Abstract
CD4+ T cells are critical for inducing and maintaining efficient humoral and cellular immune responses to pathogens. The CD4+ T-cell response in human T-lymphotropic virus 1 (HTLV-1) infection has not been studied in detail. However, CD4+ T cells have been shown to predominate in early lesions in HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP). We present direct estimates of HTLV-1 Env- and Tax-specific CD4+ T-cell frequencies in patients infected with HTLV-1. We first showed that there was a strong bias toward the Th1 phenotype in these HTLV-1–specific CD4+ T cells in patients with HAM/TSP. We then demonstrated significantly higher frequencies of HTLV-1–specific Th1-type CD4+ T cells in HAM/TSP patients than in asymptomatic HTLV-1 carriers. The majority of these HTLV-1–specific CD4+ T cells did not express HTLV-1 Tax and were therefore unlikely to be infected by HTLV-1. High frequencies of activated HTLV-1–specific CD4+ T cells of the Th1 phenotype might contribute to the initiation or pathogenesis of HAM/TSP and other HTLV-1–associated inflammatory diseases.
Introduction
Human T-cell lymphotropic virus type 1 (HTLV-1) was the first human retrovirus discovered.1 It is endemic in many tropical countries particularly Melanesia, the Caribbean, West Africa, Central/South America, and in Southern Japan and Iran. It is estimated to infect between 10 and 20 million people worldwide.2 Unlike human immunodeficiency virus (HIV), HTLV-1 does not cause disease in the majority of infected subjects (asymptomatic carriers [ACs]). Approximately 2% to 3% develop adult T-cell leukemia/lymphoma and another 2% to 3% develop chronic inflammatory disease of which HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP) is the most commonly recognized, but polymyositis, alveolitis, arthritis, thyroiditis, uveitis, and other end-organ inflammatory diseases have also been diagnosed.3
HTLV-1 possesses the 3 main genomic regions of env,gag, and pol (similar to other retroviruses), but unlike other leukemia viruses, it has an additional region calledpX that encodes at least 2 other transcriptional regulatory proteins, the Tax and Rex proteins.3 These proteins are the homologues of the Tat and Rev proteins of HIV.4 The Rex protein stabilizes viral messenger RNAs and regulates their splicing and transport. The Tax protein is crucial to virus dynamics because as well as transactivating viral transcription, it is thought to drive host cell proliferation. The Tax protein is also the dominant target antigen recognized by HTLV-1–specific cytotoxic T lymphocytes (CTLs) in most responding individuals.5-9 So far, most investigations of the immune response to HTLV-1 have focused on the CTL response.
The CD4+ T-cell response to HTLV-1 is also important, for the following reasons: (1) CD4+ T cells are the main subset of cells infected with HTLV-1 in vivo10,11; (2) HTLV-1–infected CD4+ T cells spontaneously secrete proinflammatory, neurotoxic cytokines such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α)12,13 and high levels of these cytokines have been demonstrated in the sera, cerebrospinal fluid (CSF), and spinal cord lesions of patients with HAM/TSP14-16; (3) CD4+ T-cell help is required for optimal CD8+ and antibody responses17,18 and HTLV-1 infection of CD4+ T cells may impair T-helper function19,20; and (4) CD4+ T cells are the predominant subset of infiltrating lymphocytes detected in the central nervous system (CNS) lesions of patients with HAM/TSP in the early phase of disease.21 This observation suggests that these cells (whether they are themselves infected with HTLV-1 or not) play a role in the pathogenesis of HAM/TSP.
Efforts to study the CD4+ T-cell response to HTLV-1 in infected patients have so far been hampered by the phenomena of “spontaneous proliferation”22-24 and spontaneous cytokine production12 25 of peripheral blood mononuclear cells (PBMCs) after several days of culture in vitro. We have therefore developed short-term (6 hours) Elispot and intracellular cytokine detection assays to overcome these problems. We have also adopted the strategy of using overlapping peptide panels that span the entire Env and Tax proteins to examine the total CD4+ T-cell responses to all potential epitopes in these proteins, regardless of the major histocompatibility complex (MHC) class II specificities of the individual. Therefore, the aims of this study were to test the hypotheses that the frequency of HTLV-1–specific CD4+ T cells differs between patients with HAM/TSP and asymptomatic carriers, that there is a bias toward the Th1 phenotype in HTLV-1–infected subjects, and that HTLV-1–specific CD4+ T cells are selectively infected with HTLV-1.
Our results showed significantly higher frequencies of HTLV-1 Env- and Tax-specific CD4+ T cells in patients with HAM/TSP compared to ACs. Patients with HAM/TSP also showed a significant predominance of the Th1 phenotype in these HTLV-1–specific CD4+ T cells. Furthermore, most HTLV-1–specific CD4+ T cells did not express Tax and were therefore not susceptible to lysis by HTLV-1–specific CTLs. These results have implications for the initiation or pathogenesis of HTLV-1–associated inflammatory diseases.
Patients, materials, and methods
Subjects and cells
Subjects were asymptomatic HTLV-1 carriers and patients with HAM/TSP attending the HTLV-1 clinic at St Mary's Hospital. Infection with HTLV-1 was confirmed by the presence of antibodies to HTLV-1 gag (p19 and p24) and env (rgp21 and rgp46-I) antigens in sera by Western blot (Genelabs HTLV 2.4, Singapore). The diagnosis of HAM/TSP was made according to World Health Organization criteria.26 All patients gave informed consent. The PBMCs were isolated via density gradient centrifugation on Histopaque-1077 (Sigma, Dorset, United Kingdom) and washed 3 times with phosphate-buffered saline (PBS). Cells were then stored frozen in liquid nitrogen in fetal calf serum (FCS; Sigma) supplemented with 10% dimethyl sulfoxide (DMSO; Sigma).
After thawing and washing in cold sterile PBS (2 times), cells were cultured in complete medium (CM), which is RPMI 1640 medium (Sigma) supplemented with 10% FCS, 2 mM glutamine (Gibco, Paisley, United Kingdom), 100 IU/mL penicillin (Gibco), and 100 μg/mL streptomycin (Gibco). All cultures were undertaken in this medium unless stated otherwise. To induce nonspecific cytokine production by PBMCs, the combination of 0.1 ng/mL phorbol myristate acetate (PMA; Sigma) and 0.5 μg/mL A23187 (Sigma) was added to the culture medium. In certain experiments, 10 μg/mL anti–MHC class I monoclonal antibody (mAb; W6/32, IgG2a; Serotec, Oxford, United Kingdom), or 10 μg/mL anti–MHC class II mAb (Tu39, IgG2a; Becton Dickinson, Oxford, United Kingdom) were also added to the culture medium.
The PBMCs were depleted of CD8+ T cells using magnetic microbeads (Miltenyi Biotec, Surrey, United Kingdom) according to the manufacturer's instructions. Fifty thousand cells from the CD8-depleted PBMCs were also stained and analyzed by flow cytometry to measure the percentage positivity for the required surface markers of CD4 and CD8; to derive the numbers of CD4+ T cells (responding CD4+ T cells, which corresponds to the CD4+, CD3+ population [data not shown]), and CD8+ cells left after CD8+ depletion. Typically, there were less than 3% CD8+ cells left after depletion.
Synthetic peptides
Peptide libraries spanning the entire length of HTLV-1 Env and Tax proteins (strain ATK)27 were synthesized by Mimotopes Pty (formerly Chiron Mimotopes, Chiron Technologies, Victoria, Australia). Purity was checked by reverse-phase high-performance liquid chromatography and ion spray mass spectrometry and was more than 84%. Env peptides: 20mer peptides offset by 5 (total of 95). Tax peptides: 13mer peptides offset by 4 (total of 86). (Full details are available on request.)
Peptides were grouped in pools of 20, and added to the cell culture medium to achieve a final concentration of 1 μM for each peptide, prior to incubation (either Elispot or flow cytometric assays) at 37°C.
Elispot assays for IFN-γ and interleukin 4
Flat-bottomed 96-well polyvinylidene difluoride (PVDF) membrane-backed plates (MAIPS45, Millipore, Bedford, United Kingdom) were first washed with sterile PBS. Each well was then precoated with the primary capture antibody to IFN-γ or interleukin-4 (IL-4) (respectively clones 1-D1K or IL-4-I, Mabtech, Nacka, Sweden) at a concentration of 15 μg/mL in sterile PBS. Antibody was allowed to bind to the membrane overnight at 4°C.
Plates were then washed 6 times with sterile PBS. CM was then added to block nonspecific binding of cytokines and antibodies, and incubated at 37°C for 3 hours.
The blocking solution was then discarded and cells were then dispensed at predefined input cell numbers per well.
Stimulatory mAbs to CD28 (clone CD28.2, Pharmingen, Becton Dickinson, Oxford, United Kingdom) and CD49d (clone HP2/1, Serotec), both at 0.5 μg/mL, were then added for CD4+ T-cell assays.28 Peptides were then added directly to the supernatant at a final concentration of 1 μM each and the plates incubated for 6 hours at 37°C in 5% CO2.
The cells were then discarded and the plate washed 6 times with PBS/0.05% Tween 20. Plates were then incubated at room temperature for 2 hours with the second-layer biotinylated antibody to IFN-γ or IL-4 (7-B6-1-biotin or IL4-II-biotin, Mabtech). The medium was then discarded and plates washed 6 times with PBS/0.05% Tween 20. Streptavidin–alkaline phosphatase conjugate (diluted 1:1000 in sterile PBS) was added and incubated at room temperature for 1 hour. This solution was then discarded and the plates washed 6 times with PBS/0.05% Tween 20. Chromogenic alkaline phosphatase substrate (nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate [NBT/BCIP], Biorad, Hertfordshire, United Kingdom) was prepared according to the manufacturer's instructions and added at a volume of 100 μL/well. The plates were incubated for 0.5 to 1 hour at room temperature and then the reaction was terminated by washing with tap water. Plates were then allowed to air-dry and the number of spots in each well was then counted in a digital image with AID software (AID Elispot Reader, Strassberg, Germany). A response was defined as positive if the number of spots exceeded the mean + 2 SDs of the spot count in the negative control wells (no peptides added). The frequency of cytokine-secreting CD4+ T cells was then derived by the formula: number of spots/number of CD4+ T cells per well (as ascertained previously by flow cytometry).
Flow cytometry
Cell preparation and antigen stimulation.
For antigen-specific cytokine responses, 1 × 106 cells (PBMCs depleted of CD8+ cells) were placed in 16 × 125-mm round-bottom polystyrene tissue culture tubes (Corning Costar, Cambridge, MA) with 1 mL CM, supplemented with peptides each at 1 μM final concentration and costimulatory mAbs as above. Culture tubes were incubated at 37°C in a humidified 5% CO2atmosphere for a total of 6 hours, with the last 5 hours including a final concentration of 10 μg/mL Brefeldin A (Sigma) to inhibit secretion of cytokines from the cell. After incubation, the cells were harvested for subsequent staining.
Cell surface staining.
Harvested cells were washed in PBS containing 7% normal goat serum (NGS; Sigma) and then incubated with NGS for 30 minutes at 4°C to block the Fc receptor sites on cells of the monocyte/macrophage lineage. After one wash, cells were incubated with the relevant mAbs (each at 15 μg/mL) to surface markers, that is, phycoerythrin-cyanine 5.1 (PC5)–labeled anti-CD4, energy-coupled dye (ECD)–labeled anti-CD8 (ECD = phycoerythrin [PE] + Texas red), PE-labeled anti-CD69 (Beckman Coulter, Bedfordshire, United Kingdom) for 30 minutes at 4°C. The stained cells were washed and fixed with 4% paraformaldehyde (Sigma) in PBS (pH 7.4) for 5 minutes at room temperature and washed again. Cells were then resuspended in PBS at 4°C awaiting analysis (if surface staining alone was required) or processed further for intracellular cytokines and HTLV-1 Tax protein detection.
Detection of intracellular Tax protein and cytokines.
Cells were permeabilized with PBS/7% NGS containing 0.2% saponin (permeabilization buffer [PB]) for 10 minutes at room temperature and then washed. The cells were then resuspended in this solution with PE-labeled or fluorescein isothiocyanate (FITC)–labeled anti-IFN-γ mAb (Beckman Coulter), anti-Tax mAb (LT4), isotype controls, or mAbs to other cytokines, for example, IL-4, as appropriate for 20 minutes at room temperature. The cells were washed twice with PB and then resuspended in PB with FITC- or PE-labeled goat F(ab′)2antimouse IgG3 (Southern Biotechnology, Birmingham, AL) as appropriate for 20 minutes at room temperature. Finally, the cells were washed twice with PB, resuspended in PBS, and analyzed by flow cytometry on a Coulter EpicsXL (Beckman Coulter) with Coulter Expo 32 software.
Results
Detection of HTLV-1–specific CD4+T cells by in vitro activation with HTLV-1 Env and Tax peptides
There has been no previous report of experimental estimates of HTLV-1–specific CD4+ T-cell frequencies in HTLV-1–infected subjects. Conventional proliferation assays cannot be used to quantify HTLV-1–specific CD4+ T-cell frequencies because of the high level of spontaneous cytokine production12,25 and proliferation22-24 in PBMCs from HTLV-1–infected people when cells are cultured in vitro. We have recently shown that HTLV-1–infected CD4+ T cells spontaneously produce detectable cytokines (initially IFN-γ) only after more than 6 hours of in vitro cultivation (no cytokines are detectable ex vivo).13 We have therefore devised flow cytometric and Elispot assays to directly detect HTLV-1–specific CD4+ T cells by short-term (6 hours) in vitro stimulation with Env and Tax peptides. We used peptide libraries encompassing the full-length sequences of Env and Tax to ensure that CD4+ T cells responding to all potential epitopes in these 2 proteins would be detected, regardless of their class II MHC specificity.
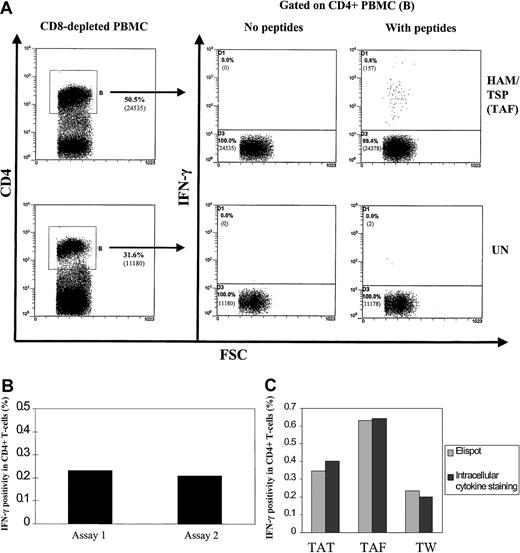
Figure 1A shows that HTLV-1 peptides induced detectable IFN-γ production only in CD4+ lymphocytes from the patient with HAM/TSP and not in cells from an uninfected control subject. The absence of IFN-γ+ cells in the uninfected control demonstrates the specificity of the assay. The use of an isotype control mAb also confirmed the specificity of IFN-γ staining (data not shown). This suggested that the 6-hour assay could be used for detection of HTLV-1 peptide-specific CD4+ T cells. When cultivation was extended to 12 hours or longer for samples from infected patients, the background IFN-γ staining became unacceptably high and meaningful results could not be obtained because over 10% of Tax-expressing CD4+ T cells expressed IFN-γ at 12 hours and this figure rose to about 20% at 24 hours.13
Detection of HTLV-1–specific CD4+ T cells by in vitro activation with Env and Tax peptides.
(A) Dot plots showing intracellular cytokine staining for IFN-γ production in CD4+ T cells from a patient with HAM/TSP (TAF) and an uninfected control (UN). PBMCs were depleted of CD8+ T cells, cultivated with Env and Tax peptides for 6 hours in vitro, then harvested and stained. One representative experiment from 3 patients with HAM/TSP is shown. Numbers in brackets show actual numbers of events acquired. (B) Comparison of HTLV-1–specific CD4+ T-cell frequencies determined by independent Elispot assays at different time points from a single blood sample. One representative experiment from 3 patients with HAM/TSP is shown. IFN-γ SFCs were divided by the number of CD4+ T cells present in each well, then multiplied by 100 to provide the data shown. A response was defined as positive if the number of spots exceeded the mean + 2 SDs of the spot count in the negative control wells (no peptides added). (C) Comparison of HTLV-1–specific CD4+ T-cell frequencies determined by Elispot and intracellular cytokine staining from the same blood sample. Results from 3 patients with HAM/TSP (TAT, TAF, TW) are shown. IFN-γ SFCs are divided by the number of CD4+ T cells present in each well, then multiplied by 100 to provide the Elispot data shown. Flow cytometric data shown is derived from IFN-γ+, CD4+ T cells divided by the total number of CD4+ T cells present, then multiplied by 100.
Detection of HTLV-1–specific CD4+ T cells by in vitro activation with Env and Tax peptides.
(A) Dot plots showing intracellular cytokine staining for IFN-γ production in CD4+ T cells from a patient with HAM/TSP (TAF) and an uninfected control (UN). PBMCs were depleted of CD8+ T cells, cultivated with Env and Tax peptides for 6 hours in vitro, then harvested and stained. One representative experiment from 3 patients with HAM/TSP is shown. Numbers in brackets show actual numbers of events acquired. (B) Comparison of HTLV-1–specific CD4+ T-cell frequencies determined by independent Elispot assays at different time points from a single blood sample. One representative experiment from 3 patients with HAM/TSP is shown. IFN-γ SFCs were divided by the number of CD4+ T cells present in each well, then multiplied by 100 to provide the data shown. A response was defined as positive if the number of spots exceeded the mean + 2 SDs of the spot count in the negative control wells (no peptides added). (C) Comparison of HTLV-1–specific CD4+ T-cell frequencies determined by Elispot and intracellular cytokine staining from the same blood sample. Results from 3 patients with HAM/TSP (TAT, TAF, TW) are shown. IFN-γ SFCs are divided by the number of CD4+ T cells present in each well, then multiplied by 100 to provide the Elispot data shown. Flow cytometric data shown is derived from IFN-γ+, CD4+ T cells divided by the total number of CD4+ T cells present, then multiplied by 100.
Elispot assays produced an equivalent result, that is, only CD4+ T cells cultivated with HTLV-1–derived peptides for 6 hours in 96-well plates showed positive responses, defined as a spot-forming cell (SFC) count that significantly exceeded the negative controls (see “Patients, materials, and methods”).
Comparison of independent replicate Elispot assays on cells from a single blood sample, performed on different dates, showed high reproducibility (Figure 1B). We then compared data from both Elispot and intracellular cytokine staining assays on cells obtained from single blood samples. Figure 1C shows that the results were similar.
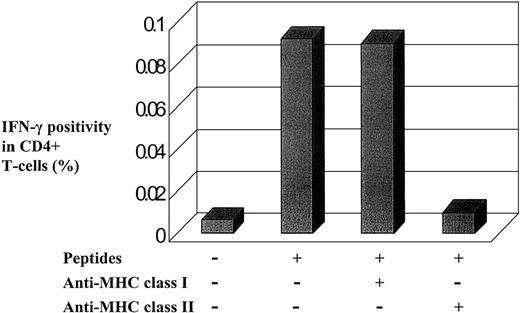
To further confirm that the peptide-induced responses were MHC class II mediated, we added anti-MHC class I and II mAbs to the culture medium to inhibit class I and II restricted responses, respectively. As expected, the responses were inhibited by anti–class II MHC antibodies but not anti–class I MHC antibodies (Figure2).
MHC class II participates in IFN-γ induction by HTLV-1 Env and Tax peptides.
Flow cytometric data showed that IFN-γ production was inhibited by the addition of an anti–class II mAb, whereas the addition of an anticlass I mAb had no effect. PBMCs were isolated from one infected individual, cultivated in vitro with or without peptides and with or without the respective blocking antibodies.
MHC class II participates in IFN-γ induction by HTLV-1 Env and Tax peptides.
Flow cytometric data showed that IFN-γ production was inhibited by the addition of an anti–class II mAb, whereas the addition of an anticlass I mAb had no effect. PBMCs were isolated from one infected individual, cultivated in vitro with or without peptides and with or without the respective blocking antibodies.
The Elispot assay was subsequently used to screen larger numbers of HTLV-1–infected subjects, because this assay requires fewer PBMCs per well (105) than intracellular cytokine staining (106).
The Th1 phenotype is dominant among HTLV-1–specific CD4+T cells in HAM/TSP patients
Circulating CD4+ T cells from patients with HAM/TSP have previously been reported to have predominantly a Th1 phenotype when stimulated with nonspecific T-cell mitogens.29However, only the overall Th1/Th2 ratio in circulating CD4+T cells was estimated because no information could be derived on HTLV-1–specific T cells. This estimate may not reflect the Th1/Th2 ratio among HTLV-1–specific T cells.
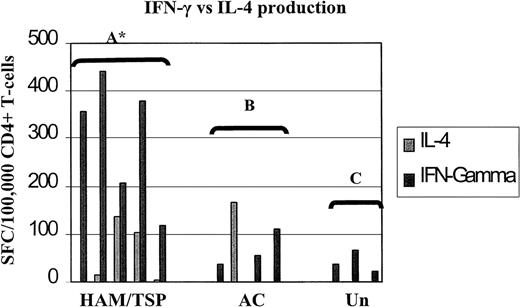
Therefore, to answer this question, we characterized the Th1/Th2 phenotypes of HTLV-1 Env and Tax peptide-specific T cells in 5 patients with HAM/TSP, 4 ACs, and 3 uninfected controls in parallel Elispot assays for IFN-γ and IL-4. The results are summarized in Figure3. Statistical analyses with the paired Student t test showed there was a strong predominance of the Th1 phenotype among HTLV-1 Env- and Tax-specific CD4+ T cells in patients with HAM/TSP, but there was no such predominance in either asymptomatic HTLV-1 carriers or uninfected controls (Figure 3).
The Th1 phenotype is dominant among HTLV-1 Env- and Tax-specific CD4+ T cells in patients with HAM/TSP.
This shows frequencies of IFN-γ and IL-4 SFCs/105CD4+ T cells by parallel Elispot assays in 5 patients with HAM/TSP, 4 asymptomatic carriers (AC), and 3 uninfected controls (Un). Statistical analyses was performed within groups using the paired Student t test. Group A: 2-tailed P = .0222 (asterisk indicates significant), IL-4 responses ranged from 0% (undetectable) to 0.14% of CD4+ T cells (median, 0.014%), IFN-γ responses ranged from 0.12% to 0.44% (median, 0.36%). Group B: 2-tailed P = .8930 (not significant), IL-4 responses ranged from 0% to 0.17% (median, 0%), IFN-γ responses ranged from 0% to 0.11% (median, .045%). Group C: 2-tailedP = .0912 (not significant), IL-4 responses were undetectable, IFN-γ responses ranged from 0.021% to 0.066% (median, 0.035%).
The Th1 phenotype is dominant among HTLV-1 Env- and Tax-specific CD4+ T cells in patients with HAM/TSP.
This shows frequencies of IFN-γ and IL-4 SFCs/105CD4+ T cells by parallel Elispot assays in 5 patients with HAM/TSP, 4 asymptomatic carriers (AC), and 3 uninfected controls (Un). Statistical analyses was performed within groups using the paired Student t test. Group A: 2-tailed P = .0222 (asterisk indicates significant), IL-4 responses ranged from 0% (undetectable) to 0.14% of CD4+ T cells (median, 0.014%), IFN-γ responses ranged from 0.12% to 0.44% (median, 0.36%). Group B: 2-tailed P = .8930 (not significant), IL-4 responses ranged from 0% to 0.17% (median, 0%), IFN-γ responses ranged from 0% to 0.11% (median, .045%). Group C: 2-tailedP = .0912 (not significant), IL-4 responses were undetectable, IFN-γ responses ranged from 0.021% to 0.066% (median, 0.035%).
The failure to detect IL-4 could be due to poor sensitivity of the Elispot assay used. To investigate this possibility, PBMCs depleted of CD8+ cells from all the patients were stimulated with polyclonal activators (PMA and A23187) and spots detected with our IL-4 Elispot assay. Responses ranged from 0.01% to 0.82% of CD4+ T cells (median frequency, 0.21%). This showed that a significant proportion of the CD4+ T cells retained the ability to secrete IL-4 and that these responses were detectable. The spontaneous IL-4 responses to peptides were detectable in 5 of 9 patients and ranged from 0.0020% to 0.17% of their CD4+ T cells (median, 0.10%).
High frequencies of HTLV-1 Env- and Tax-specific CD4+T cells in HAM/TSP patients
Having established that the Th1 phenotype is predominant among HTLV-1–specific CD4+ T cells in patients with HAM/TSP, we determined the frequencies of IFN-γ–secreting HTLV-1 Env- and Tax-specific CD4+ T cells in 9 patients with HAM/TSP, 7 ACs, and 3 uninfected controls.
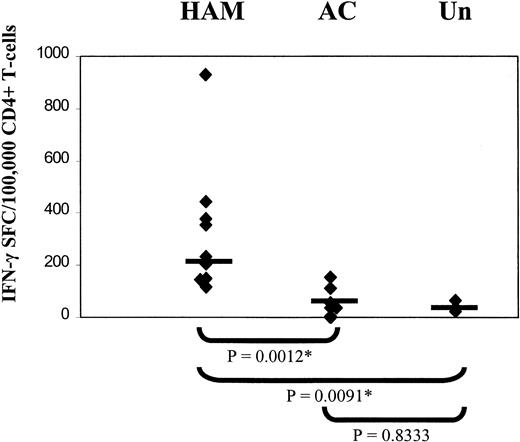
The results (Figure 4) show that patients with HAM/TSP had a significantly higher frequency of HTLV-1–specific CD4+ T cells than both healthy ACs and uninfected controls. These responses varied from 0.12% to 0.93% (median, 0.23%) of circulating CD4+ T cells for patients with HAM/TSP, compared to ACs in whom the frequency varied from undetectable to 0.34% (median frequency, 0.038%). Uninfected controls showed a basal level of response (median frequency, 0.035%). There was no significant difference between the responses seen in ACs and the basal level seen in uninfected controls. Experiments are now under way to define the precise peptides recognized by each individual.
High frequencies of HTLV-1 Env- and Tax-specific CD4+ T cells in patients with HAM/TSP.
Elispot assays were carried out to compare the frequencies of IFN-γ-producing HTLV-1 Env- and Tax-specific CD4+ T cells in patients with HAM/TSP, asymptomatic carriers (AC), and uninfected controls (Un). Statistical analysis was performed using the Mann-Whitney U test. Horizontal bar indicates median frequency.
High frequencies of HTLV-1 Env- and Tax-specific CD4+ T cells in patients with HAM/TSP.
Elispot assays were carried out to compare the frequencies of IFN-γ-producing HTLV-1 Env- and Tax-specific CD4+ T cells in patients with HAM/TSP, asymptomatic carriers (AC), and uninfected controls (Un). Statistical analysis was performed using the Mann-Whitney U test. Horizontal bar indicates median frequency.
Detection of Tax expression in PBMCs from HTLV-1–infected patients
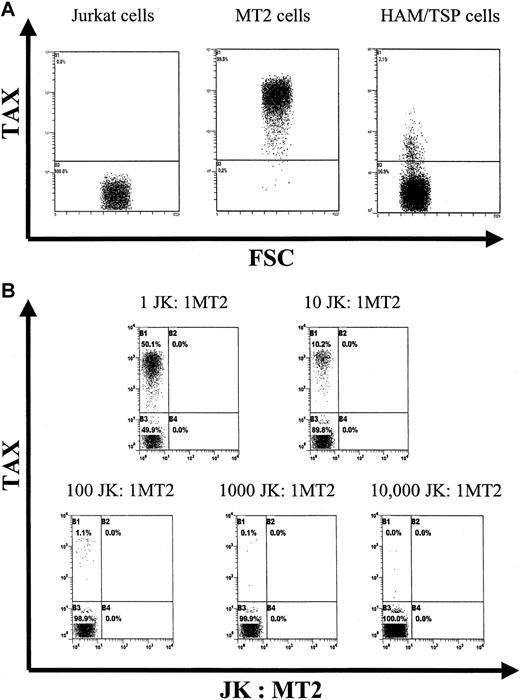
We have recently devised a sensitive flow cytometric assay to detect intracellular Tax protein expression.11 We have now further characterized the sensitivity and specificity of the assay. Figure 5A shows the result of Tax staining in MT-2 cells (chronically infected with HTLV-1), Jurkat cells (uninfected), and a patient with HAM/TSP's PBMCs (cultivated in vitro for 6 hours). Figure 5B shows the results of staining differing ratios of MT-2 cells with Jurkat cells. These data confirmed that the Tax staining assay was able to detect one MT-2 cell among 10 000 uninfected cells.
Sensitivity and specificity of intracellular Tax protein detection.
(A) Dot plots showing Tax expression in Jurkat (an uninfected T-cell line) cells, MT2 cells (persistently infected with HTLV-1), and PBMCs from a patient with HAM/TSP after 6 hours of in vitro culture. (B) Dot plots showing experiments where different ratios of MT2 to Jurkat cells were mixed, stained for intracellular Tax, and analyzed by flow cytometry. All plots show 20 000 events except for the final plot, which shows 50 000 events. There are 5 cells positive for Tax seen in the final plot.
Sensitivity and specificity of intracellular Tax protein detection.
(A) Dot plots showing Tax expression in Jurkat (an uninfected T-cell line) cells, MT2 cells (persistently infected with HTLV-1), and PBMCs from a patient with HAM/TSP after 6 hours of in vitro culture. (B) Dot plots showing experiments where different ratios of MT2 to Jurkat cells were mixed, stained for intracellular Tax, and analyzed by flow cytometry. All plots show 20 000 events except for the final plot, which shows 50 000 events. There are 5 cells positive for Tax seen in the final plot.
Proportion of HTLV-1–specific CD4+T cells that express Tax protein
We wished to know whether HTLV-1–specific CD4+ T cells were selectively infected with HTLV-1 because infection has been shown to alter the function of T-helper cells.19,20 Recent data from our laboratory suggested that there is preferential infection of HTLV-1–specific CTLs.30
Therefore, staining for intracellular Tax expression was combined with the 6-hour flow cytometric assay for intracellular IFN-γ. One representative experiment from 5 subjects is shown in Figure6.
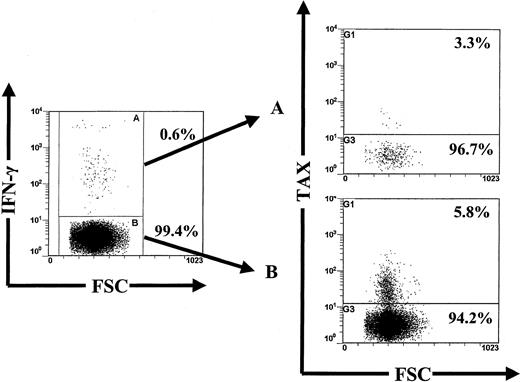
The majority of HTLV-1 Env- and Tax-specific CD4+ T cells are not infected with HTLV-1.
This figure shows concomitant detection of intracellular Tax and IFN-γ expression in CD4+ T cells after 6 hours in vitro activation with peptides from a patient with HAM/TSP (TAF). Dot plots showed that the vast majority of Tax+cells came from the IFN-γ− fraction. This was representative of experiments on 5 different subjects; refer to Table 1.
The majority of HTLV-1 Env- and Tax-specific CD4+ T cells are not infected with HTLV-1.
This figure shows concomitant detection of intracellular Tax and IFN-γ expression in CD4+ T cells after 6 hours in vitro activation with peptides from a patient with HAM/TSP (TAF). Dot plots showed that the vast majority of Tax+cells came from the IFN-γ− fraction. This was representative of experiments on 5 different subjects; refer to Table 1.
Table 1 shows that Tax expression was less frequent in HTLV-1–specific CD4+ T cells than CD4+ T cells of other specificities, in 2 patients with HAM/TSP, whereas Tax expression was more frequent in HTLV-1–specific CD4+ T cells in both ACs and a third patient with HAM/TSP. This contrasts with our previous finding that Tax expression in CD8+ T cells was detected only among HTLV-1–specific cells (in 5 of 5 individuals) and not among Epstein-Barr virus (EBV)–specific cells (0 of 4 individuals).30
Detection of Tax protein expression in CD4+ T cells from HTLV-1-infected subjects
| . | Percentage of Tax-expressing cells . | |
|---|---|---|
| IFN-γ+ cells . | IFN-γ− cells . | |
| TAF | 3.3 | 5.8 |
| TAT | 0.4 | 6.1 |
| TW | 1.7 | 0.6 |
| HAY | 10.7 | 0.4 |
| HT | 5.9 | 0.8 |
| . | Percentage of Tax-expressing cells . | |
|---|---|---|
| IFN-γ+ cells . | IFN-γ− cells . | |
| TAF | 3.3 | 5.8 |
| TAT | 0.4 | 6.1 |
| TW | 1.7 | 0.6 |
| HAY | 10.7 | 0.4 |
| HT | 5.9 | 0.8 |
The 6-hour flow cytometric assay for intracellular IFN-γ was combined with staining for Tax protein expression. Patients with HAM/TSP have codes beginning with T and ACs have codes beginning with H.
The fact that most HTLV-1–specific CD4+ T cells were not infected with transcriptionally active virus, also implies that they were not susceptible to CTL-mediated killing in vivo. Thus, the majority of circulating HTLV-1–specific CD4+ T cells remain capable of producing proinflammatory cytokines on encountering their cognate antigen.
These data do not exclude the possibility that some infected HTLV-1–specific CD4+ cells are unable to produce cytokines when activated with their respective antigen and thus escape detection in these assays. Testing of this point may have to await the advent of MHC class II tetramer or similar technology.
Discussion
The role of the cellular immune response in HTLV-1 infection is not fully understood. To date, the focus has been on the CTL response to the virus and there are few published data on the CD4+T-cell response to HTLV-1. However, the importance of the CD4+ T-cell response should not be underestimated, because it has been shown to be essential for efficient humoral and cellular immune responses, including the CTL response.17,18 The situation is complicated further by the fact that the main subset of lymphocytes infected with HTLV-1 in vivo is the effector/memory CD4+ T cell.10 11 The paucity of data on the HTLV-1–specific CD4+ T-cell response reflects the problems encountered with the phenomena of spontaneous lymphocyte proliferation and cytokine production seen in PBMCs from these infected patients.
Using short (6-hour) cultures we have eliminated the effect of spontaneous proliferation of lymphocytes from our assays. This has enabled us to demonstrate HTLV-1 peptide-specific CD4+T-cell responses in subjects infected with HTLV-1. We have found that these cells exhibit a Th1 phenotype and we therefore postulate a role for these cells in the pathogenesis of HTLV-1–associated inflammatory disease, especially in HAM/TSP. Pathologic examination of the spinal cord has shown that in patients with HAM/TSP with a short duration of symptoms, the characteristic perivascular lymphocyte infiltrate consists predominantly of CD4+ T cells21; only later do CD8+ T cells predominate, and finally an atrophic picture is seen.31
Whether the CD4+ T cells present in HAM/TSP lesions are predominantly HTLV-1 infected or HTLV-1 specific has not been shown. However, if HTLV-1–specific CD4+ T cells of the Th1 phenotype are also frequent among the CD4+ T cells that infiltrate CNS lesions (as they are in peripheral blood, which we show in this paper), then such cells may be important in the pathogenesis of HAM/TSP, particularly in early lesions, through the secretion of neurotoxic cytokines such as IFN-γ. A similar role has been suggested for HTLV-I Tax-specific CTLs,32 which predominate later in disease. However, relatively little is known of the functions of the infiltrating lymphocytes; both cell types may contribute to pathogenesis, in varying proportions, throughout the course of the disease.
This observation that HTLV-1–specific CD4+ T cells in HAM/TSP patients are mainly of the Th1 phenotype is important because a dominant Th1 response could be either beneficial, harmful, or both. A strong Th1 response could be beneficial because it is associated with a strong antiviral CTL response and therefore with effective control of HTLV-1 infection, or harmful because the production of proinflammatory cytokines could lead to “bystander damage” to uninfected cells.33 It is possible that the balance of good and harm done by HTLV-1–specific CD4+ T cells depends on factors such as the proviral load, in a manner analogous to the mechanism we have proposed in the CD8+ T-cell response to HTLV-1.34
Furthermore, we showed that the frequencies of HTLV-1 Env- and Tax-specific CD4+ T cells are significantly greater in HAM/TSP patients than in ACs. Activated Th1 cells (but not Th2) are reported to have a strong tendency to cross vessel walls (including the blood-brain barrier) and infiltrate areas of inflammation.35 Therefore, we suggest that these abundant Th1-type HTLV-1–specific CD4+ T cells are likely to encounter cognate antigen in the tissues (eg, the CNS) when the proviral load is high (as in patients with HAM/TSP), because the frequencies of both the Th1 CD4+ T cells and infected CD4+ T cells are high. The high frequencies of specific CD4+ T cells suggest that there is continuous antigen stimulation in vivo, implying in turn that the virus is not latent.
It is well established that HTLV-1 infection is associated with a high proviral load in peripheral blood.36 It is also known that the main burden of HTLV-1 infection in vivo is carried by the activated memory subset of CD4+ T cell (CD3+, CD4+, CD45RO+).10,11 Furthermore, we have previously shown that a majority of these infected cells were clustered in the effector memory subset (CCR7−, CD62L−).13 Therefore, it was important to know whether the majority of the HTLV-1–specific CD4+ T cells were infected with HTLV-1. Recent work from this laboratory suggested that HTLV-1–specific CD8+ CTLs were preferentially infected by HTLV-1, whereas EBV-specific CD8+ CTLs were not.30
Infection of HTLV-1–specific CD4+ T cells would have the following implications: (1) disruption of normal in vivo function by up-regulation and transactivation of cellular gene expression by Tax, and (2) viral protein expression in infected T cells would render them susceptible to killing by autologous CTLs. Taken together, these could seriously impair or weaken the CD4+ T-cell immune response to HTLV-1. Using our recently devised sensitive and specific flow cytometric assay, we have shown that the majority of these HTLV-1 Env- and Tax-specific CD4+ T cells did not express the Tax protein after 6 hours of culture in vitro. This observation suggested that most HTLV-1–specific CD4+ T cells were not infected by transcriptionally active HTLV-1 and therefore remained capable of normal cytokine production on encountering antigen.
In conclusion, we demonstrate significantly higher frequencies of Env- and Tax-specific CD4+ T cells in patients with HAM/TSP than in ACs. We also show that the Th1 phenotype is dominant among these cells in patients with HAM/TSP. The majority of these Th1-type HTLV-1–specific CD4+ T cells are also shown not to express Tax protein after short in vitro culture and thus remain capable of responding normally when encountering cognate antigen. We suggest that these cells are involved in the initiation and pathogenesis of inflammatory disease in patients with HAM/TSP by the production of proinflammatory cytokines when activated by HTLV-1 antigens in the CNS and other end organs.
We would like to thank the staff and patients of St Mary's Hospital Medical Trust, Dr Paul Klenerman of the Nuffield Department of Medicine, and John Radcliffe Hospital, Oxford, United Kingdom for his help in the critical review of the manuscript.
Supported by the Wellcome Trust, United Kingdom, (P.K.C.G., C.R.M.B.) and the Fonds National de la Recherche Scientifique (FNRS), Belgium (E.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Charles Bangham, Department of Immunology, Imperial College Faculty of Medicine, St Mary's Campus, Norfolk Pl, London W2 1PG, United Kingdom; e-mail: c.bangham@ic.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal