Abstract
Glucocorticoids are integral to successful treatment of childhood acute lymphoblastic leukemia (ALL) and other lymphoid malignancies. A large body of data indicates that in various model systems, elevation of cyclic adenosine monophosphate (cAMP) can potentiate glucocorticoid response, although this has not been well evaluated as a potential leukemia treatment. Although cAMP analogs have been studied, little data exist regarding the potential toxicity to leukemia cells of pharmacologic elevation of cAMP levels in leukemic blasts. Using MTT assays of cell proliferation on CEM ALL cells, we found that aminophylline and other nonspecific phosphodiesterase (PDE) inhibitors suppress cell growth. This effect is replicated by the PDE4-specific PDE inhibitor rolipram, but not by specific inhibitors of the PDE1 or PDE3 classes. We found that PDE inhibitors cause increased dexamethasone sensitivity and a synergistic effect with the adenylyl cyclase activator forskolin. We observed several important cellular characteristics associated with this treatment, including elevation of cAMP, induction of p53 and p21WAF1/CIP1proteins, G1 and G2/M cell cycle arrest, and increased apoptosis. Sensitivity to forskolin and rolipram is shared by at least 2 pediatric ALL cell lines, CEM and Reh cells. Some cell lines derived from adult-type lymphoid malignancies also show sensitivity to this treatment. These findings suggest that PDE inhibitors have therapeutic potential in human ALL and characterize the molecular mechanisms that may be involved in this response.
Introduction
Glucocorticoids have been among the first known effective agents for the treatment of childhood acute lymphocytic leukemia (ALL). Despite the variety of chemotherapeutic drugs with activity in this disease, the importance of glucocorticoids consistently is highlighted by clinical data.1,2 In particular, the results from a series of European trials have indicated that the in vivo response to an initial 7-day course of prednisone monotherapy is a highly reliable prognostic factor in childhood ALL.3
The relationship between the expression level of glucocorticoid receptors (GRs) in leukemic blasts and clinical responses has been demonstrated repeatedly in childhood ALL.4-7 A single-institution study demonstrated a higher induction failure rate and relapse rate in patients with lower blast GR levels.8The early data from a Pediatric Oncology Group study indicated an average of 9900 GR/cell in 291 patients who achieved subsequent remission induction, compared to an average of 4400 GR/cell in 13 patients who did not achieve remission status.9 Long-term follow up showed that a higher GR expression is a favorable prognostic factor in childhood ALL, in which the 5-year event-free survival rate was 47% for patients with fewer than 8000 GR/cell, and 61% for those with more than 8000 GR/cell.10 These clinical studies suggest that either higher GR content confers enhanced glucocorticoid susceptibility on the leukemic blasts or, alternatively, that this GR content is a marker for generalized drug susceptibility. The former hypothesis is more attractive, due to the proven importance of glucocorticoids in childhood ALL therapy.
Glucocorticoids induce apoptosis in a variety of lymphoid cell types. These include primary thymocytes, ALL cells, chronic lymphocytic leukemia (CLL), and multiple myeloma cells. A relationship between sensitivity to glucocorticoid-induced growth arrest and intracellular concentration of GR in lymphoid cell lines has been reported.11-13 In addition, a basal level of GR expression is required to induce apoptosis by glucocorticoids in glucocorticoid-sensitive T cells.14 The data derived from analysis of cell lines in culture reflect the relationship between lymphocytotoxicity and GR content seen in childhood ALL blasts treated in vivo.
Elevation of cyclic adenosine monophosphate (cAMP) level is another stimulus that can induce growth arrest or cell death (or both) in many cultured lymphoid cells, including resting B cells, germinal center B cells, T lymphocytes, and thymocytes.15-19 cAMP also induces cell death in cells derived from lymphoid malignancies, including murine lymphoma cell line S49.1, B-CLL cells, and multiple myeloma cells.20-22 Kiefer et al found that GR is required for glucocorticoid-independent, cAMP-induced apoptosis in a subclone of CEM T-cell ALL cells.23 This result implies some cross-talk between 2 apoptotic pathways and suggests that the cAMP pathway may cause ligand-independent activation of GR. Similar ligand-independent activation of another steroid receptor, progesterone receptor (PR), has been linked to cAMP-induced phosphorylation of PR by protein kinase A.24 The mechanism is complex, however, because cAMP appears necessary but not sufficient for this activation.
Elevation of cAMP can potentiate glucocorticoid effect in at least some cell types.25,26 This effect may be stimulated in part by cAMP induction of the GR gene promoter, elevating GR content.27 However, cAMP potentiation of glucocorticoid effect can be seen even without rises in GR content.28 29These results led us to investigate whether other pharmacologic manipulation of intracellular cAMP levels might enhance the killing of human lymphocytic leukemia cells by glucocorticoid. Such pharmacologic activity would have specific clinical relevance.
Many details of the cAMP signal transduction system have been learned in recent years. Activation of specific cell surface receptors, such as adrenergic receptors, results in activation of one of several isozymes of adenylyl cyclase, leading to cAMP synthesis.30 cAMP causes activation of protein kinase A, with diverse consequences in different cell types. Inactivation of cAMP is catalyzed by cyclic nucleotide phosphodiesterases (PDEs), which are divided into at least 10 classes and more than 40 enzymes based on biochemical features, including substrate specificity, response to selective inhibitors, transcription regulation, kinetics, and DNA sequence.30-32Normal lymphocytes and several lymphoid leukemia cell lines express at least 2 high-affinity cAMP PDEs, cyclic guanosine monophosphate (cGMP)–inhibited cAMP PDE (PDE3) and the cAMP-specific PDE (PDE4), whereas PDE4 is dominant in monocytes and neutrophils and PDE3 in platelets.33-40 Vinpocetine, an inhibitor of PDE1, induces apoptosis in ALL cells.41 Rolipram, an inhibitor of PDE4 enzymes, selectively induces apoptosis in CLL patient cells in vitro.42 The nonspecific PDE inhibitor aminophylline prolongs the survival of mice injected with human ALL cells.43 Because PDE inhibitors such as caffeine, theophylline, rolipram, and vinpocetine have been used therapeutically in humans, it is attractive to consider whether these agents have significant activity against human ALL cells. In this report we show that PDE inhibitors induce growth suppression and apoptosis in human ALL cells, primarily mediated through inhibition of the PDE4 subclass, and that additive or synergistic effects are induced by adding an activator of adenylyl cyclase.
Materials and methods
Cell culture
Human acute T-lymphocytic leukemia cells CEM-GH (CEM) were routinely cultured at 37°C in 5% CO2 in RPMI-1640 medium (Life Technologies, Bethesda, MD) supplemented with 10% fetal bovine serum (FBS) in the concentration between 1 × 105/mL and 1 × 106/mL. Human pre-B lymphocytic leukemia cells Reh, human acute T-lymphocytic leukemia cells Jurkat, RPMI-8402 cells and Molt-16 cells and human B-lymphoma cells KARPASS 422 were maintained in RPMI-1640 with 10% FBS. Human Burkitt cells, Ramos, ST486, DW6, and Epstein-Barr virus–infected human B lymphoblastoid cells were cultured in Iscoves modified Dulbecco medium (IMDM) with 10% FBS. Peripheral blood mononuclear cells (PBMCs) were freshly purified from a healthy donor by a gradient method of Ficoll-Paque from Pharmacia (Piscataway, NJ). CEM-GH cells were a gift of Dr Curt Civin (Johns Hopkins Oncology Center, Baltimore, MD). This subclone of CCRF-CEM cells is relatively resistant to dexamethasome, resembling subclone CEM-C1.28
Reagents
Forskolin, dexamethasone, aminophylline, enprophylline, caffeine, and pentoxyfylline were obtained from Sigma (St Louis, MO), dyphylline from Fluka (Milwaukee, WI), and rolipram, trequinsin, and vinpocetine from Calbiochem (La Jolla, CA). Stock solutions were made in water, ethanol, and dimethyl sulfoxide (DMSO). Working solutions of these compounds were made on the day of use in RPMI 1640 with 10% FBS. In all cases, final concentration of ethanol or DMSO during treatment did not exceed 0.1%, and appropriate controls with vehicle alone were prepared in parallel.
Modified MTT assay
Cell viability was assessed indirectly by modified MTT assay, based on the enzymatic reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma) to form formazan crystal by mitochondria and cellular dehydrogenase enzymes.44 Briefly, 50-μL aliquots of the cell suspension at concentrations of 3 × 104 to 1 × 105/mL were dispensed into 96-well flat-bottomed microplates containing 50 μL of the various chemical dilutions in 6 replicate rows. Plates were incubated in a humidified incubator in 5% CO2 for 4 days at 37°C. Then 20 μL MTT solution (5 mg/mL MTT in phosphate-buffered saline [PBS] stored at −20°C) was added and incubated for 4 hours at 37°C in 5% CO2. Formazan crystals were dissolved with 100 μL lysis buffer (20% [wt/vol] sodium dodecyl sulfate in 1:1 dimethyl-formamide/water, 2.5% [wt/vol] 1 N acetic acid, 2.5% [wt/vol] 1 N HCl, final pH 4.7), followed by overnight incubation at 37°C. Each sample point was assayed with 6 replica points per assay, and experiments were performed at least 4 times. Absorbance at 550 nm (A550) of the solubilized formazan was measured using a Bio-Tek Instruments (Winooski, VT) microplate reader zeroed on the reagent blanks to correlate the colored product with the number of cells, and the results were expressed as a ratio of the treated cells over the untreated vehicle cells, according to the following formula: Viability = [(A550 of treated cells)−(A550of vehicle control)]/[(A550 of control cells)−(A550 of vehicle control)].
cAMP enzyme immunoassay
Total cellular cAMP levels were measured by Biotrak cAMP direct enzyme immunoassay from Amersham Pharmacia Biotech (Piscataway, NJ). CEM cells were cultured in 96-well microplates with a concentration at 4 × 105 cells/well overnight at 37°C. Forskolin, rolipram, and dexamethasone were added to cell culture and incubated for 60 minutes at 37°C. Buffer containing 2.5% dodecyltrimethylammonium bromide was added to lyse cells. Then, 100 μL cell lysate was transferred to appropriate wells in microplates conjugated with donkey antirabbit IgG and incubated at 3°C to 5°C for 2 hours before 50 μL cAMP-peroxidase conjugate was added. After incubating at 3°C to 5°C for 1 hour, all wells were washed 4 times with wash buffer. Immediately, enzyme substrate was dispensed into all wells and plates were incubated at room temperature. To terminate the reaction, 100 μL 1 M sulfuric acid was added into the wells, and the optical density at 450 nm was measured.
Caspase-3 activity and adenosine triphosphate assays
The caspase-3 assay kit from Pharmingen (San Diego, CA) was used according to the manufacturer's directions. CEM cells were treated with or without 20 μM forskolin and 20 μM rolipram for up to 72 hours. Lysates from similarly treated cells were assayed for adenosine triphosphate (ATP) content using the ATP Bioluminescence CLS II Assay (Roche Diagnostics, Indianapolis, IN), as specified by the manufacturer.
Analysis of apoptosis by annexin V binding
To detect apoptosis induced by cAMP-elevating agents and dexamethasone, a translocation from the inner to the outer leaflet of the plasma membrane phospholipid phosphatidylserine (PS), one of the earliest feature of apoptosis, was detected using the ApoAlert enhanced green fluorescent protein (EGFP)-annexin V kit from Clontech (Palo Alto, CA) according to the manufacturer's directions. Signals generated by EGFP–annexin V and propidium iodide (PI) were analyzed on a Becton Dickinson flow cytometer (San Jose, CA) using a single laser emitting excitation light at 488 nm.
Sub-G1 hypodiploidy peak and cell cycle analysis by PI staining
Cells treated under various conditions were harvested and fixed with cold 70% ethanol and then stained with 100 mg/mL PI containing 100 mg/mL RNase, NP-40, and sodium citrate. Analysis by flow cytometry using 488-nm excitation was performed, gating out doublets and clumps. The percentage of cells to the left of G0/1peak, representing hypodiploid cells, was taken as the percentage of apoptotic cells. Further cell cycle profiles were analyzed using ModFit LT Cell Cycle Analysis application software (Verity Software House, Topsham, ME) to calculate phase percentages.
Immunoblots
Cells were collected by centrifugation and lysed by heating in 1% sodium dodecyl sulfate (SDS) to 100°C for 5 minutes. Protein concentrations were measured by the BCA kit (Pierce Chemical, Rockford, IL) and equal amounts of protein were analyzed by immunoblot using anti-p53 (Oncogene Research Products, Boston, MA), anti-p21 (Calbiochem, San Diego, CA), and antiactin (Sigma, St Louis, MO) antibodies by enhanced chemiluminescence (Pierce Chemical, Rockford, IL).
Results
Nonspecific PDE inhibitors suppress cell growth in human T-cell ALL cells
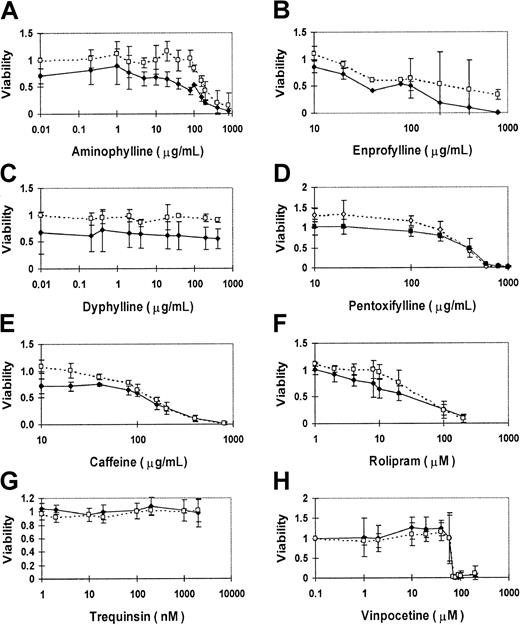
We evaluated the activity of PDE inhibitors in a variety of malignant lymphoid cell lines. Because of the hypothetical potentiation of GR activity, we focused initially on the cell lines derived from pediatric ALL patients, in which the role of GR is best defined. The best characterized of these cell lines is the relatively glucocorticoid-resistant cell line CEM-CCRF, derived from a child with T-cell ALL.45 To test whether methylxanthines can suppress leukemic cell growth, CEM cells were exposed to methylxanthine derivatives in vitro for 4 days, and the fraction of viable cells was measured by the MTT assay. CEM cells displayed a dose-related growth suppression in response to increasing concentrations of aminophylline (Figure1A). Compared to mock-treated CEM cells, the effective concentration of aminophylline that inhibited viability of treated CEM cells to 50% of control cells (EC50) was 400 μg/mL. This implies that aminophylline has either a direct lymphocytotoxic or growth inhibitory effect. Because of the relative glucocorticoid resistance of CEM cells, the addition of 1 μM dexamethasone alone normally caused no more than 15% growth suppression in this assay. However, concurrent treatment of CEM cells with 1 μM dexamethasone reduced the aminophylline EC50 to100 μg/mL, implying an additive effect of glucocorticoid and methylxanthine agents.
PDE inhibitors suppress the growth of human CEM ALL cells in culture.
CEM cells were cultured with each drug for 4 days, and MTT assay measurements are expressed as a viability ratio of drug-treated cells to untreated control cells in each experiment. Error bars represent standard deviation ranges for each data point. Nonspecific inhibitors include aminophylline (A), enprofylline (B), dyphylline (C), pentoxyfylline (D), and caffeine (E). Specific inhibitors include PDE4-specific rolipram (F), PDE3-specific trequinsin (G), and relatively PDE1-specific vinpocetine (H). The dashed lines and open symbols depict the sensitivity profile of each drug alone on CEM cells. The solid lines and closed symbols delineate the sensitivity profile of each drug when combined with 1 μM dexamethasone.
PDE inhibitors suppress the growth of human CEM ALL cells in culture.
CEM cells were cultured with each drug for 4 days, and MTT assay measurements are expressed as a viability ratio of drug-treated cells to untreated control cells in each experiment. Error bars represent standard deviation ranges for each data point. Nonspecific inhibitors include aminophylline (A), enprofylline (B), dyphylline (C), pentoxyfylline (D), and caffeine (E). Specific inhibitors include PDE4-specific rolipram (F), PDE3-specific trequinsin (G), and relatively PDE1-specific vinpocetine (H). The dashed lines and open symbols depict the sensitivity profile of each drug alone on CEM cells. The solid lines and closed symbols delineate the sensitivity profile of each drug when combined with 1 μM dexamethasone.
Other methylxanthines also induced this toxic effect in CEM cells. A slightly lower concentration of caffeine was required for this effect (EC50 200 μg/mL), but dexamethasone provided less additive effect to caffeine (EC50 150 μg/mL; Figure 1E). Enprophylline (3-propylxanthine), another nonspecific PDE inhibitor, provided an EC50 value at 500 μg/mL, reduced to less than 100 μg/mL by concurrent treatment with 1 μM dexamethasone (Figure1B). Pentoxyfylline also caused dose-related growth suppression, but with no additive effect from dexamethasone (Figure 1D). Dyphylline (7-[2,3-dihydroxypropyl] theophylline), a methylxanthine that is only one fifth as potent as theophylline as a bronchodilator, did not suppress cell growth in CEM cells, even with 1 μM dexamethasone (Figure 1C).
We conclude that several nonspecific PDE inhibitors cause growth inhibition in human T-ALL cells in culture and that they augment glucocorticoid lymphocytotoxicity in a glucocorticoid-resistant cell line. None of the other nonspecific PDE inhibitors provided a superior effect to aminophylline (Table 1).
Effective concentrations of PDE inhibitors producing an EC50 of cultured CEM cell number
| Drug . | EC50alone . | EC50 with 1 μM DEX . | Fold increase in DEX sensitivity . |
|---|---|---|---|
| Aminophylline | 180 μg/mL | 54 μg/mL | 3.3 |
| Enprophylline | 240 μg/mL | 100 μg/mL | 2.4 |
| Caffeine | 150 μg/mL | 150 μg/mL | 1 |
| Pentoxifylline | 350 μg/mL | 350 μg/mL | 1 |
| Dyphylline | > 400 μg/mL | > 400 μg/mL | 1 |
| Rolipram | 44 μM (12 μg/mL) | 26 μM (7 μg/mL) | 1.7 |
| Drug . | EC50alone . | EC50 with 1 μM DEX . | Fold increase in DEX sensitivity . |
|---|---|---|---|
| Aminophylline | 180 μg/mL | 54 μg/mL | 3.3 |
| Enprophylline | 240 μg/mL | 100 μg/mL | 2.4 |
| Caffeine | 150 μg/mL | 150 μg/mL | 1 |
| Pentoxifylline | 350 μg/mL | 350 μg/mL | 1 |
| Dyphylline | > 400 μg/mL | > 400 μg/mL | 1 |
| Rolipram | 44 μM (12 μg/mL) | 26 μM (7 μg/mL) | 1.7 |
DEX indicates dexamethasone.
Cell growth is suppressed by inhibition of PDE4 activity but not by inhibitors of other cAMP-specific PDEs
Rolipram sensitivity has been used to define PDE4 activity, which strongly hydrolyzes cAMP, with little effect on cGMP. We found that rolipram had a strong growth suppressive effect on CEM cells, with an EC50 of approximately 44 μM (Figure 1F). The rolipram EC50 was further lowered to 27 μM by concurrent treatment with 1 μM dexamethasone. In addition to PDE4, lymphocytes express PDE3 activity.39 However, the PDE3 inhibitor trequinsin caused little effect on CEM cell growth, even at concentrations well above the inhibitory concentration of 50% (IC50) for PDE3 activity in vitro of 0.3 nM (Figure 1G). Similarly, the PDE1-specific inhibitor vinpocetine induced no growth suppression of CEM cells until the drug rose to concentrations at which PDE subclass selectivity is lost (IC50 for PDE1 activity in vitro = 20 μM; Figure 1H). These results indicate that PDE inhibitors known to elevate intracellular cAMP suppress CEM cell growth. This implies that the growth inhibitory effect of PDE is mediated by elevated intracellular cAMP levels, consistent with published data that cAMP analogs are lymphocytotoxic. This also suggests a possible role for PDE4 activity in growth regulation in leukemic lymphoblasts.
Inhibition of PDE4 activity increases the sensitivity of CEM cells to glucocorticoids
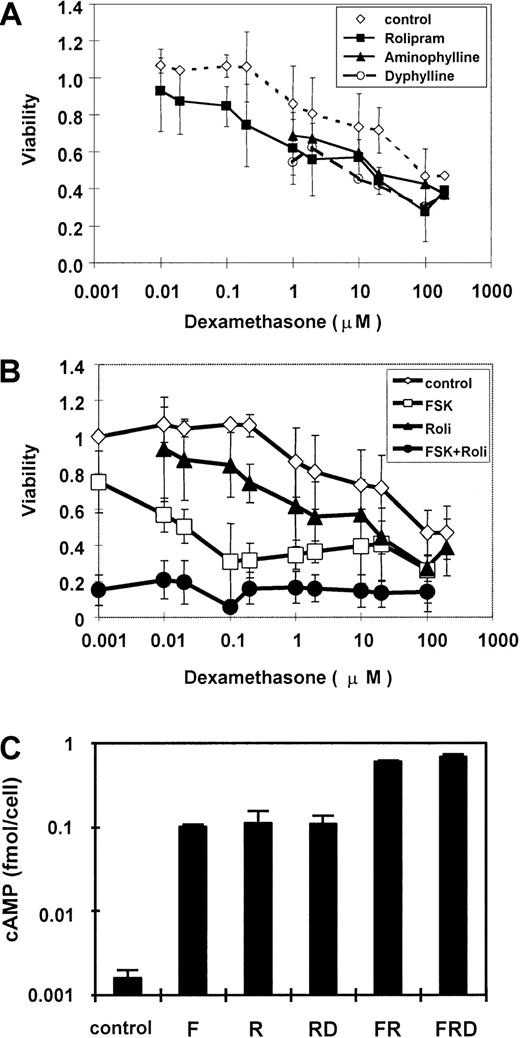
To evaluate the modifying effect of PDE inhibitors on glucocorticoid lymphocytotoxicity in CEM cells, we analyzed the dose-response relationship of dexamethasone to inhibition of CEM cell growth, with and without these inhibitors. We found that dexamethasone slightly suppressed growth at 1 μM, with an EC50 value of 100 μM (Figure 2A). This high EC50 value demonstrates the relative glucocorticoid resistance of this cell line, known to harbor an inactivating mutation in one of the GR alleles.46 When CEM cells are cultured with nonspecific PDE inhibitors, 40 μg/mL aminophylline or 40 μg/mL dyphylline, the EC50 value of dexamethasone is reduced to 20 μM, thus indicating a 5-fold greater sensitivity to glucocorticoid. This effect is fully replicated by the PDE4-specific inhibitor rolipram at a concentration of 20 μM. We conclude that inhibition of PDE activity can partially restore glucocorticoid sensitivity to a resistant cell line, and that much or all of this effect is mediated specifically through inhibition of PDE4 activity.
PDE inhibitors increase sensitivity of CEM cells to glucocorticoid, with concurrent elevation of cAMP.
(A) CEM cells were cultured with 40 μg/mL aminophylline, 40 μg/mL dyphylline, or 20 μM rolipram in the presence of various concentrations of dexamethasone. (B) CEM cells were cultured with 20 μM forskolin and 20 μM rolipram, each alone and in combination, with various concentrations of dexamethasone. MTT assay measurements are expressed as a viability ratio of drug-treated cells to untreated control cells in each experiment. Error bars represent SD ranges for each data point. (C) CEM cells were cultured with combinations of forskolin, rolipram, and 1 μM dexamethasone for 30 minutes, and total cellular cAMP was measured. Cellular cAMP is expressed as a ratio compared to untreated cells and plotted on a logarithmic scale. Error bars represent SD.
PDE inhibitors increase sensitivity of CEM cells to glucocorticoid, with concurrent elevation of cAMP.
(A) CEM cells were cultured with 40 μg/mL aminophylline, 40 μg/mL dyphylline, or 20 μM rolipram in the presence of various concentrations of dexamethasone. (B) CEM cells were cultured with 20 μM forskolin and 20 μM rolipram, each alone and in combination, with various concentrations of dexamethasone. MTT assay measurements are expressed as a viability ratio of drug-treated cells to untreated control cells in each experiment. Error bars represent SD ranges for each data point. (C) CEM cells were cultured with combinations of forskolin, rolipram, and 1 μM dexamethasone for 30 minutes, and total cellular cAMP was measured. Cellular cAMP is expressed as a ratio compared to untreated cells and plotted on a logarithmic scale. Error bars represent SD.
Activation of adenylyl cyclase provides synergy with PDE4 inhibition to suppress CEM growth
In addition to cAMP hydrolysis by PDE, intracellular cAMP levels also are controlled by the rate of cAMP synthesis by adenylyl cyclase. We hypothesized that activators of adenylyl cyclase might provide a synergistic effect to PDE inhibitors. We compared the effects on cell growth provided by rolipram, the adenylyl cyclase activator forskolin, and a combination of the 2. As before, we found that 20 μM rolipram conferred on CEM cells at least a 6-fold increase in glucocorticoid sensitivity (Figure 2B). In contrast, 20 μM forskolin decreased the dexamethasone EC50 to less than 0.1 μM, conferring more than a 1000-fold increase in glucocorticoid sensitivity. A combination of the 2 agents suppressed cell growth by approximately 85%, much more than either agent alone and a maximal value that is not further increased by dexamethasone. This was the same maximal value obtained in this assay by the highest doses of dexamethasone alone. These results indicate that combined activation of adenylyl cyclase activity and inhibition of PDE4 activity provide pharmacologic synergy in inhibiting the growth of CEM leukemia cells.
Activation of adenylyl cyclase provides synergy with PDE4 inhibition to elevate cellular cAMP
To confirm the hypothesis that forskolin and rolipram jointly increase cAMP level in CEM cells, total cellular cAMP levels were measured. In CEM cells, 20 μM rolipram and 20 μM forskolin each elevated the cAMP level about 70-fold to 0.1 fmol/cell (Figure 2C). Combination of both agents stimulated cAMP to 0.6 fmol/cell, a 400-fold elevation over background and 6-fold over either forskolin or rolipram alone. This rise was more than additive, implying pharmacologic synergy of these agents in elevating cellular cAMP concentration. Dexamethasone has little effect on cAMP level, as reported previously.28
cAMP-elevating agents induce G1 and G2/M arrest in CEM cells
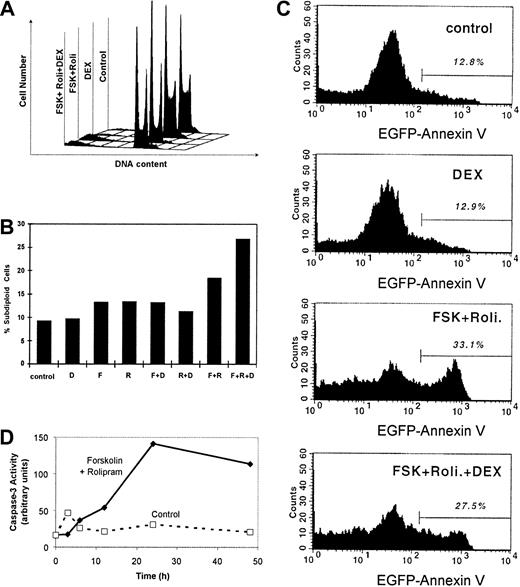
The human T-lymphoblastic leukemia CEM cells used in this experiment proliferated rapidly. Doubling was about 20 hours (data not shown). We determined the cell cycle profiles of treated and untreated CEM cells (Figure 3A). The cell cycle profile of untreated CEM cells during logarithmic growth revealed that 56% of cells were in S phase, 29% in G1, and 15% in G2/M, this high S-phase fraction consistent with deregulated cell growth (Table 2). Treatment of CEM cells with 1 μM dexamethasone alone for 72 hours slightly reduced the S-phase fraction to 31%, with rises in both G1 and G2/M phase. More strikingly, a combination of cAMP-elevating agents, 20 μM forskolin and 20 μM rolipram, suppressed the S-phase fraction to 20%, driving cells into both the G1 and G2/M phases. When dexamethasone was added to these 2 cAMP-elevating drugs, the S-phase fraction remained low at 20%. Interestingly, the fraction of cells accumulating in G1 and G2/M fraction was approximately midway between those cells treated with dexamethasone alone and those treated with forskolin and rolipram. We conclude that cAMP elevation by rolipram and forskolin induces G1 and G2/M phase cell cycle arrest in CEM cells.
Forskolin and rolipram induce G1 and G2/M cell cycle arrest and apoptosis in CEM cells.
CEM cells were cultured with 20 μM forskolin, 20 μM rolipram, and 1 μM dexamethasone in various combinations for 48 hours. (A) DNA content per cell was measured by PI staining and flow cytometry. Deconvolution analysis of the cell cycle profiles was performed to estimate the percentages of cells in G1, S, and G2/M phases (Table 2), as well as (B) the percentage of cells with subdiploid DNA content, indicative of apoptosis. (C) The fluorescence profile of cells bound to EGFP–annexin V fusion protein is plotted with the percentage of highly fluorescent cells shown above the bar, indicating apoptotic cells. (D) Lysate of untreated cells (broken line) and cells treated with forskolin and rolipram (solid line) for various time intervals were assayed for caspase-3 activity using a fluorescent substrate. Activity is measured in arbitrary units of relative fluorescence.
Forskolin and rolipram induce G1 and G2/M cell cycle arrest and apoptosis in CEM cells.
CEM cells were cultured with 20 μM forskolin, 20 μM rolipram, and 1 μM dexamethasone in various combinations for 48 hours. (A) DNA content per cell was measured by PI staining and flow cytometry. Deconvolution analysis of the cell cycle profiles was performed to estimate the percentages of cells in G1, S, and G2/M phases (Table 2), as well as (B) the percentage of cells with subdiploid DNA content, indicative of apoptosis. (C) The fluorescence profile of cells bound to EGFP–annexin V fusion protein is plotted with the percentage of highly fluorescent cells shown above the bar, indicating apoptotic cells. (D) Lysate of untreated cells (broken line) and cells treated with forskolin and rolipram (solid line) for various time intervals were assayed for caspase-3 activity using a fluorescent substrate. Activity is measured in arbitrary units of relative fluorescence.
Results of cell cycle analysis of CEM cells treated with dexamethasone (DEX), forskolin and rolipram (F + R), or all three agents (F + R + D) for 24, 48, and 72 hours
| Treatment . | Duration of treatment . | % G1 . | % S . | % G2/M . |
|---|---|---|---|---|
| Control | None | 29 | 56 | 15 |
| DEX | 24 h | 24 | 55 | 21 |
| 48 h | 35 | 30 | 35 | |
| 72 h | 41 | 31 | 28 | |
| F + R | 24 h | 35 | 40 | 26 |
| 48 h | 39 | 28 | 33 | |
| 72 h | 56 | 20 | 24 | |
| F + R + D | 24 h | 37 | 29 | 34 |
| 48 h | 43 | 27 | 30 | |
| 72 h | 50 | 20 | 31 |
| Treatment . | Duration of treatment . | % G1 . | % S . | % G2/M . |
|---|---|---|---|---|
| Control | None | 29 | 56 | 15 |
| DEX | 24 h | 24 | 55 | 21 |
| 48 h | 35 | 30 | 35 | |
| 72 h | 41 | 31 | 28 | |
| F + R | 24 h | 35 | 40 | 26 |
| 48 h | 39 | 28 | 33 | |
| 72 h | 56 | 20 | 24 | |
| F + R + D | 24 h | 37 | 29 | 34 |
| 48 h | 43 | 27 | 30 | |
| 72 h | 50 | 20 | 31 |
cAMP-elevating agents induce apoptosis through caspase-3 activation in CEM cells
At this point, it is evident that cAMP-increasing agents induce cell growth suppression and cell cycle arrest in CEM cells; however, it is not clear whether these agents induce apoptosis. To detect apoptosis in CEM cells, 3 complementary assays were used. First, the percentage of cells with subdiploid DNA content was measured (Figure 3). After 24 hours of dexamethasone treatment, the percentage of apoptotic cells increased to 16.5%, compared to 7.8% in control cells (Figure 3B). Rolipram alone and forskolin alone each caused a peak percentage of apoptotic cells at 48 hours (Table 2). The combination of forskolin and rolipram induces a peak percentage of apoptosis of 26.1% at 72 hours.
In the second assay, apoptotic cells were detected by annexin V binding, measured by flow cytometry. Similar to the first assay, at 24 hours dexamethasone induced a 2-fold in annexin V binding compared to control (Figure 3C). The combination of forskolin and rolipram induced annexin V binding in 33.1% of CEM cells after 48 hours. These results confirm those from enumeration of subdiploid cells above.
In the third assay of apoptosis, caspase-3 activity was measured. One of the earliest indications of developing apoptosis, caspase-3 activity was markedly induced by 24 hours in CEM cells treated with forskolin and rolipram (Figure 3D). Minimal caspase-3 activity was detected in untreated control cells during 48 hours of culture. This result indicated that cAMP-elevating agents induce apoptosis in CEM cells involving the caspase-3 pathway.
It is conceivable that robust stimulation of adenylyl cyclase might deplete endogenous ATP levels as a potential cause of apoptosis. We measured cellular ATP content of cells that were untreated or treated with forskolin and rolipram for up to 96 hours. Although ATP content declined with the duration of culture in untreated cells, it was virtually identical in cells treated with forskolin and rolipram for similar intervals (data not shown).
Forskolin and rolipram suppress growth of several human malignant lymphoid cell lines
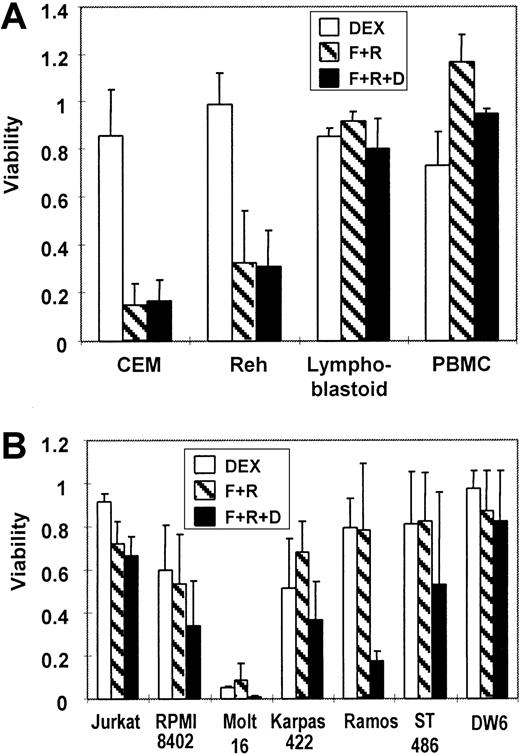
To verify a growth suppressive effect by cAMP-elevating agents, various human lymphocytic cells were exposed to (1) dexamethasone alone, (2) a combination of forskolin and rolipram, or (3) a combination of forskolin, rolipram, and dexamethasone. The 2 best characterized pediatric ALL cell lines, CEM cells (T cell) and Reh cells (B lineage), both demonstrated considerable resistance to dexamethasone alone (Figure 4A). However, the growth of both cell lines was significantly suppressed by forskolin plus rolipram, with no interference by added dexamethasone. In contrast, no significant reduction in viability was seen in treated lymphoblastoid cells or in peripheral blood mononuclear cells. This may be due in part to their low proliferative rate (data not shown). We also tested several other available lymphoid lines. The T-cell lines Jurkat and RPMI-8402 showed slight sensitivity to both dexamethasone and the combination of forskolin and rolipram, and an additive effect of glucocorticoid with cAMP-elevating agents was observed (Figure 4B). T-lymphocytic Molt-16 cells were very sensitive to either dexamethasone or the combination of forskolin and rolipram. Other tested B-lineage cells were resistant to both dexamethasone and the combination of forskolin and rolipram, except for Ramos Burkitt lymphoma cells, in which dexamethasone resistance was reversed markedly by forskolin plus rolipram.
Forskolin and rolipram suppress the growth of several malignant lymphoid lines.
(A) MTT assays demonstrate that 20 μM forskolin and 20 μM rolipram (F+R, cross-hatched bars) suppress proliferation of 2 pediatric ALL lines: T-cell ALL line CEM and the B-precursor ALL line Reh. There is minimal effect from 1 μM dexamethasone (DEX, open bars) alone, and no additive effect seen in combination with forskolin and rolipram (F+R+D, filled bars). Lymphoblastoid cells and PBMCs freshly obtained from a healthy volunteer are virtually unaffected by the same treatments. (B) MTT assays of cell lines derived from adult lymphoid malignancies show variable degrees of growth suppression by combinations of dexamethasone, forskolin, and rolipram. The cell lines that were assayed include Jurkat, RPMI-8402, Molt-16, Karpas-422, Ramos, ST-486, and DW-6. Error bars represent SD.
Forskolin and rolipram suppress the growth of several malignant lymphoid lines.
(A) MTT assays demonstrate that 20 μM forskolin and 20 μM rolipram (F+R, cross-hatched bars) suppress proliferation of 2 pediatric ALL lines: T-cell ALL line CEM and the B-precursor ALL line Reh. There is minimal effect from 1 μM dexamethasone (DEX, open bars) alone, and no additive effect seen in combination with forskolin and rolipram (F+R+D, filled bars). Lymphoblastoid cells and PBMCs freshly obtained from a healthy volunteer are virtually unaffected by the same treatments. (B) MTT assays of cell lines derived from adult lymphoid malignancies show variable degrees of growth suppression by combinations of dexamethasone, forskolin, and rolipram. The cell lines that were assayed include Jurkat, RPMI-8402, Molt-16, Karpas-422, Ramos, ST-486, and DW-6. Error bars represent SD.
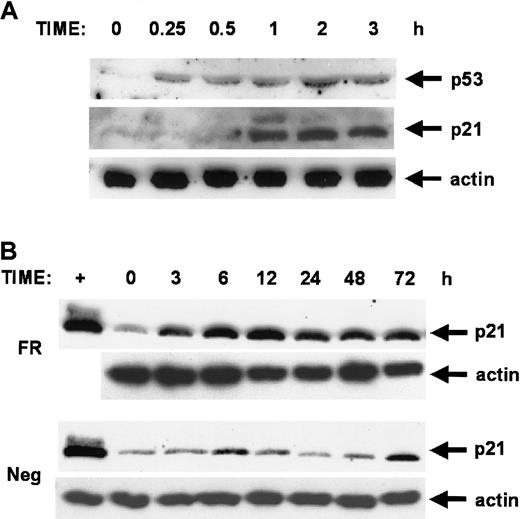
Forskolin and rolipram stimulation leads to sequential induction of p53 and p21 proteins
We observed the resemblance of the cell cycle arrest patterns seen in forskolin- and rolipram-treated cells to that seen in cells overexpressing the growth suppressors p53 or p21WAF1/CIP1. We evaluated the protein levels of these as a function of time after stimulation of CEM cells with forskolin and rolipram. Anti-p53 immunoblot shows rapid induction of p53 protein within 15 minutes (Figure 5A). Anti-p21 immunoblot demonstrates subsequent induction of p21WAF1/CIP1 protein by 1 hour of stimulation. The level of p21 protein induction was comparable to that seen in etoposide-treated NIH 3T3 fibroblasts, in which p53 is known to sharply induce p21 gene transcription after etoposide treatment (Figure 5B). This induction of p21 protein was sustained for at least 72 hours of treatment with forskolin and rolipram. The timing of sequential induction of p53 and p21 proteins was consistent with cAMP induction of p53, followed by expected p53 induction of the p21 promoter. These results suggest that p53 and p21 proteins may play a role in cAMP-induced growth suppression and apoptosis.
Forskolin and rolipram stimulation leads to sequential induction of p53 and p21 proteins.
CEM cells were stimulated with 20 μM forskolin and 20 μM rolipram, and cell extracts obtained at various time points were analyzed by parallel immunoblots using anti-p53, anti-p21, and antiactin antibodies. (A) An early time course shows that induction of p53 protein is detected by 15 minutes of treatment with forskolin and rolipram, with p21 protein subsequently detected by 1 hour. The actin control demonstrates equivalent loading in all lanes. (B) Prolonged induction of p21 protein is seen during a longer time course in cells treated with forskolin and rolipram (FR), with a peak at 12 hours, but sustained elevation is still seen at 72 hours. Extracts collected from negative control cells at parallel time points (Neg) show only very low level increases in p21 levels compared to the robust induction in treated cells. The magnitude of p21 protein induction is comparable to that seen in positive control extracts from NIH 3T3 fibroblasts treated with etoposide for 8 hours (first lane, +).
Forskolin and rolipram stimulation leads to sequential induction of p53 and p21 proteins.
CEM cells were stimulated with 20 μM forskolin and 20 μM rolipram, and cell extracts obtained at various time points were analyzed by parallel immunoblots using anti-p53, anti-p21, and antiactin antibodies. (A) An early time course shows that induction of p53 protein is detected by 15 minutes of treatment with forskolin and rolipram, with p21 protein subsequently detected by 1 hour. The actin control demonstrates equivalent loading in all lanes. (B) Prolonged induction of p21 protein is seen during a longer time course in cells treated with forskolin and rolipram (FR), with a peak at 12 hours, but sustained elevation is still seen at 72 hours. Extracts collected from negative control cells at parallel time points (Neg) show only very low level increases in p21 levels compared to the robust induction in treated cells. The magnitude of p21 protein induction is comparable to that seen in positive control extracts from NIH 3T3 fibroblasts treated with etoposide for 8 hours (first lane, +).
Discussion
Our results document for the first time the efficacy of a large panel of PDE inhibitors in inducing growth suppression and apoptosis in a human ALL line. Our goal was to test and compare the activities of a series of PDE inhibitors, primarily ones that are already available for clinical use. The activities of several nonspecific PDE inhibitors in our assay confirm the results seen with aminophylline on a B-lineage ALL cell line.43 Our data extend this finding and demonstrate at least additive effects with either a glucocorticoid or an adenylyl cyclase activator. We find that such combinations of drugs induce cell cycle arrest and apoptosis in human ALL cells and that this effect involves inhibition of the PDE4 subclass of PDE inhibitors. This contributes to a growing body of evidence that PDE inhibitors can have antitumor activity in lymphoid leukemias and some solid tumors.
Our data indicate that the nonspecific methylxanthine drugs, aminophylline, caffeine, enoprophylline, and pentoxyfylline, each can suppress the growth of T-cell ALL cells in culture. This finding extends several other reports relating to the effects of methylxanthine in normal and malignant hematopoietic cells.47-50 In our analysis of the nonspecific methylxanthines, caffeine was the most potent in achieving growth suppression, although the EC50value is approximately 10 times the maximum clinically achievable serum levels. This suggests that the nonspecific PDE inhibitors probably are impractical for clinical use as a single agent in lymphoid leukemias and that either more specific inhibitors, or drug combinations involving such inhibitors, would be superior to drugs such as aminophylline or caffeine.
We find that CEM cells are sensitive to the PDE4-specific inhibitor rolipram in a manner that reproduces the sensitivity to nonspecific PDE inhibitors. This indicates that the important activity of nonspecific inhibitors for this effect is mediated through PDE4 inhibition. Moreover, rolipram-induced cell death is augmented by the adenylate cyclase activator forskolin, suggesting that cAMP elevation is part of this mechanism. Similar effects from rolipram have been reported in CLL cells.42 We saw no significant effect from inhibition of the PDE1 pathways, although involvement of the Ca++-calmodulin–dependent PDE (PDE1) pathway has been implicated in apoptosis in the human lymphoblastoid B-cell line RPMI-8392.51 We also saw no growth suppression from PDE3 inhibition, even though 39% of PDE activity is PDE3 specific in the related Jurkat cell line.36 The differential sensitivity of leukemic cells to PDE inhibition may relate to their high level of PDE activity, up to 10-fold that of peripheral blood lymphocytes. Because PDE4 activity comprises only 20% to 40% of the total PDE activity, it is likely that PDE4 occupies a disproportionate role in growth and apoptosis.36 40 In summary, it appears that specific PDE4 inhibition induces apoptosis or growth suppression in models of human CLL and some acute myelogenous leukemia, and our data extend this to childhood ALL cells.
Our results show that PDE inhibitors reverse glucocorticoid resistance in childhood ALL cell lines. Presumably, this is primarily due to elevation of intracellular cAMP levels, because there is abundant documentation of interactions between the cAMP and GR pathways and induction of apoptosis in lymphoid cells by both.23,52-54The mechanism of cAMP-induced apoptosis itself is unclear, but it appears to require presence of the GR, even in the absence of exogenous glucocorticoid.23,55 Recently published findings suggest that forskolin can stimulate p53 activity, in a pathway that appears to involve a ternary complex of p53, cAMP response element binding protein (CREB), and CREB binding protein (CBP).56 This pathway may pertain to our results from CEM cells, because we also observed induction of the p53-regulated p21WAF1 protein in CEM cells treated with forskolin and rolipram. Induction of p53 activity may not be critical, or mutations can be bypassed in certain circumstances, because CEM, Jurkat, and Ramos cells all are reported to have null p53 activity, yet 2 of these cell lines responded in our experiments to forskolin and rolipram with or without dexamethasone. Furthermore, p53 activity is not required for glucocorticoid induction of apoptosis in thymocytes, but it is required for radiation- or etoposide-induced apoptosis.57 Diverse mechanisms of interaction appear to connect the cAMP and GR pathways, and it is likely that the cell type or genetic status of the cell may influence the importance of specific pathways.
cAMP has been reported to cause cell cycle arrest in a variety of cell models, including G1 arrest in CEM cells.58 In our model, the combination of rolipram and forskolin induces both a G1 and G2/M arrest, consistent with a p21WAF1-induced pattern of arrest. A similar arrest has been seen in murine macrophages treated with cAMP.59 It is unclear whether this cell cycle arrest contributes to the degree of apoptosis seen with cAMP treatment. In MCF7 breast cancer cells, apoptosis induced by tumor necrosis factor (TNF) is inhibited by concurrent induction of p21WAF1.60 Suppression of p21WAF1 induction prevents cell cycle arrest and augments apoptosis induced by TNF. It may be possible that similar mechanisms may pertain to cAMP-induced apoptosis in lymphoid cells, but this has not been evaluated.
Our data demonstrate for the first time that class-specific PDE inhibitors may have a therapeutic potential in the treatment of ALL, especially childhood types. These findings confirm and extend those demonstrating effectiveness of nonspecific PDE inhibitors in ALL and CLL, and of rolipram in CLL.42,43,47 Considering the therapeutic importance of glucocorticoids in childhood ALL therapy, the reversal of glucocorticoid resistance by rolipram seen in our data is intriguing. Furthermore, the additive effects of adenylyl cyclase activation and rolipram seen in our results suggest that clinically relevant, specific adenylyl cyclase activators could enhance the potential therapeutic effect of a rolipram-glucocorticoid combination. Our laboratory is currently evaluating such combinations. Pharmacologic manipulation of intracellular cAMP levels offers potentially much more specificity than the use of cAMP analogs. The antileukemic and anti-inflammatory potential of drugs targeting the cAMP pathway is becoming better recognized.61,62 Newer, more specific PDE4 inhibitors are being produced, primarily intended for asthma therapy.63 64 It will be important to evaluate these drugs for antileukemic activity.
The authors would like to acknowledge the scientific support of Dr Chi Dang and the clerical support of Linda Thomas and Phyllis Crockett.
Supported in part by the National Institutes of Health grant R29-CA71546, the American Cancer Society, the Leukemia and Lymphoma Society, the Alexander and Margaret Stewart Trust, the Concern Foundation, the Johns Hopkins Clinician Scientist Award (to G.J.K.), and National Institutes of Health grant K08-CA75120 (to M.B.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Gregory J. Kato, Ross Research Building, Rm 1125, 720 N Rutland Ave, Baltimore, MD 21205; e-mail: gkato@jhmi.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal