. Humphries, Terry Fox Laboratory, Vancouver, BC) to generate a MSCV-BCR-ABL-IRES-GFP vector (p210). Helper-free virus was obtained by transfecting amphotropic Phoenix packaging cells30 cultured in Dulbecco modified Eagle medium (DMEM) plus 10% fetal calf serum (FCS; StemCell Technologies, Vancouver, BC) using CaPO4 and harvesting the virus-containing medium (VCM) 32 to 72 hours later as described.31 After filtration through a 0.45-μm filter, the medium was stored at −190°C. Titers of the p210 and MIG virus were 2 to 5 and 5 to 9 × 105/mL, respectively, as assessed by green fluorescence protein (GFP) expression in NIH 3T3 cells.
Cells
The CB cells were obtained with informed consent from mothers undergoing cesarean delivery of healthy, full-term infants and low-density (< 1.077 g/mL) cells isolated using Ficoll-Hypaque (Amersham Pharmacia, Piscataway, NJ). CD34+-enriched cells (50% ± 10%, n = 10) were isolated by immunomagnetic removal of lineage marker-positive (lin+) cells (using StemSep columns, StemCell).
Retroviral transduction
Cells to be transduced were first cultured at 1 to 2 × 105/mL for 48 hours in complete serum-free medium (SFM) consisting of Iscoves medium supplemented with bovine serum albumin (BSA), insulin, and transferrin (BIT, StemCell), 40 μg/mL low-density lipoproteins, and 10−4 M 2-mercaptoethanol (both from Sigma Chemicals, St Louis, MO) to which the following 5 purified recombinant human growth factors (GFs) were added: Flt-3 ligand (FL; 100 ng/mL, Immunex Corporation, Seattle, WA), Steel factor (SF; 100 ng/mL, produced in the Terry Fox Laboratory), interleukin 3 (IL-3; 20 ng/mL, Novartis, Basel, Switzerland), interleukin-6 (IL-6; 20 ng/mL, Cangene, Mississauga, ON), and granulocyte colony-stimulating factor (G-CSF; 20 ng/mL, StemCell). At the end of this 2-day prestimulation period, the cells were resuspended in filtered VCM supplemented with the same 5 GFs except for the studies shown in Table3 where FL (100 ng/mL) plus granulocyte-macrophage CSF (GM-CSF, 20 ng/mL, Novartis) and G-CSF (20 ng/mL) were used as the GFs added during the period of exposure to VCM. Protamine sulfate (5 μg/mL, Sigma) was also added to the medium and the cells then placed in fibronectin-coated Petri dishes previously “loaded” twice with VCM (each time for 30 minutes). This infection procedure was repeated on each of the next 2 consecutive days and another 24 hours later the cells were transferred to fresh SFM plus GFs but no VCM and incubated for a further 24 hours before harvesting and further analysis. In 3 experiments, 0.25 μmol STI571 (Novartis) was added to the medium of some of the transduction cultures.
Flow cytometry
Viable (propidium iodide–negative [PI−]) GFP+ and GFP− CD34+ CB cells and various subpopulations of CD34+ CB cells (CD45RA−CD71−; CD45RA+CD71−; CD45RA−CD71+) were isolated at a purity of more than 99% using a 3-laser FACStar+ (Becton Dickinson, San Jose, CA) as described previously.2 Bulk cells were collected in Eppendorf tubes containing complete SFM. Sorted single cells were collected (using the single-cell deposition unit of the FACS) into the individual round-bottom wells of 96-well plates that had been prefilled with 100 μL of complete SFM. For the phenotype analyses, cells were stained with anti-CD34 (8G12)-Cy5 and either anti–CD15-phycoerythrin (PE) or anti–CD41a-PE (the latter both from Pharmingen, Mississauga, ON) or anti–glycophorin-A-PE (10F7) or anti–CD71-PE (OKT-9) or anti–CD13-PE or anti–CD33-PE or anti–c-kit-PE or anti–CD36-PE (the last 4, all from Becton Dickinson). They were then analyzed on a FACScalibur using Cell Quest software (Becton Dickinson) with gates set to exclude more than 99.9% of cells stained with irrelevant antibodies labeled with the corresponding fluorochromes.
Cultures
Transduced cells were cultured either in bulk cultures in complete SFM with FL, SF, IL-3, IL-6, and G-CSF, or as single cells in 96-well plates in the same medium with or without erythropoietin (epo, 3 U/mL, StemCell) as indicated. In some experiments 0.25 μmol STI 571 was also added. Colony-forming cell (CFC) assays were performed as described32 in SFM-containing methylcellulose cultures (StemCell) with human SF (50 ng/mL), 20 ng/mL each of IL-3, IL-6, G-CSF, and GM-CSF with or without epo (3 U/mL), or with FL (100 ng/mL), and 20 ng/mL G-CSF and GM-CSF, as indicated.
Reverse transcriptase–polymerase chain reaction analyses
Total RNA was extracted from aliquots of 104 cells as described previously.33 The reverse transcriptase (RT) reaction was performed in 20 μL with superscript II RNase H− RT (Gibco/BRL, Rockville, MD) using random hexamer oligonucleotides as primers (Pharmacia). Then, 5 μL of the RT reaction was used for polymerase chain reaction (PCR) amplification in 50 μL volumes of 1 times PCR buffer (Gibco/BRL) containing 20 mmol Tris-HCl (pH 8.4), 50 mmol KCL, 2 mM MgCl2, 200 μmol of each dNTP (Amersham Pharmacia), 1 unit of Taqpolymerase, and 10 pmol of specific primers for BCR-ABL 5′-CAGGGTGCACAGCCGCAACGGCAA-3′ and 5′-GTCCAGCGAGAAGGTTTTCCTTGGA-3′), actin 5′-GTGCGTGACATTAAGGAGAA-3′ and 5′-GGAGGGGCCGGACTCGTCA-3′) to give DNA fragments of 374 base pairs (bp; BCR-ABL) and 471 bp (actin). Thirty-five cycles of amplification (94°C for 30 seconds, 62°C for 1 minute, 72°C for 1 minute) were then performed.
Southern blot analysis
High-molecular-weight DNA from p210- or MIG-transduced cells was isolated using DNAzol (Gibco/BRL) and 15 μg DNA was digested withXbaI (which cuts once in the LTRs of the provirus) to release a full-length proviral fragment. The digested DNA was then electrophoresed on 0.8% agarose gel and processed as previously described34 using a 32P-dCTP–labeled (Amersham Pharmacia) GFP probe derived from the MIG retroviral vector.
Western analyses
Cells (105 cells per treatment group) were lysed in phosphorylation solubilization buffer (PSB; 50 mmol HEPES buffer, 100 mmol NaF, 10 mmol sodium pyrophosphate, 2 mmol Na3VO4, 4 mmol EDTA) containing 0.5% Nonidet P-40 and then Western analyses performed as previously described35 using a mouse monoclonal anti-Abl antibody (8E9, Pharmingen) and antiphosphotyrosine antibody (4G10, Upstate Biotechnology, Lake Placid, NY).
Statistical analysis
Results are shown as the mean ± SEM of values obtained in independent experiments. Differences between groups were assessed using the 2-tailed Student t test.
Results
BCR-ABL–transduced CD34+ human CB cells generate predominantly erythroid cells in suspension cultures containing FL, SF, IL-3, IL-6, and G-CSF but no epo
Lin−CD34+ CB cells were transduced with a p210-GFP or MIG (control GFP) MSCV-IRES vector as described in “Materials and methods” using a 6-day culture procedure in which the cells were maintained in FL, SF, IL-3, IL-6, and G-CSF and exposed on the third, fourth, and fifth days to fresh virus. FACS analysis of GFP expression 2 days after the last exposure to fresh virus showed that the overall efficiency of transduction by the p210 and MIG viruses was similar at 17% ± 6% versus 25% ± 8%, respectively (n = 5). Corresponding proportions of GFP+ cells among the CD34+ present at the end of the transduction procedure were also not significantly different (10% ± 2% and 21% ± 8%, respectively). Because the yield of CD34+ cells at the end of the transduction procedure was similar between the 2 groups, the absolute yields of GFP+ CD34+ cells were also similar (1.8 ± 1.0 and 5.7 ± 5.1, respectively, per 100 initial CD34+ cells, P = .3).
FACS-sorted GFP+ CD34+ cells isolated from both transduced populations were then cultured further in the presence of the same growth factors (FL, SF, IL-3, IL-6, and G-CSF). As shown in Figure 1, both transduced populations expanded rapidly under these conditions but the growth of the p210-transduced cells was much faster (∼105- versus ∼103-fold over the first 14 days, P < .05, n = 3). Southern blot analysis confirmed the presence of the expected full-length BCR-ABL proviral sequence in DNA extracted from the expanded p210-transduced cells (Figure2A, lane 1) and RT-PCR analyses demonstrated that they were expressing BCR-ABL messenger RNA (mRNA; Figure 2B). Interestingly, as shown in Figure 2C, the p210-transduced CB cells produced a higher level of p210BCR-ABL than was seen in K562 cells (an erythroleukemic cell line isolated from a patient with CML in blast crisis36) and the p210BCR-ABL in the transduced CB cells also appeared to be more highly tyrosine-phosphorylated.
p210-transduced CD34+ CB cells expand more rapidly than MIG-transduced cells and this is normalized when STI571 is present.
Total viable (trypan blue-excluding) cells were measured at the times shown in serum-free cultures initiated with 1 to 5 × 104p210-transduced (triangles) or MIG-transduced (squares) (GFP+) CD34+ CB cells and containing FL, SF, IL-3, IL-6, and G-CSF either with (open symbols) or without (solid symbols) 0.25 μmol STI 571. Results are from 3 experiments.
p210-transduced CD34+ CB cells expand more rapidly than MIG-transduced cells and this is normalized when STI571 is present.
Total viable (trypan blue-excluding) cells were measured at the times shown in serum-free cultures initiated with 1 to 5 × 104p210-transduced (triangles) or MIG-transduced (squares) (GFP+) CD34+ CB cells and containing FL, SF, IL-3, IL-6, and G-CSF either with (open symbols) or without (solid symbols) 0.25 μmol STI 571. Results are from 3 experiments.
Molecular analyses of p210-transduced CB cells.
(A) Southern analysis of genomic DNA isolated from p210-tranduced CB cells 14 days after transduction after digestion with XbaI and hybridization with a GFP probe showing the expected full-length (13.6-kb) integrated BCR-ABL. (B) RT-PCR analysis of RNA isolated from MIG or p210-transduced CB cells 7 days after transduction showing evidence of BCR-ABL transcripts (upper gel) in the p210-transduced cells only and actin transcripts in all samples. Lane 1 contained no RT (negative control for RT-PCR). Lane 2 contained water (negative control for PCR). Lanes 3 to 5 contained amplified products from MIG-transduced CB cells (3 different experiments). Lanes 6 to 10 contained amplified products from p210-transduced CB cells (5 different experiments). Lane 11 contained amplified products from K562 cells (positive control). The expected sizes, in base pairs, are indicated for both PCR products. (C) Increased expression and phosphorylation of p210BCR-ABL in p210-transduced CB cells as compared to K562 cells. A Western blot analysis of total cell lysates probed with an antiphosphotyrosine antibody is shown in the lower panel. The membrane was then stripped and reprobed with an anti-ABL antibody (upper panel). The positions of p210BCR-ABL and the endogenous p145c-ABL(endogenous loading control) are indicated. Total cell lysates from MIG-transduced CB cells and from the K562 cell line were used as negative and positive controls, respectively.
Molecular analyses of p210-transduced CB cells.
(A) Southern analysis of genomic DNA isolated from p210-tranduced CB cells 14 days after transduction after digestion with XbaI and hybridization with a GFP probe showing the expected full-length (13.6-kb) integrated BCR-ABL. (B) RT-PCR analysis of RNA isolated from MIG or p210-transduced CB cells 7 days after transduction showing evidence of BCR-ABL transcripts (upper gel) in the p210-transduced cells only and actin transcripts in all samples. Lane 1 contained no RT (negative control for RT-PCR). Lane 2 contained water (negative control for PCR). Lanes 3 to 5 contained amplified products from MIG-transduced CB cells (3 different experiments). Lanes 6 to 10 contained amplified products from p210-transduced CB cells (5 different experiments). Lane 11 contained amplified products from K562 cells (positive control). The expected sizes, in base pairs, are indicated for both PCR products. (C) Increased expression and phosphorylation of p210BCR-ABL in p210-transduced CB cells as compared to K562 cells. A Western blot analysis of total cell lysates probed with an antiphosphotyrosine antibody is shown in the lower panel. The membrane was then stripped and reprobed with an anti-ABL antibody (upper panel). The positions of p210BCR-ABL and the endogenous p145c-ABL(endogenous loading control) are indicated. Total cell lysates from MIG-transduced CB cells and from the K562 cell line were used as negative and positive controls, respectively.
Surprisingly, both morphologic (Figure 3) and phenotype analyses (Figure 4) revealed that, within 1 week in culture (after the transduction procedure was completed), most of the cells generated by the p210-transduced CD34+ cells were glycophorin A+erythroblasts even though no epo had been added to the medium. By 2 weeks after transduction, the number of erythroid cells present in these cultures had increased another 100-fold (Figure5A,B) and many showed evidence of terminal differentiation as indicated both by the presence of large numbers of orthochromatic erythroblasts in Wright-Giemsa–stained cytospin preparations and the red pellets obtained when the cells were centrifuged. In contrast, the MIG-transduced cells continued to expand less rapidly and did not produce recognizable erythroid cells. Interestingly, the massive production of erythroblasts in the cultures of p210-transduced cells was accompanied by a marked absolute decrease in the output of terminally differentiating myeloid cells (Figure5C,D). Follow-up studies of the cultures of p210-transduced cells after more prolonged times showed that terminally differentiating erythroid cells continued to be produced and were maintained as the predominant cell type for many weeks (data not shown), in contrast to the MIG-transduced cells that remained predominantly granulopoietic.
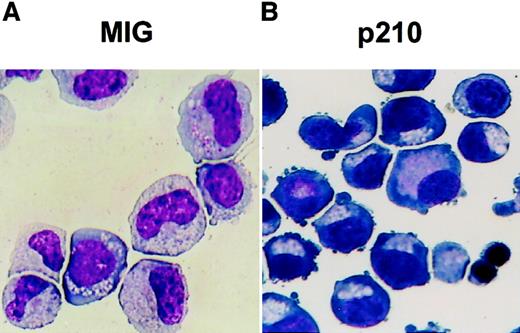
Altered differentiation of cultured p210-transduced CB cells as shown by Wright staining.
Representative cytospins prepared from 7 day-old serum-free cultures of MIG (A) and p210-transduced (B) (GFP+) CD34+ CB cells maintained in FL, SF, IL-3, IL-6, and G-CSF but no added epo. These show the prevalence of recognizable granulocyte (metamyelocyte, band forms) and erythroid (basophilic erythroblasts) precursors, respectively, in the cultures initiated with these 2 types of cells (× 400 magnification). At later times, large numbers of polychromatophilic erythroblasts were also seen in the cultures of p210-transduced cells.
Altered differentiation of cultured p210-transduced CB cells as shown by Wright staining.
Representative cytospins prepared from 7 day-old serum-free cultures of MIG (A) and p210-transduced (B) (GFP+) CD34+ CB cells maintained in FL, SF, IL-3, IL-6, and G-CSF but no added epo. These show the prevalence of recognizable granulocyte (metamyelocyte, band forms) and erythroid (basophilic erythroblasts) precursors, respectively, in the cultures initiated with these 2 types of cells (× 400 magnification). At later times, large numbers of polychromatophilic erythroblasts were also seen in the cultures of p210-transduced cells.
Increased erythroid cells and decreased myeloid cells in cultures of p210-transduced CB cells.
Representative FACS profiles of cells from 7-day-old serum-free cultures of MIG (left panel) and p210-transduced (right panel) (GFP+) CD34+ CB cells maintained in FL, SF, IL-3, IL-6, and G-CSF but no added epo illustrating the differences seen in granulopoietic (CD15) and erythroid (glycophorin A) marker expression. The reduced level of GFP expression seen here is due to the fact that the cells were cultured in the absence of serum as shown by the fact that subsequent addition of serum increased the average level of GFP fluorescence several-fold (data not shown).
Increased erythroid cells and decreased myeloid cells in cultures of p210-transduced CB cells.
Representative FACS profiles of cells from 7-day-old serum-free cultures of MIG (left panel) and p210-transduced (right panel) (GFP+) CD34+ CB cells maintained in FL, SF, IL-3, IL-6, and G-CSF but no added epo illustrating the differences seen in granulopoietic (CD15) and erythroid (glycophorin A) marker expression. The reduced level of GFP expression seen here is due to the fact that the cells were cultured in the absence of serum as shown by the fact that subsequent addition of serum increased the average level of GFP fluorescence several-fold (data not shown).
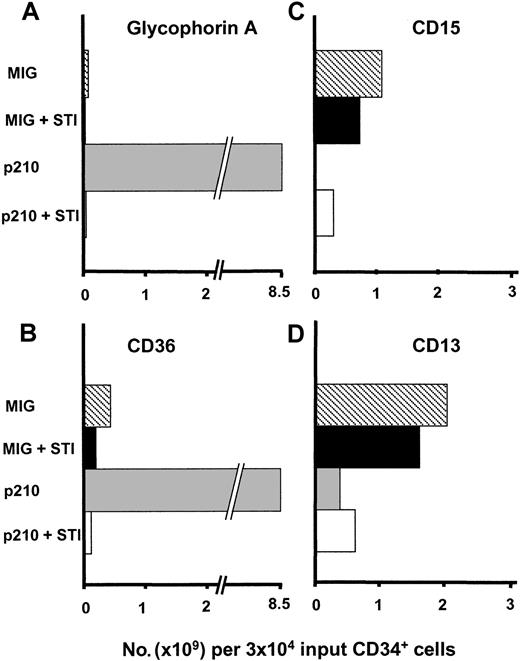
Transduction of p210 causes an STI571-dependent increase in the absolute output of erythroid cells and a decrease in the absolute output of myeloid cells.
An increased number of cells expressing erythroid markers (glycophorin A, panel A; CD36, panel B) and a decreased number of cells expressing markers of myeloid cells (CD15, panel C; CD13, panel D) were produced from the p210-transduced (GFP+) CB cells after culturing them for 2 weeks as described in Figure 1 and these were largely normalized in the presence of 0.25 μmol STI571. STI 571 had little or no effect on the differentiation of MIG-transduced (GFP+) cells. Results shown are from a representative experiment (of 4 with no STI571 and another 2 with and without STI571).
Transduction of p210 causes an STI571-dependent increase in the absolute output of erythroid cells and a decrease in the absolute output of myeloid cells.
An increased number of cells expressing erythroid markers (glycophorin A, panel A; CD36, panel B) and a decreased number of cells expressing markers of myeloid cells (CD15, panel C; CD13, panel D) were produced from the p210-transduced (GFP+) CB cells after culturing them for 2 weeks as described in Figure 1 and these were largely normalized in the presence of 0.25 μmol STI571. STI 571 had little or no effect on the differentiation of MIG-transduced (GFP+) cells. Results shown are from a representative experiment (of 4 with no STI571 and another 2 with and without STI571).
BCR-ABL transduction of CD34+ human CB cells alters the types of CFCs detectable immediately after transduction
To determine which and how many progenitors were responsible for the altered growth and differentiation seen in bulk cultures of p210-transduced CD34+ CB cells, aliquots of the same transduced CD34+ GFP+ cells isolated immediately after transduction were plated in standard semisolid methylcellulose assays (both with and without epo). When epo was present, the p210-transduced cells made significantly fewer pure granulopoietic colonies than did the same number of MIG (control)–transduced cells (11 ± 3/100 versus 50 ± 7/100 initial CD34+ cells, P < .05, n = 5; see the footnote to Table 1 for a more detailed description of this normalization.). Under the same (plus epo) conditions, the p210-transduced cells also usually made fewer colonies containing both erythroid and granulopoietic cells. However, the numbers of these were more variable between experiments and overall the difference with the control cells did not reach significance (69 ± 17/100 versus 106 ± 18/100 initial CD34+ CB cells, respectively, P = .08). Conversely, an approximately 3-fold higher number of erythroid colonies was produced by the p210-transduced cells in the same assays, although the variability in these values again made the difference not significant (300 ± 150/100 versus 110 ± 52/100 initial CD34+ CB cells, P = .13). When epo was not added to the methylcellulose medium, the yield of granulopoietic colonies was the same as in the assays to which epo was added. However, in the absence of epo, the p210-transduced cells also produced many obviously hemoglobinized (red) colonies. As expected, these were not seen in parallel assays of the MIG-transduced cells (data not shown). Taken together, these findings suggest that overexpression of p210BCR-ABL in CD34+ CB cells can suppress normal granulopoietic differentiation and promote erythroid differentiation both in pluripotent and in apparently committed granulopoietic progenitors (rather than simply stimulating the amplified outgrowth of a subset of progenitors already restricted to differentiation along the erythroid pathway).
Number of different types of CFCs detected before and after transduction of different subsets of CD34+ CB cells with MIG or p210 in the presence of FL, SF, IL-3, IL-6, and G-CSF
| Progenitors . | CD34+CD45RA−CD71−(stem cell candidates) . | CD34+CD45RA+CD71−(granulopoietic fraction) . | CD34+CD45RA−CD71+(erythroid fraction) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pretransduction* . | Posttransduction* . | Pretransduction* . | Posttransduction* . | Pretransduction* . | Posttransduction* . | ||||
| MIG . | P210 . | MIG . | P210 . | MIG . | P210 . | ||||
| CFU-E | 0 ± 0 | 0 ± 0 | 21 ± 5† | 0 ± 0 | 0.3 ± 0.3 | 10 ± 3† | 0.4 ± 0.3 | 25 ± 6 | 55 ± 21 |
| BFU-E | 1.6 ± 0.3 | 95 ± 17 | 349 ± 95† | 0.2 ± 0.1 | 2 ± 1 | 45 ± 8† | 18 ± 3 | 573 ± 93 | 672 ± 198 |
| CFU-GEMM | 12 ± 1 | 143 ± 10 | 112 ± 19 | 1.3 ± 0.1 | 15 ± 5 | 7 ± 1 | 7 ± 3 | 127 ± 33 | 193 ± 56 |
| CFU-GM | 6 ± 1 | 85 ± 12 | 18 ± 4† | 14 ± 2 | 140 ± 59 | 27 ± 9† | 1 ± 0.3 | 9 ± 1 | 1 ± 1† |
| Total CFC | 20 ± 2 | 323 ± 28 | 500 ± 107 | 15 ± 2 | 158 ± 63 | 89 ± 12 | 26 ± 1 | 733 ± 124 | 920 ± 232 |
| Progenitors . | CD34+CD45RA−CD71−(stem cell candidates) . | CD34+CD45RA+CD71−(granulopoietic fraction) . | CD34+CD45RA−CD71+(erythroid fraction) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pretransduction* . | Posttransduction* . | Pretransduction* . | Posttransduction* . | Pretransduction* . | Posttransduction* . | ||||
| MIG . | P210 . | MIG . | P210 . | MIG . | P210 . | ||||
| CFU-E | 0 ± 0 | 0 ± 0 | 21 ± 5† | 0 ± 0 | 0.3 ± 0.3 | 10 ± 3† | 0.4 ± 0.3 | 25 ± 6 | 55 ± 21 |
| BFU-E | 1.6 ± 0.3 | 95 ± 17 | 349 ± 95† | 0.2 ± 0.1 | 2 ± 1 | 45 ± 8† | 18 ± 3 | 573 ± 93 | 672 ± 198 |
| CFU-GEMM | 12 ± 1 | 143 ± 10 | 112 ± 19 | 1.3 ± 0.1 | 15 ± 5 | 7 ± 1 | 7 ± 3 | 127 ± 33 | 193 ± 56 |
| CFU-GM | 6 ± 1 | 85 ± 12 | 18 ± 4† | 14 ± 2 | 140 ± 59 | 27 ± 9† | 1 ± 0.3 | 9 ± 1 | 1 ± 1† |
| Total CFC | 20 ± 2 | 323 ± 28 | 500 ± 107 | 15 ± 2 | 158 ± 63 | 89 ± 12 | 26 ± 1 | 733 ± 124 | 920 ± 232 |
CFU-E indicates colony-forming unit-erythroid; BFU-E, burst-forming unit-erythroid; CFU-GEMM, colony-forming unit granulocyte, erythroid, macrophage, megakaryocyte; and CFU-GM, colony-forming unit granulocyte-macrophage.
Before refers to CFC assays performed on freshly isolated FACS-purified subpopulations of lin−CD34+ CB cells just before beginning the 6-day transduction protocol. After refers to CFC assays performed on GFP+ cells isolated immediately following the 6-day transduction protocol. All values have been normalized to a common starting cell number (100 freshly isolated cells of the same subset). This calculation assumes that the pre–CFC values obtained for each initial population would have applied to the cells within that population that were subsequently transduced with either vector. Results are from 4 experiments.
Significantly different from the MIG-transduced cells (P < .05).
Evidence for altered programming of committed human CB progenitors by p210BCR-ABL
To examine more rigorously the types of progenitors that could be stimulated to generate erythroid progeny following transduction with BCR-ABL, the following experiment was performed. Lin−CD34+ CB cells were first physically separated by FACS into 3 biologically distinct subsets according to their levels of expression of CD45RA and CD7137before being transduced. As confirmed in Table 1, which shows the CFC content of the 3 fractions of CD34+ cells isolated (as assessed immediately after their isolation), this sort strategy reproducibly yields subpopulations of CD34+ cells in which more than 90% of the CFCs are either granulopoietic (in the CD34+CD45RA+CD71− fraction) or erythroid (in the CD34+CD45RA−CD71+ fraction), or contain the majority of the pluripotent CFCs (in the CD34+CD45RA−CD71− fraction). The remainder of the cells isolated in each of these CD34+subsets were then transduced with either the p210 or the MIG virus using the same 6-day transduction protocol as used before for the unseparated CD34+ CB cells.
Analysis of the proportion of GFP+ cells in each fraction at the end of the transduction procedure showed that the efficiency of transduction of the CD34+CD45RA−CD71+ (erythropoietic) cells with p210 and MIG was similar (23% ± 7% versus 33% ± 9%, P = .21). However, for both the CD34+CD45RA+CD71−(granulopoietic) and the CD34+CD45RA−CD71− (candidate stem cell) fractions, the efficiency of transduction by p210 was lower (4% ± 1% versus 18% ± 3%, P < .001, and13% ± 5% versus 23% ± 4%, P < .05, respectively, n = 5). Molecular analyses were performed on cells derived from 7-day liquid suspension cultures of GFP+ cells obtained from each of the transduced subpopulations of CD34+ cells, as in the experiments with unseparated CD34+ cells (described above). These showed that the p210-transduced cells obtained from all 3 CD34+ subsets had integrated the full-length BCR-ABL proviral sequence (Figure 2A, lanes 2-4), were expressing BCR-ABL mRNA (data not shown), and contained levels of the p210BCR-ABL oncoprotein that were higher on a per cell basis than those seen in simultaneously analyzed K562 cells (data not shown).
The immediate effect of expressing p210BCR-ABL in the progenitors present in the various subpopulations of transduced CD34+ cells was first assessed by isolating the GFP+ cells from each of the 6 transduced populations by FACS (directly at the end of the transduction procedure) and then plating these cells in equal numbers either in methylcellulose colony assays (plus epo, Table 1) or in single-cell liquid suspension cultures (both with and without epo, Table 2). Because all types of CFCs are amplified during the 6 days of GF stimulation used in the transduction procedure, all values have been normalized to 100 initial cells in each of the 3 CD34+subsets. The magnitude of these increases can thus be seen by comparison of the “pretransduction” values and the corresponding “post-(MIG) transduction” values. Interestingly, when the results of the assays of the p210 and MIG-transduced cells are compared, there is no significant difference in the total numbers of colonies produced from any of the 3 subsets of CD34+ cells transduced. However, p210 transduction of the granulopoietic (CD34+CD45RA+CD71−) fraction did significantly reduce (P < .04) the number of granulopoietic colonies generated and in the same assays there was a significant (P < .002) increase in the number of exclusively erythroid colonies seen. In the assays of cells from the population that had been enriched in pluripotent progenitors prior to transduction (the CD34+CD45RA−CD71− cells), similar differences between the p210 and MIG (control) groups were seen (P < .05). In both of these fractions the average yield of mixed colonies was also lower. However, these differences were not significant and were even reversed in the population that had been enriched in erythroid progenitors before transduction. Notably, this latter population also showed no significant preferentialincrease in erythroid colony numbers by the p210-transduced cells (P > .05). This finding argues strongly against an ability of p210BCR-ABL to promote erythropoiesis by stimulating an early and selective expansion of already committed erythroid progenitors during the transduction procedure.
Numbers and erythroid differentiation of clones produced by different subsets of CB34+ CB cells transduced with MIG or p210 and plated in single-cell serum-free liquid cultures
| Subsets of CB cells . | Epo . | Total clones (%) . | No. of erythroid clones/no. analyzed (%)* . | ||
|---|---|---|---|---|---|
| MIG . | p210 . | MIG . | p210 . | ||
| CD34+CD45RA−CD71−(stem cell candidates) | + | 69/192 (36) | 103/192 (54) | 8/14 (57) | 20/21 (95) |
| − | 24/96 (25) | 41/96 (43) | 8/17 (47) | 12/16 (75) | |
| CD34+CD45RA+CD71−(granulopoietic) | + | 62/192 (32) | 84/192 (44) | 7/23 (30) | 21/24 (88) |
| − | 17/96 (18) | 22/96 (23) | 1/10 (10) | 8/14 (57) | |
| CD34+CD45RA−CD71+(erythroid) | + | 102/192 (53) | 139/192 (72) | 20/22 (91) | 22/22 (100) |
| − | 8/96 (8) | 2/96 (2) | 8/8 (100) | 3/3 (100) | |
| Subsets of CB cells . | Epo . | Total clones (%) . | No. of erythroid clones/no. analyzed (%)* . | ||
|---|---|---|---|---|---|
| MIG . | p210 . | MIG . | p210 . | ||
| CD34+CD45RA−CD71−(stem cell candidates) | + | 69/192 (36) | 103/192 (54) | 8/14 (57) | 20/21 (95) |
| − | 24/96 (25) | 41/96 (43) | 8/17 (47) | 12/16 (75) | |
| CD34+CD45RA+CD71−(granulopoietic) | + | 62/192 (32) | 84/192 (44) | 7/23 (30) | 21/24 (88) |
| − | 17/96 (18) | 22/96 (23) | 1/10 (10) | 8/14 (57) | |
| CD34+CD45RA−CD71+(erythroid) | + | 102/192 (53) | 139/192 (72) | 20/22 (91) | 22/22 (100) |
| − | 8/96 (8) | 2/96 (2) | 8/8 (100) | 3/3 (100) | |
Data shown are the number of clones detected/number of wells analyzed (expressed as a % value in parentheses) for both total clones (≥ 32 cells per well, assessed by direct visualization in situ after 7 days of culture after transduction) and for erythroid clones. The frequency of erythroid clones was determined by morphologic analysis of Wright-Giemsa–stained cytospin preparations made individually on randomly selected 7-day-old clones of ≥ 32 cells/well. Data are from 2 experiments with epo (+) and 1 experiment without epo (−). All wells contained FL, SF, IL-3, IL-6, and G-CSF with or without (±) epo as indicated.
Because less than 40% (6%-32%) of the transduced cells from any of the CD34+ subsets were able to produce a colony in methylcellulose, the CFC data in Table 1 do not exclude the possibility that the increased numbers of erythroid CFCs and decreased numbers of mixed and granulopoietic CFCs detected could result from separate effects of p210BCR-ABL on different cell types. To address this, we also analyzed the differentiation of clones of cells produced in single-cell liquid cultures containing FL, SF, IL-3, IL-6, and G-CSF with and without epo, to maximize and minimize, respectively, the detection of cells with “normal” erythroid potential. As expected, under these conditions, the frequency of clone formation (≥ 32 cells/well) when epo was present was generally higher than 50% (Table 2). Moreover, there was no significant change in the overall cloning efficiency of any of these populations after 4, 7, or 14 days of growth (data not shown, P ≥ .1). Therefore, on day 7, individual cytospin preparations were made from randomly selected clones so that the cells they contained could be analyzed morphologically. This showed that all 3 subsets of p210-transduced cells produced more erythroid clones than did their MIG-transduced counterparts and that the magnitude of this effect was greatest for the cells that had initially been enriched in granulopoietic progenitors. Thus when epo was present, the proportion of erythroid clones generated by these cells increased from 30% in the control group to 88% in the p210-transduced group. In the absence of added epo, the corresponding values were 10% and 57%.
In 2 further experiments, the granulopoietic progenitor-enriched (CD45RA+CD71−) subset of CD34+ CB cells were incubated with GFs (FL, G-CSF, and GM-CSF) that exclusively support granulopoiesis from the time the cells were first exposed to virus. GFP+ cells were then isolated and assessed for colony formation in methylcellulose containing the same growth factors. As shown in Table 3, under these conditions, the control (MIG-transduced) cells produced only granulocyte-macrophage colonies, as expected. However, obvious erythroid colonies (10%-20% of all colonies seen) were again produced by the BCR-ABL–transduced cells and the frequency of granulocyte-macrophage colonies was still markedly reduced (∼2-fold).
Number of different types of CFCs detected before and after transduction of CD34+CD45RA+CD71−(granulopoietic) CB cells with MIG or p210 in the presence of FL, G-CSF, and GM-CSF
| Progenitor . | Before transduction3-150 . | After MIG3-150 . | After p2103-150 . |
|---|---|---|---|
| CFU-E | 0, ND | 0, 0 | 2, 3 |
| BFU-E | 0, ND | 0, 0 | 9, 5 |
| CFU-GEMM | 0, ND | 0, 0 | 0, 0 |
| CFU-GM | 12, ND | 84, 107 | 41, 51 |
| Total CFC | 12, ND | 84, 107 | 52, 59 |
| Progenitor . | Before transduction3-150 . | After MIG3-150 . | After p2103-150 . |
|---|---|---|---|
| CFU-E | 0, ND | 0, 0 | 2, 3 |
| BFU-E | 0, ND | 0, 0 | 9, 5 |
| CFU-GEMM | 0, ND | 0, 0 | 0, 0 |
| CFU-GM | 12, ND | 84, 107 | 41, 51 |
| Total CFC | 12, ND | 84, 107 | 52, 59 |
ND indicates not done.
CFC numbers were calculated as described in the footnote to Table 1. Values shown are from 2 independent experiments.
Low doses of STI571 normalize the type of progeny obtained from p210-transduced CD34+ CB cells
Because of the very high levels of p210BCR-ABL found in the transduced cells, we hypothesized that the excessive kinase activity of this oncoprotein was responsible for their increased growth rate and deregulated production of erythroid progeny. To test this hypothesis, we took advantage of the specificity of STI571 as an inhibitor of the p210BCR-ABL kinase.23 As shown in Figure 1, when CB cells were transduced and subsequently cultured in the presence of 0.25 μmol STI571, the growth rate of the p210-transduced cells became similar to that of the MIG-transduced cells. Moreover, the STI571-treated p210-transduced cells also showed a more “normal” differentiation behavior with a predominance of granulopoietic cells among those produced in the first 2 weeks (at which time the cultures were terminated for analysis, Figure 5). As expected, STI571 had no influence on the growth or differentiation of the MIG-transduced cells. Western analyses confirmed that the dose of STI 571 used caused a decrease in the level of tyrosine phosphorylated p210BCR-ABL and other proteins present in the progeny of the p210-transduced cells (data not shown).
Discussion
The human myeloid leukemias, like many malignancies, are clonal disorders believed to arise through a series of mutational events that first occur in the stem cell compartment of the tissue. Although the first event is assumed to confer a proliferative advantage on the affected cell, evolution to a “malignant” leukemia is characterized by a failure of the cells to mature normally. As a result, large numbers of cells that appear “undifferentiated” accumulate. In some cases, leukemic cells that display an abnormal, mixed lineage phenotype are also produced.38 These latter effects on differentiation are believed to be caused by the acquisition of additional mutations. Nevertheless, the nature of these mutations and how they may perturb normal mechanisms of hematopoietic progenitor commitment and differentiation remain poorly understood. Here we provide evidence that p210BCR-ABL, when expressed at sufficiently high levels in primary human hematopoietic CB cells, can alter their normal pattern of differentiation. This was first suggested by the increased output of differentiating erythroid cells and decreased output of differentiating granulopoietic cells seen in bulk suspension cultures of p210-transduced CB cells. Subsequent experiments showed this reflected a reprogramming of the majority of all progenitors in the p210-transduced CD34+ CB population, independent of their original lineage restriction, even under growth factor conditions that normally selectively promote granulopoietic differentiation.
To our knowledge this is the first report of experimentally induced altered programming of a primary human hematopoietic cell, although several examples of oncogene or growth factor–induced lineage switching in previously immortalized or transformed murine hematopoietic cell lines have been described in the past.8-11,13 Of particular relevance here is the single report of spontaneous lineage switching from a mast cell to an erythroid or myeloid phenotype in certain established lines of BCR-ABL–transformed murine cells.12 More recently p185BCR-ABL transduction of human HL60 cells (a myeloid human leukemic cell line39) was found to activate an erythroid program in these cells.40 Taken together, these findings suggest that lineage commitment mechanisms in the hematopoietic system are more flexible and susceptible to perturbation at much later stages of differentiation than previously appreciated. Such a concept has recently been provided with a mechanistic basis through emerging evidence that suppression of alternative lineage-specific transcription factors and the downstream genes they regulate may be as important to the process of lineage determination as lineage-specific gene activation.41-44 Accordingly, it will be of interest in future work to examine the sequence of gene expression and biochemical changes that occur as a result of the highly up-regulated expression of p210BCR-ABL that is achieved in the novel model described here.
The ability of STI571 to partially reverse the p210BCR-ABL– induced suppression of granulopoietic differentiation and concomitant up-regulation of the generation of erythroid cells shows that these effects require the kinase activity of p210BCR-ABL. Preliminary experiments indicate that these effects are also obtained in transduced CD34+ cells from normal adult human bone marrow. Thus, the mechanisms involved do not appear to be exclusive to ontogenically early target cells although this may influence the likelihood of obtaining the reprogramming response observed here. In contrast to the essential role of the kinase domain of p210BCR-ABL, experiments using a mutant BCR-ABL vector indicate an intact SH2 domain is not required (Y.C. et al, manuscript in preparation). It should be noted that in CML patients' CD34+ cells, a level of p210BCR-ABLequivalent to that obtained here using an MSCV LTR promoter may never be achieved in the chronic phase of the disease and perhaps only rarely in blast crisis. Interestingly in blast crisis, leukemic cells expressing markers of erythroid differentiation are sometimes seen.45,46 Even in the chronic phase, both erythroid and myeloid progenitors are proportionately increased and, although only the output of mature granulocytes is usually elevated,47occasional examples of Philadelphia chromosome–positive (Ph+) patients presenting with an erythrocytosis have been reported.48 Thus, in retrospect, the present findings may be less at odds with the pathologic features of human CML than would appear at first glance. Differences in the level of BCR-ABL expression may also explain the differences between the results described here and those of Zhao et al49 who reported an increase in granulopoietic but not erythroid progenitors in BCR-ABL–transduced CD34+ CB cells, although in their study, objective documentation of the amplified granulopoiesis described was not provided.
Our findings also provide the first reported example of epo-independent erythropoiesis induced by the introduction of an oncogene in primary human hematopoietic cells. Epo independence is a well-established, albeit variably detectable, feature of the neoplastic erythroid progenitors from patients with CML.50 The present findings confirm this to be a consequence of BCR-ABL expression in human erythroid cells, consistent with a number of previous studies with murine targets showing a similar effect of v-abl51 and more recently of BCR-ABL even in progenitor cells lacking erythropoietin receptors.52 However, further analyses will be required to determine what are the downstream targets in p210BCR-ABL–expressing human erythroid cells.
In summary, we have found that expression of high levels of the p210BCR-ABL oncoprotein in primitive human CB cells suppresses granulopoietic differentiation and simultaneously activates entry and progression down the erythroid pathway. This occurs even in apparently committed granulopoietic progenitors and is accompanied by a partial abrogation of the normal requirement of terminally differentiating erythroid cells for stimulation by exogenous epo. Further investigation of this novel transdifferentiation response may offer insights into the normal mechanisms of lineage restriction in primitive human hematopoietic cells as well as the mechanisms by which they can be perturbed in advanced stages of human myeloid leukemia.
We thank the members of the Stem Cell Assay Service of the Terry Fox Laboratory for initial cell processing; Gayle Thornbury, Giovanna Cameron, and Rick Zapf for operating the FACStar+; J. Griffin (Dana Farber Cancer Institute, Boston MA) for the BCR-ABL plasmid; K. Humphries and P. Lansdorp (both from the Terry Fox Laboratory) for various cDNA probes and antibodies; and StemCell, Novartis, Cangene, and Immunex for gifts of recombinant growth factors and other reagents.
Supported by grants from the National Cancer Institute of Canada (NCIC) with funds from the Canadian Cancer Society and the Terry Fox Run. Y.C. was supported by a Terwindt Foundation Fellowship, and C.E. was a Terry Fox Cancer Research Scientist of the NCIC.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Connie J. Eaves, Terry Fox Laboratory, 601 W 10th Ave, Vancouver, BC, V5Z 1L3, Canada; e-mail: ceaves@bccancer.bc.ca.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal