Abstract
Second mitochondria-derived activator of caspases (Smac)/DIABLO is a mitochondrial protein that is released into the cytosol along with cytochrome c (cyt c) during the execution of the intrinsic pathway of apoptosis. Smac/DIABLO promotes apoptosis by neutralizing the inhibitory effect of the inhibitor of apoptosis (IAP) family of proteins on the processing and activities of the effector caspases. Present studies demonstrate that, upon engagement of the mitochondrial pathway of apoptosis, epothilone (Epo) B derivative BMS 247550, a novel nontaxane antimicrotubule agent, as well as the death ligand Apo-2L/TRAIL (tumor necrosis factor-α–related apoptosis-inducing ligand) induce the mitochondrial release and cytosolic accumulation of Smac/DIABLO, along with cyt c, in human acute leukemia Jurkat T cells. While it had no activity alone, ectopic overexpression of Smac/DIABLO or treatment with the N-terminus heptapeptide (Smac-7) or tetrapeptide (Smac-4) of Smac/DIABLO significantly increased Epo B– or Apo-2L/TRAIL–induced processing and PARP cleavage activity of caspase-3. This produced a significant increase in apoptosis of Jurkat cells (P < .05). Increased apoptosis was also associated with the down-regulation of XIAP, cIAP1, and survivin. Along with the increased activity of caspase-3, ectopic overexpression of Smac/DIABLO or cotreatment with Smac-4 also increased Epo B– or Apo-2L/TRAIL–induced processing of caspase-8 and Bid, resulting in enhanced cytosolic accumulation of cyt c. This was not due to increased assembly and activity of Apo-2L/TRAIL–induced DISC (death-inducing signaling complex) but dependent on the feedback activity of caspase-3. These findings demonstrate that cotreatment with the N-terminus Smac/DIABLO peptide is an effective strategy to enhance apoptosis triggered by the death receptor or mitochondrial pathway and may improve the antitumor activity of Apo-2L/TRAIL and Epo B.
Introduction
Cleavage of a wide range of cellular protein targets by the activated effector caspases-3, -6 and -7 results in apoptosis.1-3 The effector caspases are activated when their proteolytically inactive proforms are cleaved at specific internal Asp residues by the upstream initiator caspases (eg, caspase-8 and caspase-9).1-3 The initiator caspases, in turn, are autoactivated by oligomerization with adaptor proteins (eg, caspase-8) by Fas-associating protein with a death domain (FADD) in the extrinsic and caspase-9 by Apaf-1 in the intrinsic or mitochondrial pathway of apoptosis.2-5 Apoptosis is initiated through the extrinsic pathway when a death ligand, eg, Apo-2L/TRAIL (tumor necrosis factor-α–related apoptosis-inducing ligand), binds to its cell surface death receptors (DRs).6,7 Apo-2L/TRAIL has been shown to induce apoptosis of a variety of tumor cell lines more efficiently than normal cells and is relatively nontoxic in vivo.8-10 Apo-2L/TRAIL can bind to DR4 and DR5, decoy receptors 1 and 2, and osteoprotegerin.4,7 DR4 and DR5 contain a cytoplasmic region consisting of a stretch of 80 amino acids, designated as the death domain (DD) responsible for transducing the death signal.4,7 Ligation by Apo-2L/TRAIL causes oligomerization of DR4 and DR5, which recruit FADD by interacting with its DD. Through its death effector domain, FADD recruits caspase-8 into a death-inducing signaling complex (DISC).4,7 This results in autoproteolytic processing and activation of caspase-8.4,7 Active caspase-8 cleaves the cytosolic p22 Bid into the BH3-only domain-containing, proapoptotic, truncated p15 tBid fragment.11 The tBid undergoes posttranslational N-myristoylation to target the mitochondria,12 where it binds to its mitochondrial proapoptotic partner Bax or Bak to trigger the release of cytochrome c (cyt c) into the cytosol.13 14Accordingly, Bid may serve to amplify and link the extrinsic pathway to the mitochondrial events mediating the intrinsic pathway of apoptosis.
A variety of apoptotic stimuli, including anticancer agents, induce the intrinsic pathway of apoptosis by triggering the loss of mitochondrial membrane integrity.15 This results in the release from the mitochondria of multiple death-promoting molecules, including holocytochrome c, apoptosis-inducing factor, endonuclease G, and second mitochondria-derived activator of caspases (Smac).16-19 In the cytosol, cyt c and deoxyadenosine triphosphate bind to Apaf-1.20,21 This causes multimerization of Apaf-1, allowing the recruitment of procaspases-9 and -3 into an Apaf-1–assembled “apoptosome.” This mediates the processing and activation of caspases-9 and -3 and apoptosis.20-22
The processing and proteolytic activity of caspase-9, followed by caspases-3 and -7, is inhibited by the inhibitor of apoptosis (IAP) family of proteins.23 IAP family members include XIAP, cIAP1, cIAP2, and survivin.23-25 All IAPs contain at least 1, while some contain 3, baculovirus IAP repeat (BIR) domains.23 The caspase-3 or -7 inhibitory segment (linker-BIR2) is distinct from the caspase-9 inhibitory region (BIR3) of XIAP.26-28 Overexpression of XIAP inhibits anticancer drug-induced caspase activity and apoptosis.29 In contrast, down-regulation of XIAP sensitizes cancer cells to apoptosis induced by chemotherapeutic drugs.29,30 Survivin is a 16 kd protein that contains a single BIR domain, followed by a C-terminal, long α helical region that is required for its binding to the microtubules in the mitotic spindle.31,32 Survivin is expressed in cancer but not in normal terminally differentiated adult tissues.31 Overexpression of survivin inhibits effector caspase activity and apoptosis induced by Fas, Bax, and anticancer drugs.33 Abrogation of the expression/function of survivin causes increased caspase-3 activity at G2/M and apoptosis as well as results in the dysregulation of mitotic progression, with supernumerary centrosomes, aberrant mitotic spindles, and polyploidy.34-36
During apoptosis, the mitochondrial Smac/DIABLO is released into the cytosol.16 By disrupting the interaction between the BIR3 with caspase-9 and linker-BIR2 with caspase-3 or -7, the cytosolic Smac/DIABLO relieves the inhibition of these caspases by XIAP.37-40 The N-terminal tetrapeptide (AVPI) of Smac/DIABLO (Smac-4) can bind across in a surface groove on BIR3 of XIAP in a mutually exclusive manner with caspase-9.40 This relieves the inhibition of caspase-9 by XIAP, thereby promoting the activity of caspase-9 followed by caspase-3 and apoptosis.38,40 Epothilone (Epo) B derivative BMS 247550 is a nontaxane antimicrotubule agent.41 Like paclitaxel (Taxol), it induces tubulin polymerization and mitotic arrest followed by apoptosis.41,42 Epo B has been shown to exert in vitro and in vivo cytotoxic effects against a variety of cancer cells, including paclitaxel and platinum-resistant cells.41 In the present studies, in human acute leukemia Jurkat T cells, we show that the ectopic overexpression of the full-length Smac/DIABLO or cotreatment with Smac-7 or Smac-4 peptide enhances Epo B– or Apo-2L/TRAIL–induced cytosolic accumulation of cyt c, caspases-9 and -3 activities, and apoptosis. While this was also associated with increased caspase-8 and Bid processing, cotreatment with N-terminus Smac-7 or Smac-4 peptide did not enhance Epo B– or Apo-2L/TRAIL–induced DISC activity in Jurkat cells.
Materials and methods
Reagents
The recombinant human trimeric form of Apo-2L/TRAIL was produced in Escherichia coli and was a gift from Genentech (South San Francisco, CA).43 Epo B derivative BMS 247550 was a gift from Bristol-Myers Squibb Pharmaceutical Research Institute (Princeton, NJ).41 Anti-Bid and anti-Smac/DIABLO antibodies11 16 were kindly provided by Dr Xiaodong Wang of the University of Texas, Southwestern School of Medicine (Dallas, TX). Monoclonal anti-XIAP antibody was purchased from Boehringer Mannheim (Indianapolis, IN). Polyclonal anti–poly(adenosine diphosphate ribose) polymerase (anti-PARP) and monoclonal anti–cIAP1, anti–caspase-9, and anti–caspase-3 antibodies were purchased from Pharmingen (San Diego, CA). Polyclonal anti–caspase-8 antibody was purchased from Upstate Biotechnology (Lake Placid, NY), while monoclonal antisurvivin was purchased from Alpha Diagnostic (San Antonio, TX). DR4 antibody was purchased from Alexis (San Diego, CA). Polyclonal anti-DR5 was obtained from Cayman Chemicals (Ann Arbor, MI). Monoclonal anti-Flag antibody was purchased from Sigma (St Louis, MO). Monoclonal anticytochrome oxidase-2 antibody was purchased from Molecular Probes (Eugene, OR), and z-VAD-FMK and DEVD-CHO were purchased from Calbiochem (San Diego, CA).
Cells
Jurkat T-cell leukemia and SKW6.4 B lymphoblast cells were obtained from American Tissue Culture Collection (Manassas, VA). Cells were maintained in culture as previously described.44
Creation of Smac/DIABLO transfectants and N-terminus Smac/DIABLO peptides
Viable Jurkat and SKW6.4 cells were transiently transfected with the pcDNA3.1 plasmid containing either the 719–base pair full-length coding region of Smac/DIABLO complementary DNA (cDNA) with a Flag tag at the C-terminus or the control vector (pcDNA3.1 Zeo),16 utilizing LipofectAMINE PLUS reagent (Invitrogen, Carlsbad, CA) and as previously described.44 The untreated or drug-treated transfectants were incubated for the designated time intervals in a humidified 5.0% CO2 environment in RPMI medium supplemented with 100 U penicillin per milliliter, 100 μg streptomycin per milliliter, 1% nonessential amino acids, 1% essential amino acids, and 10% bovine calf serum (Invitrogen, Baltimore, MD). Following these treatments, the cells were harvested for preparation of S-100 fractions and Western analyses (see below). The N-terminus 4 (Smac-4) or 7 (Smac-7) amino acids of Smac/DIABLO-containing peptides and their respective fluorescein-tagged counterparts were custom-synthesized by ResGen (Huntsville, AL). The purity of the peptides as determined by high-performance liquid chromatography was 99%. Following exposure to 10 μM for 24 hours, the intracellular uptake of fluorescein-tagged Smac-4 and Smac-7 peptides in Jurkat and SKW6.4 cells was confirmed by immunofluorescence microscopy.
Preparation of S-100 and heavy membrane fraction and Western analysis of cytosolic cyt c
Untreated and drug-treated cells were harvested by centrifugation at 1000g for 10 minutes at 4°C. The cell pellets were washed once with ice-cold phosphate-buffered saline (PBS) and resuspended with 5 volumes of buffer (20 mM HEPES-KOH [pH 7.5], 10 mM KCl, 1.5 mM MgCl2, 1 mM sodium ethylenediaminetetraacetic acid, 1 mM sodium ethyleneglycotetraacetic acid, 1 mM dithiothreitol, and 0.1 mM phenylmethylsulfonyl fluoride [PMSF]) containing 250 mM sucrose. The cells were homogenized with a 22-gauge needle, and the homogenates were centrifuged at 1000g for 10 minutes at 4° C. The pellet was lysed using lysis buffer (25 mM Tris-HCl [pH 7.2], 150 mM NaCl, 25 mM NaF, 1 mM benzamidine, 1.0% Triton X-100, 2 μg/mL aprotinin, 2 μg/mL leupeptin, 1 μg/mL pepstatin-A, and 0.1 μg/mL PMSF) to get the mitochondria-enriched heavy membrane fraction, whereas the supernatants were further centrifuged at 100 000g for 30 minutes. The resulting supernatants (S-100) were collected, and the protein concentrations were determined by Bradford method (Bio-Rad, Hercules, CA). A total of 10 to 20 μg of the S-100 protein was used for Western blot analysis of cyt c.19,45 The purification of S-100 was determined by Western blot using anticytochrome oxidase-2 antibody.19
Western analyses of proteins
Western analyses of DR4, DR5, Apo-2L, caspase-8, caspase-9, caspase-3, Bid, PARP, XIAP, cIAP, survivin, and β-actin were performed using specific antisera or monoclonal antibodies according to previously reported protocols.45 Horizontal scanning densitometry was performed on Western blots by using acquisition into Adobe Photo Shop (Apple, Cupertino, CA) and analysis by the NIH Image Program (U.S. National Institutes of Health, Bethesda, MD). The expression of β-actin was used as a control.
Apoptosis assessment by annexin V staining
After drug treatments, cells were resuspended in 100 μL staining solution (containing Annexin V fluorescein and propidium iodide in a HEPES buffer, Annexin-V-FLUOS Staining Kit, Boehringer Mannheim). Following incubation at room temperature for 15 minutes, cells were analyzed by flow cytometry.46 Annexin V binds to those cells that express phosphotidylserine on the outer layer of the cell membrane, and propidium iodide stains the cellular DNA of those cells with a compromised cell membrane. This allows for the discrimination of live cells (unstained with either fluorochrome) from apoptotic cells (stained only with annexin V) and necrotic cells (stained with both annexin V and propidium iodide).46
Morphology of apoptotic cells
After drug treatment, 50 × 103 cells were washed with PBS (pH 7.3) and resuspended in the same buffer. Cytospin preparations of the cell suspensions were fixed and stained with Wright stain. Cell morphology was determined by light microscopy. In all, 5 different fields were randomly selected for counting of at least 500 cells. The percentage of apoptotic cells was calculated for each experiment, as described previously.45
Apo-2L/TRAIL–induced DISC analysis
Untreated or drug-treated Jurkat cells were suspended at a final concentration of 106/mL in a prewarmed, complete RPMI media. Cells were treated with 100 ng/mL Apo-2L/TRAIL for 2 hours at 37°C, followed by washing with 1 mL ice-cold PBS. Cells were lysed in 500 μL lysis buffer (25 mM Tris-HCl [pH 7.2], 150 mM NaCl, 25 mM NaF, 1 mM benzamidine, 1.0% Triton X-100, 2 μg/mL aprotinin, 2 μg/mL leupeptin, 1 μg/mL pepstatin-A, and 0.1 μg/mL PMSF) for 30 minutes on ice.47 In the untreated controls, 100 ng/mL Apo-2L/TRAIL was added after lysis of cells to immunoprecipitate nonstimulated Apo-2L/TRAIL receptors. A total of 100 μg of the lysates was incubated at 4°C for 2 hours with 1 μg each of anti-Apo-2L/TRAIL receptors 1 and 2 (DR4 and DR5) antibodies, kindly provided by Immunex (Seattle, WA). The immunecomplexes were incubated overnight at 4°C with 20 μL protein A–agarose beads (Roche, Indianapolis, IN). The beads were recovered by centrifugation and washed twice with the lysis buffer. The pellet was resuspended in the sample buffer and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblot analysis using antibodies against caspase-8, DR5, DR4, and FADD.47 48
Statistical analysis
Significant differences between values obtained in a population of leukemic cells treated with different experimental conditions were determined using the Student t test.
Results
Ectopic overexpression of Smac/DIABLO sensitizes Jurkat cells to Epo B– and Apo-2L/TRAIL–induced apoptosis
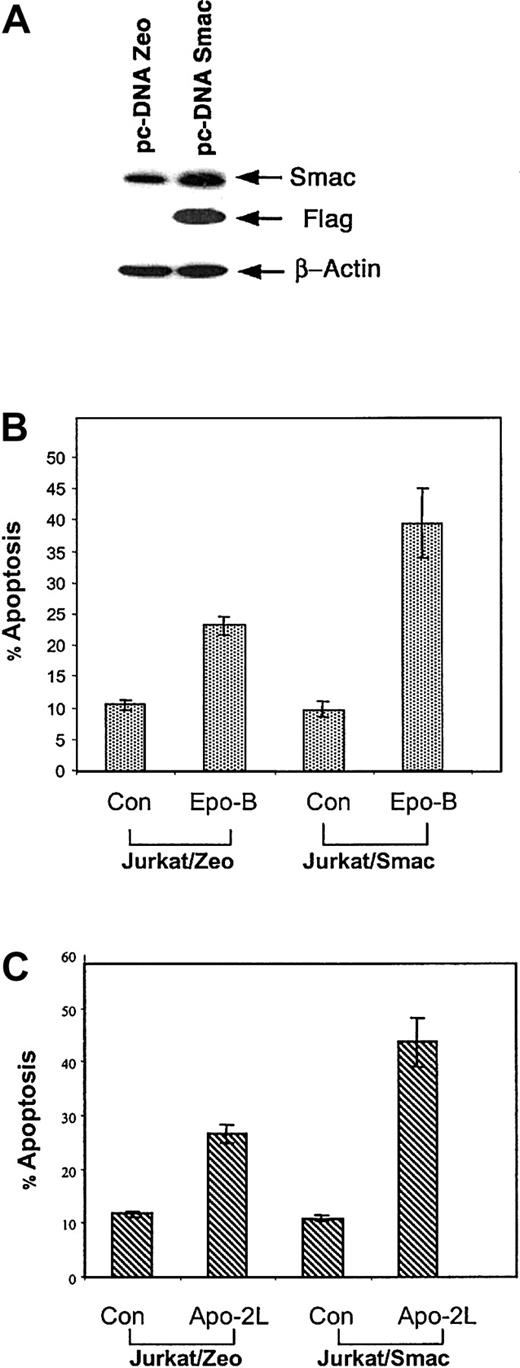
First we determined the apoptotic effect of Epo B and Apo-2L/TRAIL on Jurkat cells. Exposure to Epo B or Apo-2L/TRAIL for 24 hours induced a dose-dependent increase in apoptosis of Jurkat cells, as determined by annexin V staining followed by flow cytometry and confirmed by light-microscopic morphologic examination of the Wright-stained cells (data not shown). Treatment with more than 50 nM Epo B or 5.0 ng/mL Apo-2L/TRAIL induced apoptosis of more than 50% of Jurkat cells. Next, we transiently transfected pcDNA3.1 containing the Flag tagged Smac/DIABLO cDNA or the vector with the zeomycin resistance gene into Jurkat and SKW6.4 cells. The transfection efficiency utilizing the LipofectAMINE PLUS reagent ranged between 30% to 50%. After 48 hours of transfection, cell lysates were immunoblotted with anti-Flag or anti-Smac/DIABLO antibodies. Figure 1A shows that Smac/DIABLO is overexpressed in the cells transfected with the Smac/DIABLO cDNA (Jurkat/Smac) but not in the control cells (Jurkat/Zeo). The full-length cDNA of Smac/DIABLO has been shown to contain an N-terminus, 55-residue mitochondrial targeting sequence, which is cleaved after mitochondrial import to generate the mature mitochondrial Smac/DIABLO. Therefore, we determined whether the ectopic overexpression of Smac/DIABLO was localized to the mitochondria of the untreated cells. Immunoblot analyses of the heavy membrane (mitochondrial) versus the S-100 cytosolic fractions of the untreated transfectants revealed that the ectopically expressed Smac/DIABLO was localized only in the heavy membrane fraction containing the mitochondria (data not shown), as has been previously reported, utilizing immunofluorescent confocal microscopy.16Consistent with this, the ectopic overexpression of Smac/DIABLO was not associated with apoptosis of Jurkat/Smac cells (Figure 1A,B) because the mature cytosolic form of Smac/DIABLO can counter the inhibitory activity of IAPs only to promote apoptosis due to apoptotic stimuli. Treatment with Epo B or Apo-2L/TRAIL induced significantly more apoptosis of Jurkat/Smac versus Jurkat/Zeo cells (Figure 1B,C). The percentages of nonviable cells detected by propidium iodide uptake were 39.5% and 43.7%, versus 23.1% and 26.7% in Jurkat/Smac versus Jurkat/Zeo cells, respectively (mean of 3 experiments). Evaluation of S-100 fractions revealed that both Epo B and Apo-2L/TRAIL induced greater cytosolic accumulation of Smac/DIABLO as well as cyt c in Jurkat/Smac versus Jurkat/Zeo cells (Figure2). Densitometric comparison showed 70% and 50% greater accumulation of Smac/DIABLO in the cytosol of Jurkat/Smac and Jurkat/Zeo cells, respectively, following treatment with Apo-2L/TRAIL and Epo B. Of note, Smac/DIABLO was also detectable in the cytosol of the untreated Jurkat/Smac and Jurkat/Zeo cells. This is most likely the inactive, short, alternately spliced, cytosolic isoform of Smac/DIABLO, detected in several cancer cell types, which lacks the N-terminus mitochondrial targeting sequence along with the functionally important 4–amino acid residues.49
Ectopic overexpression of Smac/DIABLO sensitizes Jurkat cells to Epo B– or Apo-2L/TRAIL–induced apoptosis.
Jurkat cells were transiently transfected with either a control vector (pcDNA3.1 Zeo) or a vector containing full coding sequence of Smac/DIABLO (pcDNA3.1 Smac). Forty-eight hours later, cells were harvested and Smac/DIABLO expression was determined by immunoblot analysis of the cell lysates by using anti-Flag and anti-Smac antibodies (A). Twenty-four hours after transfection, the cells were treated with 10 nM Epo B (B) or 2.5 ng/mL Apo-2L/TRAIL (C) for 24 hours. Following this, the percentage of apoptotic cells was determined by annexin V staining followed by flow cytometry. Values represent the mean ± SE of 3 independent experiments.
Ectopic overexpression of Smac/DIABLO sensitizes Jurkat cells to Epo B– or Apo-2L/TRAIL–induced apoptosis.
Jurkat cells were transiently transfected with either a control vector (pcDNA3.1 Zeo) or a vector containing full coding sequence of Smac/DIABLO (pcDNA3.1 Smac). Forty-eight hours later, cells were harvested and Smac/DIABLO expression was determined by immunoblot analysis of the cell lysates by using anti-Flag and anti-Smac antibodies (A). Twenty-four hours after transfection, the cells were treated with 10 nM Epo B (B) or 2.5 ng/mL Apo-2L/TRAIL (C) for 24 hours. Following this, the percentage of apoptotic cells was determined by annexin V staining followed by flow cytometry. Values represent the mean ± SE of 3 independent experiments.
Ectopic overexpression of Smac/DIABLO enhanced Epo B– or Apo-2L/TRAIL–induced cytosolic accumulation of cyt c and Smac/DIABLO in Jurkat cells.
Jurkat cells were transiently transfected with either the control vector (pcDNA3.1 Zeo) or pcDNA3.1-expressing Smac/DIABLO (pcDNA3.1 Smac). Twenty-four hours after transfection, cells were treated with 10 nM Epo B (A) or 2.5 ng/mL Apo-2L/TRAIL (B) for 24 hours. Following this treatment, the S-100 fractions were obtained from the harvested cells and used for the immunoblot analyses for the cytosolic cyt c and Smac.
Ectopic overexpression of Smac/DIABLO enhanced Epo B– or Apo-2L/TRAIL–induced cytosolic accumulation of cyt c and Smac/DIABLO in Jurkat cells.
Jurkat cells were transiently transfected with either the control vector (pcDNA3.1 Zeo) or pcDNA3.1-expressing Smac/DIABLO (pcDNA3.1 Smac). Twenty-four hours after transfection, cells were treated with 10 nM Epo B (A) or 2.5 ng/mL Apo-2L/TRAIL (B) for 24 hours. Following this treatment, the S-100 fractions were obtained from the harvested cells and used for the immunoblot analyses for the cytosolic cyt c and Smac.
Ectopic overexpression of Smac/DIABLO increases Epo B– or Apo-2L/TRAIL–induced caspase-8 and Bid processing and down-regulates levels of the IAP family of proteins
Ectopic overexpression of Smac/DIABLO did not potentiate Apo-2L/TRAIL–induced DISC assembly, as represented by recruitment of similar levels of procaspase-8 and FADD (not shown) into the DISC and similar processing of caspase-8 in the DISC in Jurkat/Smac and Jurkat/Zeo cells (Figure 3A). Notably, immunoprecipitation with anti-DR5 antibody followed by immunoblotting with anticaspase-8 antibody revealed detectable levels of procaspase-8 in the DISC, even in Jurkat cells that were not treated with Apo-2L/TRAIL. This may be due to a nonspecific association of procaspase-8 with the high levels of DR5 found in Jurkat cells. However, this is clearly insufficient to trigger the autoprocessing of procaspase-8 in the absence of ligation and oligomerization of DR5 by Apo-2L/TRAIL, which results in the induced proximity and autoprocessing of procaspase-8. This also indicates that the observed increase in Apo-2L/TRAIL–induced processing of caspase-8 and Bid in the immunoblot analyses of the cell lysates (Figure 4B), as well as increased cytosolic accumulation of cyt c (noted above), was not due to greater assembly and activity of Apo-2L/TRAIL–induced DISC in Jurkat/Smac versus Jurkat/Zeo cells. Treatment of Jurkat/Smac cells with Epo B for 24 hours also produced greater processing of caspase-8 (Figure 3B) and Bid (not shown) as well as increased cytosolic accumulation of cyt c (noted above). Again, this was also unlikely to be due to increased assembly and activity of a DISC initiated through DR4 and/or DR5, because the processing and activation of caspase-8 was not detectable after shorter exposure intervals to Epo B (Figure 3B). Therefore, the late potentiation of the processing of caspase-8 and Bid and the resulting increased accumulation of cyt c due to treatment with Epo B is likely to be due to Smac/DIABLO-mediated higher activity of caspase-3 followed by the feedback processing of caspase-8 and Bid.44 Indeed, consistent with this, Figure 4A shows that Epo B treatment (10 nM for 24 hours) induced significantly more processing and PARP cleavage activity of caspase-3 in Jurkat/Smac versus Jurkat/Zeo cells. This was also associated with Epo B–induced more down-regulation of XIAP, cIAP1, and survivin in Jurkat/Smac cells (Figure 4A). Treatment with 2.5 ng/mL Apo-2L/TRAIL for 24 hours also induced greater processing of procaspase-3 as well as more down-regulation of XIAP, cIAP1, and survivin in Jurkat/Smac versus Jurkat/Zeo cells (Figure 4B). Hence, increased processing of caspase-8 and Bid due to treatment of Jurkat/Smac cells with Apo-2L/TRAIL for 24 hours (Figure 4B) is also likely to be due to increased Apo-2L/TRAIL–induced activity of caspase-3. This conclusion was further supported by the observation that cotreatment with 50 μM z-DEVD-CHO, a caspase-3–specific inhibitor, prevented the Epo B– or Apo-2L/TRAIL– (2.5 ng/mL) induced enhanced processing of caspase-8 and Bid in Jurkat/Smac cells (data not shown).
Ectopic overexpression of Smac/DIABLO does not enhance Apo-2L/TRAIL–induced DISC and its processing of caspase-8.
Jurkat cells were transiently transfected with either the control vector (pcDNA3.1 Zeo) or pcDNA3.1-expressing Smac/DIABLO (pcDNA3.1 Smac). Twenty-four hours after transfection, cells were treated with either 100 ng/mL Apo-2L/TRAIL for 2 hours or 10 nM Epo B for 4 or 24 hours. Following this, cells were harvested and cell lysates were obtained. These were either immunoprecipitated with anti-DR4 and anti-DR5 antibodies and immunoblotted with anticaspase-8 antibody to evaluate its recruitment to and processing by DISC (A), or the cell-lysates were immunoblotted with anticaspase-8 and β-actin antibodies (β-actin serving as the loading control) (B). Immunoblots are representative of results from 3 experiments.
Ectopic overexpression of Smac/DIABLO does not enhance Apo-2L/TRAIL–induced DISC and its processing of caspase-8.
Jurkat cells were transiently transfected with either the control vector (pcDNA3.1 Zeo) or pcDNA3.1-expressing Smac/DIABLO (pcDNA3.1 Smac). Twenty-four hours after transfection, cells were treated with either 100 ng/mL Apo-2L/TRAIL for 2 hours or 10 nM Epo B for 4 or 24 hours. Following this, cells were harvested and cell lysates were obtained. These were either immunoprecipitated with anti-DR4 and anti-DR5 antibodies and immunoblotted with anticaspase-8 antibody to evaluate its recruitment to and processing by DISC (A), or the cell-lysates were immunoblotted with anticaspase-8 and β-actin antibodies (β-actin serving as the loading control) (B). Immunoblots are representative of results from 3 experiments.
Ectopic overexpression of Smac/DIABLO enhanced Epo B– or Apo-2L/TRAIL–induced processing and activity of caspase-3 and down-regulation of the levels of XIAP, cIAP, and survivin.
Jurkat cells were transiently transfected with either the control vector (pcDNA Zeo) or pcDNA-expressing Smac (pcDNA Smac). Twenty-four hours after transfection, cells were treated with 10 nM Epo B (A) or 2.5 ng/mL Apo-2L/TRAIL (B) for 24 hours. Following this, cell lysates were obtained from treated and untreated cells, and immunoblot analysis of caspase-8 (processing), Bid, PARP, caspase-3, XIAP, cIAP1, or survivin was performed; β-actin was used as loading control.
Ectopic overexpression of Smac/DIABLO enhanced Epo B– or Apo-2L/TRAIL–induced processing and activity of caspase-3 and down-regulation of the levels of XIAP, cIAP, and survivin.
Jurkat cells were transiently transfected with either the control vector (pcDNA Zeo) or pcDNA-expressing Smac (pcDNA Smac). Twenty-four hours after transfection, cells were treated with 10 nM Epo B (A) or 2.5 ng/mL Apo-2L/TRAIL (B) for 24 hours. Following this, cell lysates were obtained from treated and untreated cells, and immunoblot analysis of caspase-8 (processing), Bid, PARP, caspase-3, XIAP, cIAP1, or survivin was performed; β-actin was used as loading control.
During apoptosis induced by a variety of antileukemic agents, including Apo-2L/TRAIL, the activity of caspase-3 has been shown to be associated with down-regulation of the levels of IAP proteins.50-52The RING domain of XIAP has ubiquitin ligase activity and promotes its self-degradation through the proteasomes in response to apoptotic stimuli.53 Furthermore, during apoptosis, XIAP has been shown to be cleaved by activated caspase-3 into the amino-terminal BIR1-2 and BIR3-RING finger fragments.54 To investigate the mechanism underlying increased down-regulation of the levels of the IAP family of proteins, we determined the effect of cotreatment of Epo B or Apo-2L/TRAIL with z-VAD-fmk (a nonspecific caspase inhibitor44). These studies demonstrated that cotreatment with zVAD-fmk inhibited Epo B– or Apo-2L/TRAIL–induced down-regulation of the levels of the IAP family of proteins as well as apoptosis of Jurkat cells (data not shown). This suggests that the increased repression in the levels of the IAP proteins is caspase-dependent.
Cotreatment with N-terminus Smac-4 or Smac-7 peptide potentiates Epo B– or Apo-2L/TRAIL–induced apoptosis
First, we determined the intracellular uptake of the fluorescein-tagged Smac-7 or Smac-4 peptide (N-terminus AVPIAQK, Smac-7 or AVPI, Smac-4, amino acids of Smac/DIABLO) or their respective control peptides into Jurkat cells. Treatment with 1.0 to 10 μM levels of the peptides for 3 hours resulted in significant intracellular accumulations of the peptides without inducing apoptosis of Jurkat cells (data not shown). Figure5 shows that cotreatment with Smac-4 but not the control peptide significantly increased Epo B– or Apo-2L/TRAIL–induced apoptosis of Jurkat cells (P < .05). This was associated with more processing of procaspase-3 and PARP cleavage activity of caspase-3 (Figure6). In addition, cotreatment with Smac-4 but not the control peptide also increased Epo B– or Apo-2L/TRAIL–mediated down-regulation of the levels of XIAP and cIAP1 (Figure 6). This was again inhibited by cotreatment with zVAD-fmk. Similar potentiation of Epo B– or Apo-2L/TRAIL–induced caspase-3 activation and apoptosis of Jurkat cells was also observed following cotreatment with Smac-7 peptide (data not shown). Jurkat cells are known to be of the type II phenotype in which Apo-2L/TRAIL–induced caspase-8 activity results in the mitochondria-initiated activation of caspases-9 and -3.55 Unlike Jurkat cells, in SKW6.4 cells that have a type I phenotype, treatment with Apo-2L/TRAIL activates caspase-3 directly by activating caspase-8. Therefore, we determined the effect of cotreatment with Smac-4 on Apo-2L/TRAIL–induced apoptosis of SKW6.4 cells. Exposure of SKW6.4 cells with Smac-4 (10 μM) and Apo-2L/TRAIL (10 ng/mL) versus Apo-2L/TRAIL alone for 24 hours produced similar levels of apoptosis, ie, 27.3% versus 29.4% of cells (mean of 3 experiments, P > .05). This is consistent with the reports that Smac-4 peptide promotes the activity of caspase-9 by neutralizing the inhibitory effect of BIR3 of XIAP on caspase-9 but does not influence the inhibitory effect of linker-BIR2 on caspase-3.39 40 In contrast, ectopic overexpression (transient) of the full-length Smac/DIABLO, as in the type II Jurkat cells, significantly enhanced Epo B– and Apo-2L/TRAIL–induced apoptosis (data not shown).
Cotreatment with Smac-4 peptide enhances Epo B– or Apo-2L/TRAIL–induced apoptosis of Jurkat cells.
Jurkat cells were treated with either 10 μM of control or Smac-4 peptide for 3 hours, followed by treatment with 10 nM Epo B or 2.5 ng/mL Apo-2L/TRAIL and/or the control or Smac-4 peptide for 24 hours. Following these treatments, the percentage of apoptotic cells (hypodiploid and sub-G1 fraction) was determined by propidium iodide staining followed by flow cytometry. Values represent the mean ± SE of 3 experiments.
Cotreatment with Smac-4 peptide enhances Epo B– or Apo-2L/TRAIL–induced apoptosis of Jurkat cells.
Jurkat cells were treated with either 10 μM of control or Smac-4 peptide for 3 hours, followed by treatment with 10 nM Epo B or 2.5 ng/mL Apo-2L/TRAIL and/or the control or Smac-4 peptide for 24 hours. Following these treatments, the percentage of apoptotic cells (hypodiploid and sub-G1 fraction) was determined by propidium iodide staining followed by flow cytometry. Values represent the mean ± SE of 3 experiments.
Cotreatment with Smac-4 peptide enhanced Epo B– or Apo-2L/TRAIL–induced processing and activity of caspase-3 and down-regulation of the levels of XIAP, cIAP, and survivin.
Jurkat cells were treated with either 10 μM of control or Smac-4 peptide for 3 hours, followed by treatment with 10 nM Epo B or 2.5 ng/mL Apo-2L/TRAIL and/or the control or Smac-4 peptide for 24 hours. Following these treatments, Jurkat cells were treated with either a control peptide or 4–amino acid Smac peptide (10 μM for 3 hours), followed by treatment with Epo B (10 nM) (A) or Apo-2L/TRAIL (2.5 ng/mL) (B) for 24 hours. Following this, cell lysates were obtained from treated and untreated cells and immunoblot analysis of PARP, caspase-3, XIAP, or cIAP1 was performed; β-actin was used as a loading control.
Cotreatment with Smac-4 peptide enhanced Epo B– or Apo-2L/TRAIL–induced processing and activity of caspase-3 and down-regulation of the levels of XIAP, cIAP, and survivin.
Jurkat cells were treated with either 10 μM of control or Smac-4 peptide for 3 hours, followed by treatment with 10 nM Epo B or 2.5 ng/mL Apo-2L/TRAIL and/or the control or Smac-4 peptide for 24 hours. Following these treatments, Jurkat cells were treated with either a control peptide or 4–amino acid Smac peptide (10 μM for 3 hours), followed by treatment with Epo B (10 nM) (A) or Apo-2L/TRAIL (2.5 ng/mL) (B) for 24 hours. Following this, cell lysates were obtained from treated and untreated cells and immunoblot analysis of PARP, caspase-3, XIAP, or cIAP1 was performed; β-actin was used as a loading control.
Discussion
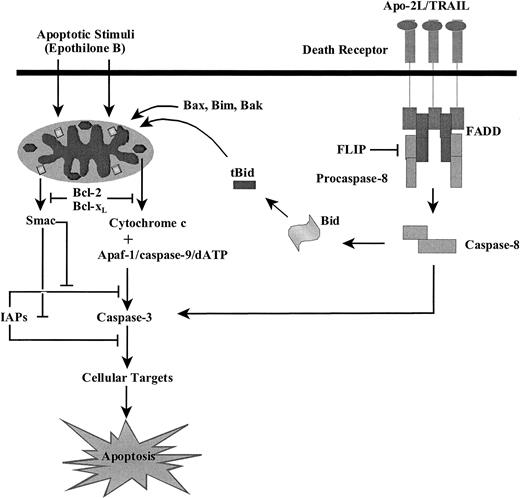
Previous reports have clearly shown that Apo-2L/TRAIL–induced DISC and caspase-8 activity results in the generation of tBid from BID and the engagement of the mitochondrial pathway of apoptosis (Figure7).7,47,50 Other reports have demonstrated that antimicrotubule agents induce cell cycle mitotic arrest, followed by mitochondrial release and cytosolic accumulation of cyt c.42 This triggers the assembly of the Apaf-1–mediated apoptosome and the activation of caspases-9 and -3 (Figure 7).56 In the present studies, we demonstrate for the first time that both Apo-2L/TRAIL and the novel nontaxane antimicrotubule anticancer drug Epo B induce the mitochondrial release and cytosolic accumulation of Smac/DIABLO along with cyt c (Figure 7). In addition, the ectopic overexpression of Smac/DIABLO sensitized Jurkat cells to Epo B– or Apo-2L/TRAIL–induced processing and activity of caspase-3 and apoptosis. These findings are consistent with the reports that Smac/DIABLO can neutralize the repressive influence of IAPs on the processing and activation of caspases-9 and -3.16,40 In this regard, because cotreatment of the type II Jurkat cells with the N-terminus Smac-4 peptide was as effective as the ectopic overexpression of Smac/DIABLO, these data are also consistent with the structural basis for the binding and neutralizing effect of Smac/DIABLO on the IAP proteins.34-37
Schematic representation of the mechanism of Smac/DIABLO- (or Smac-4–) mediated sensitization of Epo B– or Apo-2L/TRAIL–induced activation of caspases-9 and -3 and apoptosis.
Schematic representation of the mechanism of Smac/DIABLO- (or Smac-4–) mediated sensitization of Epo B– or Apo-2L/TRAIL–induced activation of caspases-9 and -3 and apoptosis.
The N-terminus 55–amino acid residues in the newly translated Smac/DIABLO precursor protein constitute its mitochondrial targeting sequence, which causes the localization of Smac/DIABLO to the mitochondria.16 Upon release from the mitochondria during apoptosis, the mature Smac/DIABLO, minus its mitochondrial targeting sequence, accumulates in the S-100 cytosolic fraction where it directly interacts with XIAP (or other IAPs that have similar structures), blocking its inhibitory effects on caspases-9, -7, and -3.16,37-39 Biochemical dissection of XIAP has shown that its caspase-3 and -7 inhibitory region is distinct from the caspase-9 inhibitory segment.37-40 A region of XIAP containing its second BIR domain and an N-terminal extension (linker-BIR2, residues 124 to 240) is the functional unit that inhibits caspases-3 and -7.26-28 In contrast, a region encompassing the third BIR domain inhibits caspase-9.37,40 The BIR antagonist action of Smac/DIABLO is due to its binding to linker-BIR2 and BIR3 in a mutually exclusive manner with caspases-3 and -7 and caspase-9, respectively. The crystal structure of mature Smac/DIABLO has shown that it is a homodimeric 3-helix bundle, and the N-terminus of Smac/DIABLO as short as 7 (Smac-7) or even 4 amino acids (Smac-4) can promote caspase-3 activation.40 In the cocrystal structure of the homodimeric Smac/DIABLO with BIR3 of XIAP, Smac-4 bound to a surface groove of BIR3, an overlap with the caspase-9 interaction site.40 Thus, Smac-4 precludes caspase-9 from binding to BIR3 of XIAP. This allows caspase-9, activated by apoptotic stimuli including Apo-2L/TRAIL and Epo B, to promote the processing and activation of caspase-3. Although there is also evidence to suggest that, as compared with full-length Smac protein, Smac-4 may be insufficient to relieve the binding of BIR2 to caspase-3,26-28 data presented here clearly demonstrate that cotreatment with Smac-4 is as effective as the ectopic overexpression of full-length Smac/DIABLO in sensitizing type II Jurkat cells to Epo B– or Apo-2L/TRAIL–induced caspase-3 activity and apoptosis (Figure 7). This equivalence in efficacy creates a strong rationale to develop and investigate small-molecule peptidomimetics of Smac-4 for their potentiating effect on apoptosis induced by anticancer agents.
Bcl-2 and Bcl-xL overexpression have been previously shown to exert their inhibitory effect on apoptosis by blocking the release of cyt c and mitochondrial ΔΨm induced by a variety of apoptotic stimuli that engage the intrinsic pathway to apoptosis (Figure7).57,58 Overexpression of Bcl-2 or Bcl-xL was also shown to inhibit Apo-2L/TRAIL–induced apoptosis of HL-60 cells.50 Apo-2L/TRAIL predominately engages the intrinsic pathway of apoptosis in these cells. This was suggested by our previous observations that the inhibition of caspase-9 activity by z-LEHD-fmk was as effective as the inhibition of caspase-8 by z-ITED-fmk in suppressing Apo-2L/TRAIL–induced apoptosis of HL-60 cells.44 Apo-2L/TRAIL–induced apoptosis was also shown to be markedly inhibited in Apaf-1–null cells.44 Thus, Jurkat and HL-60 cells are of the type II variety with respect to the DR-initiated apoptotic signaling.44 55 In type II cells that show a relative resistance to the mitochondrial pathway of apoptosis due to overexpression of Bcl-2 and/or Bcl-xL, cotreatment with Smac-4 may be an attractive strategy to partially overcome this resistance to apoptosis. Cotreatment with Smac-4 or its synthetic mimetics may show efficacy in overcoming XIAP- (or other IAP with similar structure) mediated repression of the caspase-9, thus allowing a greater activation of the effector caspases by the upstream initiator caspase-8 and enhancing Apo-2L/TRAIL–induced apoptosis. In type I SKW6.4 cells, Apo-2L/TRAIL–mediated DISC activity activates caspase-8, which directly processes and induces the activity of caspase-3. In these cells, cotreatment with Smac-4 failed to enhance Apo-2L/TRAIL–induced apoptosis.
Nontaxane novel antimicrotubule agent Epo B has been shown in early human trials to be clinically active at relatively safe dose levels.41,59 Recent in vitro studies in our laboratory have shown that Epo B induces mitotic arrest followed by apoptosis of a variety of human epithelial cancer cells and their cisplatin- or paclitaxel-resistant variants.60 Taken together with the results presented here, these finding support the rationale to investigate the in vitro and in vivo activity of Smac-4 plus Epo B against human tumor models.
Supported by NIH grants NCI CA56613 and CA63382.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kapil Bhalla, Interdisciplinary Oncology Program, Moffitt Cancer Center and Research Institute, University of South Florida, 12902 Magnolia Dr, MRC 3 East, Rm 3056, Tampa, FL 33612; e-mail: bhallakn@moffitt.usf.edu.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal