Abstract
Heavy chain ferritin (H-ferritin) is a component of the iron-binding protein, ferritin. We have previously shown that H-ferritin inhibits anti-CD3–stimulated lymphocyte proliferation and that this was due to increased production of interleukin-10 (IL-10). In the present study we have shown that induction of IL-10 production was due to effects of H-ferritin on adherent antigen-presenting cells (APCs) in blood and monocyte-derived dendritic cells (MoDCs). IL-10 was produced by a subpopulation of CD4 T cells, which expressed the CD25 component of the IL-2 receptor and the CTLA-4 receptor characteristic of regulatory T cells. The changes induced in MoDCs were compared with those induced by CD40L and their significance tested by inhibition with monoclonal antibodies. These studies indicated that H-ferritin induced relatively greater expression of CD86 and B7-H1 on MoDCs and that monoclonal antibodies against their receptors, CTLA-4 and programmed death receptor-1 (PD-1), inhibited IL-10 production from the regulatory T cells. H-ferritin did not appear to induce direct production of the cytokines IL-2, IL-4, IL-6, IL-10, IL-12, or interferon-γ from the DCs. These results are consistent with the thesis that H-ferritin induces B7-H1 and CD86 (B7-2) on APCs, which in turn induce IL-10 production from regulatory T cells. This is possibly one mechanism by which melanoma cells may induce changes in APCs in the vicinity of the tumor and result in suppression of immune responses by induction of regulatory T cells.
Introduction
The growth of melanoma, especially in its more advanced stages, can be associated with suppression of immune responses to the tumor and to other antigens.1,2 Induction of anergy to melanoma antigens due to lack of costimulatory molecules or production of reactive oxygen metabolites is one possible mechanism for such changes.3 In addition, a number of soluble factors produced by melanoma cells have been described that down-regulate immune responses via direct effects on lymphocytes or on antigen-presenting cells (APCs) in the vicinity of the tumor (reviewed by Hersey4). Such factors include vascular endothelial growth factor,5,6 interleukin-10 (IL-10),7 and transforming growth factor–β (TGF-β).8 9
We have recently reported that heavy chain ferritin (H-ferritin) appears to be another factor released by melanoma cells that may suppress immune responses by its ability to induce IL-10 production in lymphocytes.10 Ferritin is a major tissue iron-binding protein11 and in its native form has a molecular weight of approximately 500 kd. It is composed of 24 subunits consisting of acid/heavy (H) and basic/light (L) chains.12,13 The genes encoding H-ferritin and light chain ferritin (L-ferritin) are found in different chromosomes and are transcriptionally independent.14 The 24-subunit polymer may form isoferritins, which are either more acidic (H-rich) or more basic (L-rich) depending on the relative proportions of H and L chains. Liver and spleen ferritins are basic, because they are made up mainly of light chains and very few heavy chains. In contrast, heart, kidney, and placental ferritins are highly acidic, because they are composed mostly of heavy chains.15 The ferritin found in cancer cells was found to consist mainly of heavy chains.16 17
Marked variability was found in the ratios of H- to L-ferritin in supernatants from melanoma cultures. Some melanoma cells released predominantly H-ferritin, whereas others contained more equal proportions of H- and L-ferritin.10 The immunosuppressive effects of the supernatants correlated with their content of H-ferritin. These findings were consistent with previous reports that H-ferritin could suppress proliferation of T cells18-20 and E rosette formation.21 In our previous studies it was found that monoclonal antibodies (mAbs) against H-ferritin or against IL-10 could reverse the immunosuppressive effects. The latter was produced from lymphocytes exposed to H-ferritin and was associated with small decreases in IL-2 and IL-4 production. Interferon-γ (IFN-gamma) production was also increased in lymphocytes exposed to H-ferritin.10
In the present study we have examined the mechanism by which H-ferritin induces IL-10 production and the cell types in blood responsible for the production of IL-10. We report that H-ferritin appears to mediate its effects on lymphocytes by action on adherent cells and dendritic cells (DCs) generated from monocytes. The lymphocytes producing IL-10 appear to have the characteristics of regulatory T cells.
Materials and methods
Antibodies and recombinant proteins
The mAb 5H1 specific for B7-H1 is described elsewhere22 and was kindly provided by Dr Lieping Chen (Department of Immunology, Mayo Graduate and Medical schools, Rochester, MN). Anti-DR was from the WM2 clone kindly provided by Dr K. Bradstock, Westmead Hospital, Sydney, Australia.23 Anti-CD3 was the OKT3 mAb.24 The mAb against CD25 was purchased from Endogen (Woburn, MA). The mAbs against CD1a, CD28, CD83, CD80 (B7-1), CD86 (B7-2), CD152 (CTLA-4), CD4, CD8, CD45RO, and immunoglobulin G1 isotype were purchased from PharMingen, (San Diego, CA). The mAb against programmed death receptor (PD-1) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Recombinant H-ferritin and recombinant L-ferritin were kindly provided by Dr Paolo Arosio and are described elsewhere.25,26 CD40L (lot 5753-56) was supplied by Immunex (Seattle, WA) and is described elsewhere.27Recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) and recombinant IL-4 were kindly provided by Schering-Plough (Kenilworth, NJ). Recombinant IL-6 was purchased from Genzyme (Cambridge, MA).
Preparation of T-cell populations
Peripheral blood lymphocytes (PBLs) were separated from whole blood on a Ficoll-Paque gradient (Amersham Pharmacia Biotech, Buckinghamshire, England) by centrifugation at 500g for 30 minutes at 20°C. PBLs were washed twice in Dulbecco modified Eagle medium (DMEM), with centrifugation at 500g for 10 minutes at 20°C. The PBLs were then cultured in DMEM containing 10% fetal calf serum (FCS) (CSL Biosciences, Sydney, Australia) in a 75 cm2 plastic flask (Sarstedt, Newton, NC) for 2 hours in a 37°C incubator supplemented with 5% CO2. Adherent cells were then harvested or used to generate DCs. The nonadherent population was ether harvested or further purified. CD4+ and CD8+ cells were enriched by negative selection on midiMACS columns using a T-cell isolation kit according to the manufacturer's instructions (Miltenyi Biotec, Bergisch-Gladbach, Germany). CD25+ cells were enriched by positive selection on midiMACS columns. In brief, for CD25+ isolation, cells were first incubated with anti-CD25 mAb for 30 minutes on ice in phosphate-buffered saline (PBS) supplemented with 1% human serum and then incubated in the same buffer with antimouse immunoglobulin G1 MicroBeads for 15 minutes. The magnetically labeled positive fraction was retained on a midiMACS column.
Generation of DCs
DCs were generated as described by Romani et al,28 with minor modifications. Briefly, lymphocyte-depleted peripheral blood mononuclear cells (PBMCs) were cultured in DMEM containing 10% FCS (CSL Biosciences). GM-CSF and IL-4 were added at a final concentration of 800 and 500 U/mL, respectively. Cultures were fed every other day (days 2, 4, and 6). On day 7, nonadherent cells were either harvested or transferred to a 24-well flat-bottom plate (Falcon, Becton Dickinson, Franklin Lakes, NJ) and cultured further with GM-CSF and IL-4 in the presence or absence of H-ferritin, L-ferritin, or CD40L for a further 24 to 48 hours.
Proliferation assay
Proliferation assays were set up as described by Gray et al.10 Briefly, 1 × 105 nonadherent PBLs were cultured with adherent PBMCs or DCs in 200 μL DMEM containing 10% FCS (CSL Biosciences) in a 96-well U-bottom plate (Falcon, Becton Dickinson). H-ferritin, L-ferritin, or CD40L was added to the culture at 1 μg/mL along with 50 ng/mL anti-CD3. Cultures were then incubated in a 37°C incubator supplemented with 5% CO2 for 72 hours for proliferation studies or 24 hours for cytokine expression studies. For studies on the effect of purified CD25+ cells on lymphocyte proliferation, the purified subpopulations were cultured for 24 hours with H-ferritin, L-ferritin, or CD40L-pretreated DCs at a CD25+ to DC ratio of 10. The CD25+subpopulation was then harvested and subsequently treated with 25 μg/mL mitomycin-C (Kyowa Hakko Kogyo, Tokyo, Japan) at 37°C for 1 hour, before addition to the proliferation assay. For blocking studies, mAbs against B7-H1, PD-1, CTLA-4, or CD28 were prebound to the plate with 10 μg/mL anti-CD3 in carbonate buffer (0.1 M NaHCO3, pH 8.2) overnight at 4°C. For proliferation studies, 2 μCi (74 KBq) of [125I]UdR (5-[125I]Iodo-2′-deoxy uridine) (Amersham, Aylesbury, United Kingdom) was added for the final 4 hours before cell harvesting. Incorporation of [125I]UdR was then measured on a CompuGamma CS gamma counter (Wallac, Oy, Finland). The degree of inhibition of anti-CD3 stimulation was calculated as previously described elsewhere.29
Mixed lymphocyte response
The mixed lymphocyte response (MLR) was set up as described by Romani et al,28 with minor modifications. Briefly, serial dilutions (1 × 104 to 1 cells per well) of DCs were cultured in triplicate with 1 × 105 allogeneic nonadherent PBLs in 200 μL DMEM containing 10% FCS (CSL Biosciences) in a 96-well U-bottom plate (Falcon, Becton Dickinson). Cultures were then incubated in a 37°C incubator supplemented with 5% CO2 for 5 days. Then, 2 μCi (74 KBq) of [125I]UdR (Amersham) was added for the final 8 hours before cell harvesting. Incorporation of [125I]UdR was then measured on a CompuGamma CS gamma counter (Wallac). The results are expressed as the mean of triplicate cultures.
Flow cytometry
Analysis was carried out using a Becton Dickinson (Mountain View, CA) FACScan flow cytometer. Appropriate concentrations of mAbs were added to the cells in 100 μL PBS containing 1% human serum and incubated for 30 minutes at 4°C. Cells were washed twice with PBS and analyzed. For indirect labeling, cells were incubated with F(ab′)2 fragment affinity-isolated phycoerythrin- or fluorescein isothiocyanate–conjugated sheep antimouse immunoglobulin (Silenus, Amrad Biotech, Boronia, Australia) plus 1% human serum for 30 minutes at 4°C. Cells were then washed once in PBS before analysis. A minimum of 5000 cells was analyzed by flow cytometry. The percentage of positive cells was calculated as the difference in positive area between the positive and negative control histograms. The positive area was that to the right of the intersection of the 2 curves.30
IL-6 bioassay
The IL-6 bioassay was set up as described by Lu and Kerbel,31 with minor modifications. In brief, IL-6–dependent B9.9 murine cells were cultured in 100 μL DMEM containing 10% FCS (CSL Biosciences) in a 96-well U-bottom plate (Falcon, Becton Dickinson) at 5 × 103 cells per well, with or without recombinant IL-6 or supernatant collected at 24 hours from anti-CD3 proliferation assay. Cultures were then incubated in a 37°C incubator supplemented with 5% CO2 for 3 days. Then, 2 μCi (74 KBq) of [125I]UdR (Amersham) was added for the final 8 hours before cell harvesting. Incorporation of [125I]UdR was then measured on a CompuGamma CS gamma counter (Wallac). The IL-6 concentration in the supernatants was determined by comparing its proliferative activity to a known IL-6 standard. The results are expressed as the mean of triplicate cultures.
Assay for IL-2, IL-4, IL-10, TGF-β1, IL-12p70, and IFN-γ
An anti-CD3 proliferation assay was set. After 24 hours the supernatants were collected from the proliferation assay. IL-2, IL-4, IL-10, TGF-β1, IL-12p70, and IFN-γ were then assayed using a biotinylated enzyme-linked immunosorbent assay (ELISA) system (PharMingen) according to the manufacturer's instructions for each cytokine.
Results
Kinetics of IL-10 production by H-ferritin
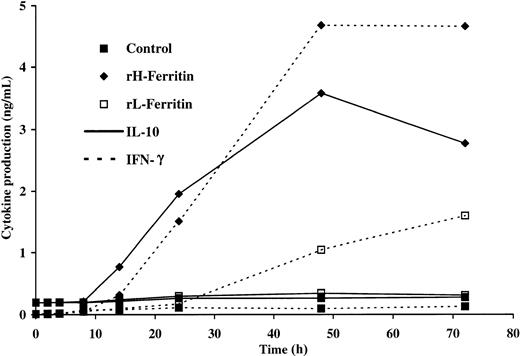
We have previously reported that H-ferritin induced a significant increase in IL-10 and IFN-γ production in anti-CD3–stimulated lymphocytes, which is consistent with a regulatory T-cell response.10 Kinetic studies have demonstrated that regulatory T cells produce IL-10 more rapidly than TH0, TH1, or TH2 cells.32 The kinetics of IL-10 production induced by H-ferritin in anti-CD3–stimulated PBLs is shown in Figure1. IL-10 was detectable by ELISA within 6 to 8 hours and peaked at 48 hours. IFN-γ was detectable by 14 hours and peaked at 48 hours.
Kinetics of IL-10 and IFN-γ production in anti-CD3–stimulated PBLs in the presence of H- and L-ferritin.
Cultures consisted of 2 × 105 PBLs, 50 ng anti-CD3, and 1 μg/mL H- or L-ferritin. Supernatants were collected at various time points, and ELISA was used to measure cytokine production.
Kinetics of IL-10 and IFN-γ production in anti-CD3–stimulated PBLs in the presence of H- and L-ferritin.
Cultures consisted of 2 × 105 PBLs, 50 ng anti-CD3, and 1 μg/mL H- or L-ferritin. Supernatants were collected at various time points, and ELISA was used to measure cytokine production.
H-ferritin induces IL-10 production and inhibition of lymphocyte proliferation by effects on adherent cells/DCs from blood
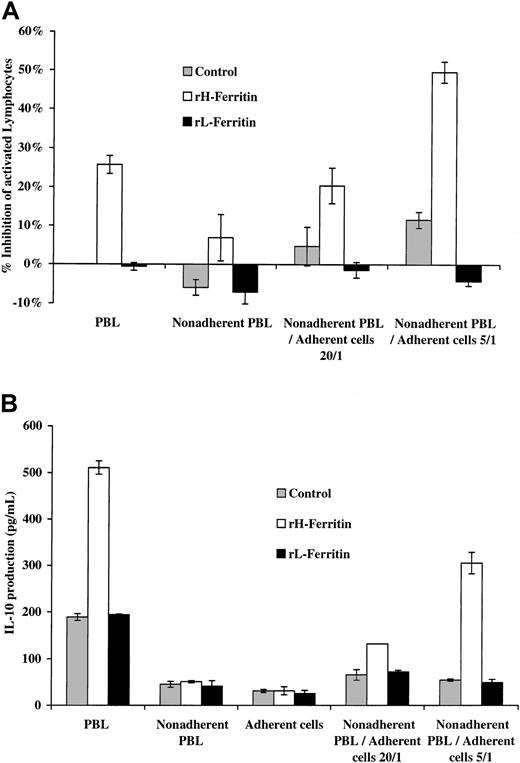
To determine which cell type was responsible for the effect of H-ferritin on lymphocyte proliferation and IL-10 production, we depleted PBLs from a normal subject of adherent cells and pretreated the populations with H-ferritin or L-ferritin at 1 μg/mL for 24 hours. As shown in Figure 2, pretreatment of the adherent and nonadherent cell populations separately had no significant effect on anti-CD3–stimulated proliferation at 3 days or IL-10 production at 24 hours, but when the pretreated adherent population was added to the nonadherent PBLs there was increased production of IL-10 and inhibition of lymphocyte proliferation induced by anti-CD3. Addition of pretreated nonadherent cells to the adherent cells did not affect their production of IL-10 or lymphocyte proliferation.
H-ferritin increases IL-10 production from adherent PBLs by effects on adherent cells.
(A) A total of 1 × 105 PBLs and nonadherent PBLs were pretreated for 24 hours with 1 μg/mL H-ferritin and stimulated with 50 ng anti-CD3 for 3 days. Inhibition of proliferation of nonadherent PBLs was seen only when mixed with 5 × 103 or 2 × 104 adherent cells that had been exposed to 1 μg/mL H-ferritin but not L-ferritin. (B) A total of 1 × 105 PBLs and nonadherent PBLs pretreated for 24 hours with 1 μg/mL H-ferritin and stimulated with 50 ng anti-CD3 for 24 hours. IL-10 production was increased only when mixed with 5 × 103 or 2 × 104 adherent cells treated with H-ferritin but not L-ferritin (1 μg/mL).
H-ferritin increases IL-10 production from adherent PBLs by effects on adherent cells.
(A) A total of 1 × 105 PBLs and nonadherent PBLs were pretreated for 24 hours with 1 μg/mL H-ferritin and stimulated with 50 ng anti-CD3 for 3 days. Inhibition of proliferation of nonadherent PBLs was seen only when mixed with 5 × 103 or 2 × 104 adherent cells that had been exposed to 1 μg/mL H-ferritin but not L-ferritin. (B) A total of 1 × 105 PBLs and nonadherent PBLs pretreated for 24 hours with 1 μg/mL H-ferritin and stimulated with 50 ng anti-CD3 for 24 hours. IL-10 production was increased only when mixed with 5 × 103 or 2 × 104 adherent cells treated with H-ferritin but not L-ferritin (1 μg/mL).
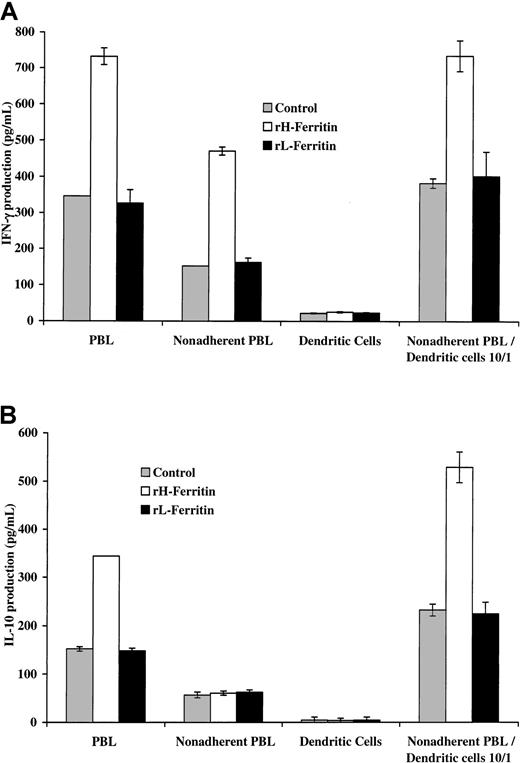
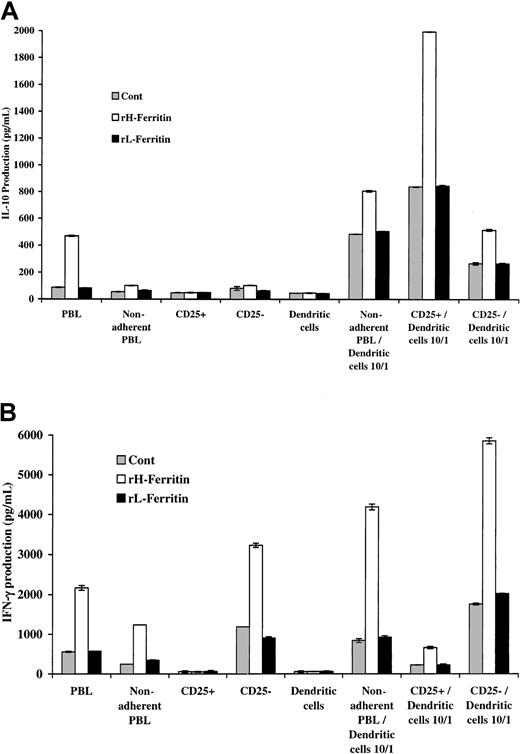
These experiments were repeated with DCs produced by culture of monocytes in IL-4 and GM-CSF for 7 days. The results shown in Figure3 indicated that addition of DCs pretreated with H-ferritin to nonadherent PBLs was associated with increased production of IL-10 and IFN-γ (measured at 24 hours). In contrast, addition of H-ferritin to nonadherent PBLs did not increase IL-10 production. There was, however, an increase in IFN-γ production from nonadherent PBLs, suggesting that H-ferritin did have some direct effect on nonadherent PBLs.
H-ferritin increases INF-γ production through a direct effect on nonadherent PBLs and IL-10 production through an indirect effect involving DCs.
(A) Pretreatment of 1 × 105 nonadherent PBLs stimulated with 50 ng anti-CD3 with H-ferritin (1 μg/mL) has a direct effect on IFN-γ production that was further enhanced by exposure to 1 μg/mL H-ferritin–pretreated 1 × 104 DCs (24 hours). (B) Pretreatment of DCs with H-ferritin for 24 hours results in increased production of IL-10 from nonadherent PBLs stimulated with anti-CD3 at 24 hours.
H-ferritin increases INF-γ production through a direct effect on nonadherent PBLs and IL-10 production through an indirect effect involving DCs.
(A) Pretreatment of 1 × 105 nonadherent PBLs stimulated with 50 ng anti-CD3 with H-ferritin (1 μg/mL) has a direct effect on IFN-γ production that was further enhanced by exposure to 1 μg/mL H-ferritin–pretreated 1 × 104 DCs (24 hours). (B) Pretreatment of DCs with H-ferritin for 24 hours results in increased production of IL-10 from nonadherent PBLs stimulated with anti-CD3 at 24 hours.
IL-10 is produced predominantly by CD4 T cells
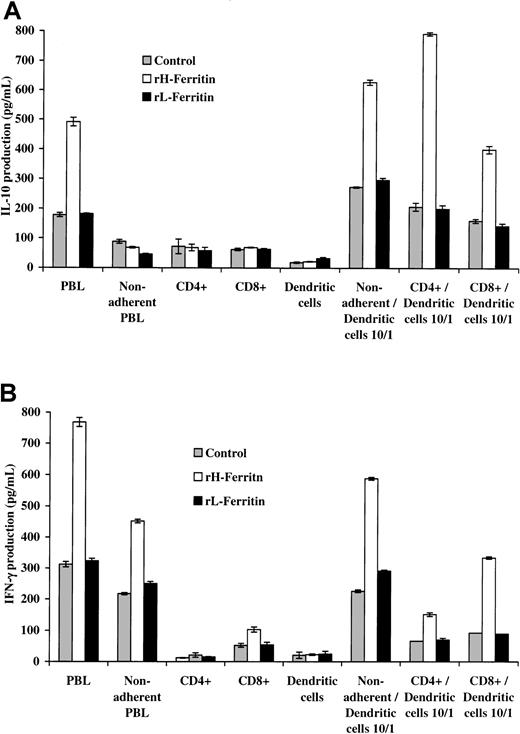
To determine which population of lymphocytes produced the IL-10 and IFN-γ, DCs were added to unseparated PBLs and purified populations of CD4 and CD8 T cells. The CD4 populations were 95% pure and the CD8 populations 94% pure. The results shown in Figure4 again showed that pretreatment with H-ferritin of nonadherent PBLs and CD4 and CD8 populations of T cells had no direct effects on IL-10 production but did increase IFN-γ production from nonadherent and CD8 T-cell populations. However, when H-ferritin–pretreated DCs were added to the lymphoid populations it was apparent that CD4 T cells were mainly responsible for the production of IL-10, whereas IFN-γ was produced mainly by the CD8 T cells.
H-ferritin increases INF-γ production through a direct effect on CD8+ T cells and IL-10 production through an indirect effect on CD4+ T cells involving DCs.
The CD4 populations were 95% pure and the CD8 populations 94% pure. (A) Addition of 1 × 104 H-ferritin– (1 μg/mL) pretreated DCs to 1 × 105 anti-CD3–stimulated nonadherent CD4 and CD8 subpopulations increased IL-10 production (24 hours) predominantly from CD4 T cells. (B) H-ferritin induces IFN-γ production in nonadherent PBLs CD8 T cells, and this was amplified by DCs pretreated with H-ferritin (1 μg/mL).
H-ferritin increases INF-γ production through a direct effect on CD8+ T cells and IL-10 production through an indirect effect on CD4+ T cells involving DCs.
The CD4 populations were 95% pure and the CD8 populations 94% pure. (A) Addition of 1 × 104 H-ferritin– (1 μg/mL) pretreated DCs to 1 × 105 anti-CD3–stimulated nonadherent CD4 and CD8 subpopulations increased IL-10 production (24 hours) predominantly from CD4 T cells. (B) H-ferritin induces IFN-γ production in nonadherent PBLs CD8 T cells, and this was amplified by DCs pretreated with H-ferritin (1 μg/mL).
IL-10 is produced by CD25+ T cells, whereas IFN-γ is produced by CD25− T cells
Previous studies have shown that regulatory T cells constitutively express the CD25 component of the IL-2 receptor. We therefore examined the possible involvement of these cells in IL-10 production by separation of the nonadherent T cells into CD25+ and CD25− populations. The CD25+ population was 83% pure. These were then added to H-ferritin–pretreated DCs. The CD25+ population was 84.7% CD4+, 5.5% CD8+, and 9.7% CD4−CD8− T cells (Table 1). The CD25+ cells also expressed CTLA-4 and were CD45RO+ and PD-1+. The results in Figure5 show that the CD25+population was mainly responsible for production of IL-10, whereas the CD25− population produced IFN-γ. IFN-γ production from the CD25− population exceeded that from the nonadherent population, suggesting that the CD25+ cells may have been inhibitory for IFN-γ production.
Characterization of CD25+ cell populations in nonadherent PBLs
| . | % Cells* . | % CD25+ cells . |
|---|---|---|
| Total CD25+ | 7.6 | |
| CD25+CD4+ | 6.44 | 84.7 |
| CD25+CD8+ | 0.42 | 5.5 |
| CD25+CD4−CD8− | 0.74 | 9.7 |
| CD25+CTLA-4+ | 6.61 | 87.0 |
| CD25+CD45RO+ | 5.20 | 68.4 |
| CD25+PD-1+ | 6.54 | 86.0 |
| . | % Cells* . | % CD25+ cells . |
|---|---|---|
| Total CD25+ | 7.6 | |
| CD25+CD4+ | 6.44 | 84.7 |
| CD25+CD8+ | 0.42 | 5.5 |
| CD25+CD4−CD8− | 0.74 | 9.7 |
| CD25+CTLA-4+ | 6.61 | 87.0 |
| CD25+CD45RO+ | 5.20 | 68.4 |
| CD25+PD-1+ | 6.54 | 86.0 |
Measured by flow cytometry as % cells stained.
T cells constitutively expressing CD25 were responsible for production of IL-10, whereas IFN-γ was produced from CD25− T cells.
The CD25+ population was 83% pure. (A) Addition of 1 × 104 H-ferritin– (1 μg/mL) pretreated DCs to 1 × 105 anti-CD3–stimulated CD25+ and CD25− subpopulations increased IL-10 production (24 hours) only in the CD25+ T-cell population. (B) H-ferritin induces IFN-γ production in CD25− T cells, and this is amplified through the depletion of CD25+ T cells.
T cells constitutively expressing CD25 were responsible for production of IL-10, whereas IFN-γ was produced from CD25− T cells.
The CD25+ population was 83% pure. (A) Addition of 1 × 104 H-ferritin– (1 μg/mL) pretreated DCs to 1 × 105 anti-CD3–stimulated CD25+ and CD25− subpopulations increased IL-10 production (24 hours) only in the CD25+ T-cell population. (B) H-ferritin induces IFN-γ production in CD25− T cells, and this is amplified through the depletion of CD25+ T cells.
H-ferritin induces maturation of DCs that are phenotypically and functionally different than mature DCs induced by CD40L
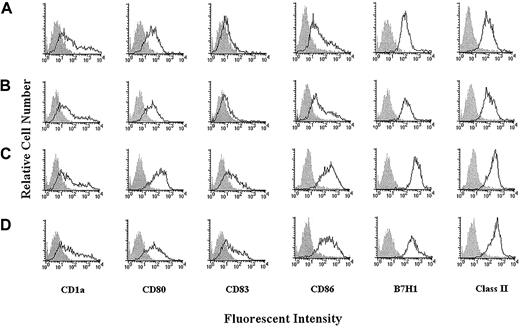
The results above suggested that H-ferritin induced changes in APCs, which preferentially induced induction of regulatory T cells. We examined whether these changes may have been reflected in expression of regulatory molecules on DCs and compared the results with phenotypic changes in DCs induced by CD40L. Pretreatment of DCs at 6 days for 24 hours with H-ferritin or CD40L was associated with similar clumping of the DCs. The phenotypes of these DCs are shown in Figure6 (donor 1) and Table2. Both H-ferritin and CD40L induced an activated mature DC phenotype, as shown by increased expression of CD83, but relative to the changes induced by CD40L, H-ferritin increased expression of CD86 and B7-H1. These results were repeated in subsequent experiments (donors 2 and 3) shown in Table 2. Statistical analysis on the median fluorescence intensity for all 3 donors found that H-ferritin significantly increased CD86 (paired F test,P = .021) and B7-H1 (P = .019). In contrast, the increase in expression induced by CD40L was not statistically significant for CD86 (P = .172) or B7-H1 (P = .091).
H-ferritin induces maturation of DCs, which are phenotypically different than DCs induced by CD40L.
Phenotypic profile of DCs derived from monocytes (A) and monocyte-derived DCs exposed on day 6 to L-ferritin (B), H-ferritin (C), or CD40L (D) (1 μg/mL) for 24 hours.
H-ferritin induces maturation of DCs, which are phenotypically different than DCs induced by CD40L.
Phenotypic profile of DCs derived from monocytes (A) and monocyte-derived DCs exposed on day 6 to L-ferritin (B), H-ferritin (C), or CD40L (D) (1 μg/mL) for 24 hours.
Effect of H-ferritin on expression of DC markers
| Donor no. . | Treatment . | CD1a* % cells (MFI) . | CD80 % cells (MFI) . | CD83 % cells (MFI) . | CD86,† % cells (MFI) . | B7-H1,† % cells (MFI) . | HLA-DR % cells (MFI) . |
|---|---|---|---|---|---|---|---|
| Donor 1 | DCs | 60.0 (4.7) | 77.4 (7.4) | 30.9 (1.7) | 77.3 (7.0) | 96.0 (18.3) | 94.2 (26.2) |
| DCs + L-ferritin | 60.7 (4.1) | 77.2 (7.1) | 29.0 (1.6) | 75.1 (5.8) | 97.6 (23.7) | 95.6 (19.3) | |
| DCs + H-ferritin | 58.1 (4.1) | 90.3 (24.4) | 58.1 (3.7) | 94.5 (37.8) | 99.5 (114.5) | 96.2 (32.5) | |
| DCs + CD40L | 60.2 (4.9) | 79.1 (10.1) | 53.3 (3.3) | 91.0 (30.0) | 99.4 (53.8) | 95.5 (52.3) | |
| Donor 2 | DCs | 34.6 (1.6) | 37.7 (1.6) | 31.4 (1.3) | 97.2 (10.9) | 71.8 (2.8) | 89.6 (5.7) |
| DCs + L-ferritin | 33.3 (1.5) | 33.3 (1.6) | 32.2 (1.4) | 95.5 (8.1) | 70.7 (3.0) | 83.6 (5.0) | |
| DCs + H-ferritin | 28.0 (1.4) | 35.3 (1.5) | 37.6 (1.6) | 99.1 (69.2) | 85.2 (4.7) | 93.6 (17.9) | |
| DCs + CD40L | 31.4 (1.6) | 28.7 (1.4) | 33.2 (1.7) | 93.3 (21.7) | 72.7 (3.3) | 89.8 (15.1) | |
| Donor 3 | DCs | NA | 68.4 (3.8) | NA | 65.6 (2.9) | 78.0 (5.8) | 79.9 (10.9) |
| DCs + L-ferritin | NA | 69.4 (4.0) | NA | 63.7 (2.4) | 93.1 (10.3) | 81.1 (8.0) | |
| DCs + H-ferritin | NA | 83.4 (6.2) | NA | 80.1 (15.7) | 95.9 (23.9) | 81.6 (13.5) | |
| DCs + CD40L | NA | 68.8 (4.8) | NA | 77.2 (12.5) | 92.8 (18.4) | 81.0 (21.7) |
| Donor no. . | Treatment . | CD1a* % cells (MFI) . | CD80 % cells (MFI) . | CD83 % cells (MFI) . | CD86,† % cells (MFI) . | B7-H1,† % cells (MFI) . | HLA-DR % cells (MFI) . |
|---|---|---|---|---|---|---|---|
| Donor 1 | DCs | 60.0 (4.7) | 77.4 (7.4) | 30.9 (1.7) | 77.3 (7.0) | 96.0 (18.3) | 94.2 (26.2) |
| DCs + L-ferritin | 60.7 (4.1) | 77.2 (7.1) | 29.0 (1.6) | 75.1 (5.8) | 97.6 (23.7) | 95.6 (19.3) | |
| DCs + H-ferritin | 58.1 (4.1) | 90.3 (24.4) | 58.1 (3.7) | 94.5 (37.8) | 99.5 (114.5) | 96.2 (32.5) | |
| DCs + CD40L | 60.2 (4.9) | 79.1 (10.1) | 53.3 (3.3) | 91.0 (30.0) | 99.4 (53.8) | 95.5 (52.3) | |
| Donor 2 | DCs | 34.6 (1.6) | 37.7 (1.6) | 31.4 (1.3) | 97.2 (10.9) | 71.8 (2.8) | 89.6 (5.7) |
| DCs + L-ferritin | 33.3 (1.5) | 33.3 (1.6) | 32.2 (1.4) | 95.5 (8.1) | 70.7 (3.0) | 83.6 (5.0) | |
| DCs + H-ferritin | 28.0 (1.4) | 35.3 (1.5) | 37.6 (1.6) | 99.1 (69.2) | 85.2 (4.7) | 93.6 (17.9) | |
| DCs + CD40L | 31.4 (1.6) | 28.7 (1.4) | 33.2 (1.7) | 93.3 (21.7) | 72.7 (3.3) | 89.8 (15.1) | |
| Donor 3 | DCs | NA | 68.4 (3.8) | NA | 65.6 (2.9) | 78.0 (5.8) | 79.9 (10.9) |
| DCs + L-ferritin | NA | 69.4 (4.0) | NA | 63.7 (2.4) | 93.1 (10.3) | 81.1 (8.0) | |
| DCs + H-ferritin | NA | 83.4 (6.2) | NA | 80.1 (15.7) | 95.9 (23.9) | 81.6 (13.5) | |
| DCs + CD40L | NA | 68.8 (4.8) | NA | 77.2 (12.5) | 92.8 (18.4) | 81.0 (21.7) |
DCs were incubated with H- or L-ferritin or CD40L at a concentration of 1 μg/mL for 24 hours prior to detection of markers. MFI indicates median fluorescence intensity; NA, not available.
Measured by flow cytometry as % cells stained.
H-ferritin significantly increased CD86 (paired F test,P = .021) and B7-H1 (P = .019).
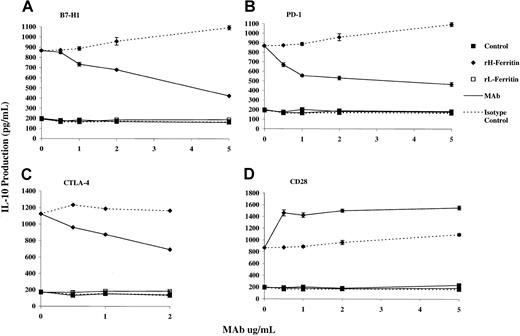
IL-10 production induced by H-ferritin–treated DCs is dependent on interaction with CTLA-4 and PD-1 on T cells, and B7-H1 ligand on adherent PBMCs
To determine whether these changes in expression of CD86 or B7-H1 may be important in induction of regulatory T cells, we carried out blocking studies with mAbs against these ligands and their receptors (CD28/CTLA-4 for CD86) and the PD-1 for B7-H1. As shown in Figure7, mAb against the ligand B7-H1 and its receptor, PD-1 (but not the isotype control), inhibited the production of IL-10 induced by DCs pretreated with H-ferritin. Similarly, mAb against CTLA-4 inhibited IL-10 production, whereas mAb against CD28 increased IL-10 production. The latter was consistent with the known costimulatory function of CD28.
H-ferritin induces IL-10 production by interaction with the CTLA-4 and PD-1, but not CD28 receptor on T cells and B7-H1 ligand on adherent PBMCs.
The mAbs against B7-H1 (A), PD-1 (B), and CTLA-4 (C) inhibited the induction of IL-10 by H-ferritin. An mAb against CD28 enhanced IL-10 production by H-ferritin (D). An isotype control had no effect on IL-10 production. A total of 2 × 105 PBLs were cultured with 1 μg/mL H- or L-ferritin in a 96-well plate that was prebound with a serial dilution of mAb and 10 μg/mL anti-CD3 for 24 hours. IL-10 was measured by ELISA in triplicate.
H-ferritin induces IL-10 production by interaction with the CTLA-4 and PD-1, but not CD28 receptor on T cells and B7-H1 ligand on adherent PBMCs.
The mAbs against B7-H1 (A), PD-1 (B), and CTLA-4 (C) inhibited the induction of IL-10 by H-ferritin. An mAb against CD28 enhanced IL-10 production by H-ferritin (D). An isotype control had no effect on IL-10 production. A total of 2 × 105 PBLs were cultured with 1 μg/mL H- or L-ferritin in a 96-well plate that was prebound with a serial dilution of mAb and 10 μg/mL anti-CD3 for 24 hours. IL-10 was measured by ELISA in triplicate.
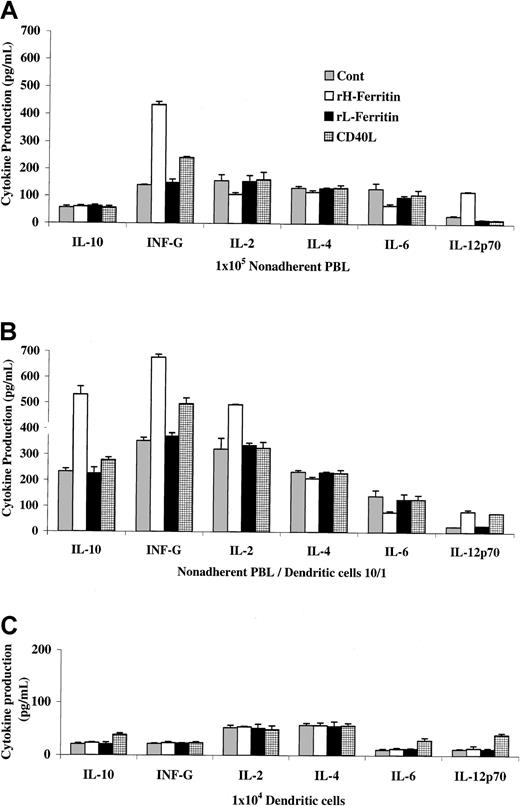
H-ferritin pretreatment of DCs induces a different cytokine profile in nonadherent lymphocytes than CD40L-pretreated DCs
We next examined whether H-ferritin may induce or suppress the production of cytokines that may be involved in induction of regulatory T cells and again compared the results to those induced by CD40L. Studies on nonadherent PBLs (Figure 8A) showed (as expected) that H-ferritin did not induce IL-10 production but did induce IFN-γ production. There was also the expected small reduction in IL-2 and IL-4 production. In addition, production of IL-6 was markedly reduced, whereas IL-12 production was increased. These effects reflected direct effects of H-ferritin on the PBLs. In contrast, CD40L had no direct effects on the nonadherent PBLs except for a small increase in IFN-γ production.
H-ferritin pretreatment of DCs induced a different cytokine profile in nonadherent lymphocytes than CD40L–pretreated DCs.
Cytokine production by 104 DCs pretreated with H- or L-ferritin for 24 hours or CD40L for 48 hours is shown in panel C and from 105 anti-CD3–stimulated nonadherent PBLs in the presences (B) or absences (A) of H- or L-ferritin or CD40L-pretreated DCs.
H-ferritin pretreatment of DCs induced a different cytokine profile in nonadherent lymphocytes than CD40L–pretreated DCs.
Cytokine production by 104 DCs pretreated with H- or L-ferritin for 24 hours or CD40L for 48 hours is shown in panel C and from 105 anti-CD3–stimulated nonadherent PBLs in the presences (B) or absences (A) of H- or L-ferritin or CD40L-pretreated DCs.
When DCs pretreated with H-ferritin were added to the nonadherent PBLs (Figure 8B) at a ratio of 10:1, the main change in cytokine production was the increase in IL-10 production and, to a lesser extent, IL-2 production. The direct effects on cytokine production from nonadherent PBLs noted in Figure 8A, such as decreased production of IL-4 and IL-6 and increased IL-12 production, was again seen. (Note: H-ferritin added to unseparated PBLs slightly inhibited IL-2 production, as noted previously.)
As shown in Figure 8C, H-ferritin had no detectable direct effects on cytokine production from the DCs alone. This contrasted with the effect of CD40L, which increased IL-12, IL-10, and IL-6 production from the DCs. These results argue against the possibility that induction of regulatory T cells was due to H-ferritin–induced changes in cytokine production from DCs, but we cannot exclude that cytokines other than those measured may have been important.
H-ferritin pretreatment of DCs stimulates a different response in T cells to CD40L–pretreated DCs
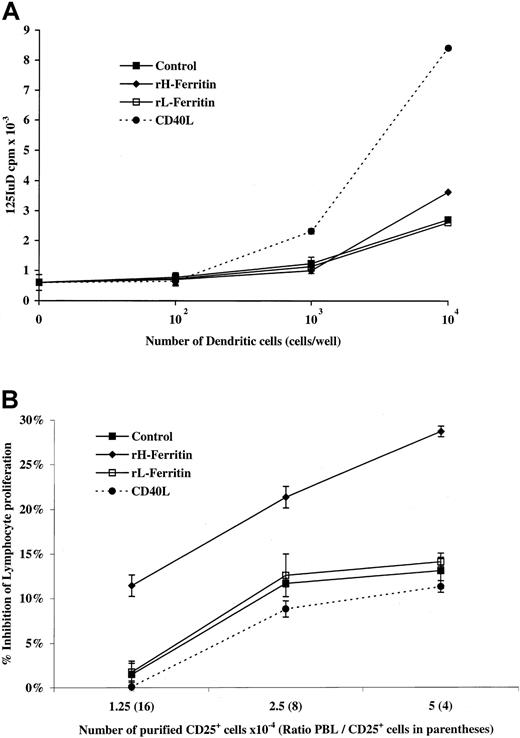
To further assess the function of H-ferritin–treated DCs, they were used as stimulators in an MLR. As shown in Figure9A, H-ferritin–treated DCs were poor stimulators of MLRs compared with CD40L-treated DCs.
H-ferritin pretreatment of DCs stimulates a different response in T cells to CD40L-pretreated DCs.
(A) Functional ability of monocyte-derived DCs exposed to 1 μg/mL H- or L-ferritin (24 hours) or CD40L (48 hours) to stimulate allo-MLRs. A serial dilution (1 × 104 to 1 cells per well) of DCs was cultured in triplicate with 1 × 105 allogeneic nonadherent PBLs responder cells and cultured for 5 days. (B) Suppression of anti-CD3 lymphocyte proliferation by CD25+ T regulatory cells is enhanced through interaction with H-ferritin–pretreated DCs. CD25+ subpopulations were precultured for 24 hours with H-ferritin–, L-ferritin–, or CD40L– (1 μg/mL) pretreated DCs, harvested, and then treated with mitomycin-C, before addition to anti-CD3–stimulated PBLs.
H-ferritin pretreatment of DCs stimulates a different response in T cells to CD40L-pretreated DCs.
(A) Functional ability of monocyte-derived DCs exposed to 1 μg/mL H- or L-ferritin (24 hours) or CD40L (48 hours) to stimulate allo-MLRs. A serial dilution (1 × 104 to 1 cells per well) of DCs was cultured in triplicate with 1 × 105 allogeneic nonadherent PBLs responder cells and cultured for 5 days. (B) Suppression of anti-CD3 lymphocyte proliferation by CD25+ T regulatory cells is enhanced through interaction with H-ferritin–pretreated DCs. CD25+ subpopulations were precultured for 24 hours with H-ferritin–, L-ferritin–, or CD40L– (1 μg/mL) pretreated DCs, harvested, and then treated with mitomycin-C, before addition to anti-CD3–stimulated PBLs.
We tested whether H-ferritin–treated DCs would increase the suppressive activity of CD25 T regulatory cells by adding purified CD25+ T cells to DCs pretreated with H-ferritin, CD40L, or L-ferritin for 24 hours. The CD25+ T cells were then added to cultures of anti-CD3–stimulated PBLs and their effect on proliferation measured at 3 days. As shown in Figure 9B, exposure to H-ferritin–treated DCs resulted in approximately a 2-fold increase in their ability to suppress lymphocyte proliferation.
Discussion
The above studies confirm that recombinant H-ferritin induces production of IL-10 from PBLs and that this is responsible for inhibition of anti-CD3–stimulated lymphocyte proliferation.10 The present studies extend these findings by showing that the induction of IL-10 production appears to be due to effects of H-ferritin on adherent cells in blood and monocyte-derived DCs produced from blood. These effects appeared specific for H-ferritin and were not due to contaminants such as endotoxins in the preparation. The latter could not be detected in the preparation of H-ferritin used in the study, and similar effects were not seen with recombinant L-ferritin produced by similar methods. Other sources of nonrecombinant H-ferritin, such as extracts of heart and melanoma cell supernatants, had similar effects as recombinant H-ferritin.10 They further show that the source of IL-10 appeared to be a particular subpopulation of CD4 T cells in blood that constitutively express CD25 and CTLA-4.
The latter finding was of much interest because much attention has been given to a subpopulation of CD4+ T cells that constitutively express the CD25 component of the IL-2 receptor and the CTLA-4 T-cell activation receptor for CD80 (B7.1) and CD86 (B7.2).33-37 These cells were shown to suppress autoimmune disease in several animal models38,39 and facilitated survival of transplants40-42—hence their description as regulatory T cells. Certain regulatory T cells were reported to also produce IFN-γ and TGF-β but not IL-2 or IL-4.34 43 The cells identified in the present study had a similar phenotype to previously described regulatory T cells but did not produce TGF-β1 in responses to H-ferritin (data not shown). H-ferritin induced IFN-γ production when added to PBLs, but subsequent analysis revealed that the IFN-γ was produced by a direct effect of H-ferritin on CD8 T cells. Whether CD8 T cells were involved in the production of IFN-γ in other studies on regulatory T cells is not known.
The mode of action of regulatory T cells is controversial. Suppression of autoimmune inflammatory bowel disease in mice by regulatory T cells appeared to be mediated by IL-10 and could not be inhibited by regulatory T cells from IL-10 knock-out mice.44 Human self–major histocompatibility complex (MHC)–reactive cells were shown to have suppressor activity against specific CD4 T cells and immunoglobulin production when cultured with non–T cells stimulated with pokeweed mitogen (PWM) or CD40L. The effects were mediated by IL-10 and/or TGF-β and involved the CTLA-4 receptor.43 In other systems, however, direct contact between regulatory T cells and responding T cells appeared necessary for suppression of immune responses.45-47 Activation of regulatory T cells was needed for their activity but, once activated, their suppression was nonspecific, perhaps due to interaction with receptors on CD25− T cells such as those of the tumor necrosis factor receptor family or those with inhibitory motifs.48 These conflicting findings may be reconciled by evidence that IL-10 is involved in the induction of regulatory T cells rather than their function.49
The processes initiating the induction of regulatory T cells are largely unknown. Interaction of high-affinity thymocytes with self-peptides was shown to induce CD4+CD25+regulatory T cells and appeared distinct from the process of positive selection or deletion.50 Other factors include chronic antigenic stimulation in mice transgenic for influenza hemagglutinin,42 chronic exposure to superantigen,51 exposure to immature DCs,52and culture of cord blood T cells in IL-10 and IFN-α.53As far as we are aware, this is the first report that effects of H-ferritin on DCs and other adherent cells may also activate regulatory T cells. Activation of the regulatory T cells in the present study appeared similar to studies on activation of regulatory self-MHC–reactive cells43 in that contact with activated APCs was required. This contact resulted in a rapid increase in the capacity of CD25+ cells to produce IL-10 and to suppress anti-CD3–stimulated lymphocyte responses.
It was examined whether H-ferritin may induce a particular DC phenotype that would explain its ability to induce regulatory T cells. Comparison with DCs exposed to CD40L suggested that there was a relatively greater increase in expression of CD86 (B7.2) and B7-H1 on the H-ferritin–stimulated DCs. These changes appeared important in that mAbs against the CTLA-4 receptor for CD86 and PD-1 for B7-H1 inhibited production of IL-10 from the T cells stimulated with H-ferritin–stimulated DCs. In contrast, mAbs against CD28 resulted in increased production of IL-10. A number of previous studies have shown that activation through the CTLA-4 receptor is needed for induction of regulatory T cells.36,37 The B7-H1 molecule was reported to costimulate T-cell proliferation and to preferentially stimulate IL-10 production.22,54,55 The receptor for B7-H1 appears to be the PD-1 molecule.56,57 Additional ligands for PD-1 have also been recently described,58 but whether H-ferritin selectively up-regulates these ligands has not been studied.
We were unable to detect direct effects of H-ferritin on cytokine production from DCs, which argued against induction of regulatory T cells by changes in cytokine production by treatment of DCs with H-ferritin. In contrast, CD40L was shown to increase production of IL-6, IL-12, and IL-10 by DCs. These results do not, however, exclude a role for other cytokines not measured in these studies. H-ferritin also had direct effects on cytokine production by nonadherent PBLs, such as increases in IFN-γ and IL-12 and inhibition of IL-2, IL-4, and IL-6, but these effects did not appear important in induction of regulatory T cells, which appeared entirely due to contact with H-ferritin–treated DCs.
On the basis of these results we postulate that production of H-ferritin by melanoma cells or other cancer cells may induce down-regulation of immune responses by action on APCs, particularly in areas adjacent to or draining the site of the tumor. Regulatory T cells generated at these sites could then be expected to suppress CD4+CD25− and possibly CD8+ T-cell responses against melanoma. H-ferritin may not be the only factor generation of regulatory T cells, because it is conceivable that self-peptides on APCs may also be involved, as described in other models.56 Whether they increase the number of activity of regulatory T cells is not known. These hypotheses need to be tested in further studies involving patients with melanoma and other malignancies producing H-ferritin.
We thank Raj Ishri from the Department of Medicine (Dermatology), University of Sydney, for assistance in production of DCs. We also thank Mrs. C. Cook for expert secretarial assistance.
Supported by the Hunter Melanoma Foundation and Melanoma and Skin Cancer Institute, New South Wales, Australia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peter Hersey, Oncology & Immunology Unit, Rm 443, David Maddison Clinical Sciences Bldg, Cnr King & Watt Streets, Newcastle, NSW 2300 Australia; e-mail: peter.hersey@newcastle.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal