Abstract
In human monocytes and macrophages, interferon-γ (IFNγ) augmented mRNA and surface expression of toll-like receptor 4 (TLR4), a crucial component of the signaling receptor complex for bacterial lipopolysaccharide (LPS). Expression of the accessory component MD-2 and of the adapter protein MyD88 was also increased. LPS increased TLR4 mRNA levels, but concomitantly decreased its surface expression. IFNγ counteracted the LPS-induced downregulation of TLR4. IFNγ-primed monocytes showed increased responsiveness to LPS in terms of phosphorylation of the interleukin-1 receptor–associated kinase (IRAK; immediately downstream of the MyD88 adapter protein), NF-kB DNA binding activity, and, accordingly, of cytokine (tumor necrosis factor α [TNFα] and interleukin-12 [IL-12]) production. These results suggest that enhanced TLR4 expression underlies the long-known priming by IFNγ of mononuclear phagocytes for pathogen recognition and killing as well as its synergism with LPS in macrophage activation.
Introduction
Mononuclear phagocytes provide a first line of defense against microorganisms which can be rapidly destroyed with no need for ensuing adaptive immune responses. Resting macrophages are relatively inefficient against pathogens unless appropriately activated. Interferon-γ (IFNγ) is a major activation signal for macrophages1-4: it activates macrophages for microbicidal and tumoricidal activity, priming their responsiveness to encounter with pathogens. Prior (priming) or concomitant exposure of macrophages to IFNγ dramatically increases their responsiveness to microbial products such as bacterial lipopolysaccharide (LPS), in terms of production of toxic mediators (eg, nitric oxide [NO]), cytokines (eg, tumor necrosis factor α [TNFα] and interleukin-12 [IL-12]), and costimulatory molecules.1-4 The molecular basis for the priming and synergism of IFNγ with microbial moieties such LPS has not been defined.
Macrophages recognize pathogens using pattern recognition receptors.5 Members of the toll-like receptor family are involved in the recognition of pathogen-associated molecular patterns.5-16 Genetic analysis identified TLR4 as a crucial component of the signaling receptor complex that interacts with LPS.6,8,14,17 The cytoplasmic portion of TLR is characterized by a toll–IL-1 receptor (TIR) domain, which recruits the adapter protein MyD88, with subsequent activation of a signaling cascade leading to NF-kB and AP-1 activation.18-21
Materials and methods
Cell culture
The cell culture medium routinely used was RPMI 1640 with 2 mM glutamine and 10% fetal calf serum (FCS) (complete medium). All reagents contained less than (0.125 EU/mL [12.5 pg/mL]) of endotoxin as checked by limulus amebocyte lysate assay (Microbiological Associates, Walkersville, MD). Circulating human monocytes were separated from buffy coats of healthy blood donors through the courtesy of Centro Trasfusionale, Ospedale Civile Formalori (Magenta, Italy), by Percoll (Pharmacia, Uppsala, Sweden) gradient centrifugation (> 95% pure as assessed by morphology) as described.25Monocyte-derived macrophages were derived from freshly isolated monocytes (3 × 106 cells/mL-5 × 106cells/mL) after incubation for 5 days in RPMI complete medium supplemented with 40% autologous serum on hydrophobic plates (Petriperm Hydrophobic; Heraeus Instruments GmbH, Munich, Germany) as described previously.26
Cytokines and antibodies
Lipopolysaccharide (Escherichia coli 005:B5) was purchased from Difco (Detroit, MI). Human recombinant IFNγ was a kind gift of Institut Roussel-Uclaf (Paris, France); human recombinant IFNα and IFNβ were from Schering (Berlin, Germany). Cycloheximide (CHX) was from Sigma Chemical (St Louis, MO). All reagents contained less than (0.125 EU/mL [12.5 pg/mL]) of endotoxin as checked by limulus amebocyte lysate assay (Microbiological Associates). The rabbit polyclonal antibody against human MyD88 was raised against a peptide sequence and affinity purified on a peptide-coated Sulphadex column (Perbio Science UK Limited, Chester, United Kingdom). It was characterized by Western blot on 293T cells transfected with MyD88. The monoclonal antibody (mAb) against human TLR4, HTA125 (mouse IgG2a), has been described previously.27 The rabbit polyclonal antihuman TLR4 antibody was raised against a peptide sequence and affinity purified against the same sequence on a peptide-coated Sulphadex column (Perbio Science UK Limited). All affinity purifications were checked by enzyme-linked immunosorbent assay (ELISA) for recognition of the peptide and of the recombinant protein. The antiserum was characterized by Western blot and immunohistochemistry on COS-cells transfected with TLR4. In Western blot, the band was lost on preabsorbtion with the peptide at a 100-fold excess. The antibody did not recognize TLR1- or TLR2-transfected COS cells by either of the 2 above methods.
Flow cytometry
Cells were incubated with saturating amounts of anti-TLR4 mAb (HTA125) or anti-CD14 mAb (UCH-M1; Santa Cruz Biotechnology, Santa Cruz, CA) or the isotype-matched nonbinding control mAb UPC10 (Sigma, St Louis, MO), followed by fluorescein isothiocyanate (FITC)–conjugated goat antimouse secondary reagent (Southern Biotechnology Associates, Birmingham, AL). In addition, experiments were also performed by incubating cells with saturating amounts of rabbit anti-TLR4 polyclonal Ab or rabbit IgG as a control, followed by FITC-conjugated goat antirabbit secondary reagent (Vector Laboratories, Burlingame, CA). Staining was performed in the presence of 100 μg/mL nonimmune human IgG to block nonspecific binding to FCγR. Cells were analyzed on a FACSCalibur (Becton Dickinson, Mountain View, CA).
Northern blot analysis
Total RNA was isolated by the guanidine isothiocyanate method with minor modifications.25 Total RNA (10 μg) was analyzed by electrophoresis through 1% agarose/formaldehyde gels, followed by Northern blot transfer to Gene Screen Plus membranes (New England Nuclear, Boston, MA). The plasmids were labeled with α-[32P]dCTP (3000 Ci/mmol [111 TBq/mmol]; Amersham, Buckinghamshire, United Kingdom). Membranes were pretreated and hybridized in 50% formamide (Merck, Rahway, NJ) with 10% dextran sulfate (Sigma), 1% sodium dodecyl sulfate (SDS; Merck), 1 M NaCl, and 100 μg/mL salmon sperm DNA at 42°C, washed twice with 2XSSC (1XSSC; 0.15 M NaCl; 0.015 M sodium citrate), and 1% SDS at 60°C for 30 minutes, and finally repeatedly washed with 0.1XSSC at room temperature. Membranes were exposed for 4 to 48 hours at −80°C with intensifying screens. RNA transfer to membranes was checked by UV irradiation, as shown in each figure. TLR4, MyD88, and MD-2 plasmids have been previously described.18 27 Densitometric analysis was performed with an AIS Image Analyzer (Imaging Research, Ontario, ON, Canada).
Electromobility shift assay analysis
Nuclear proteins were prepared as follows: 106 cells per sample were resuspended in 300 μL in buffer A (lysis buffer) (50 mM KCl, 0.5% Nonidet P-40, 25 mM HEPES pH 7.8, 1 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg/mL leupeptin, 20 μg/mL aprotinin, 100 μM dithiothreitol [DTT]), and subsequently incubated for 5 minutes in ice. Cells were collected by centrifugation at 2000 rpm, and the supernatant was decanted. The nuclei were washed in buffer A without Nonidet P-40, collected at 2000 rpm, resuspended in 25 μL buffer B (extraction buffer) (500 mM KCl, 25 mM HEPES pH 7.8, 10% glycerol, 1 mM PMSF, 10 μg/mL leupeptin, 20 μg/mL aprotinin, 100 μM DTT), and kept on ice for 5 minutes. The samples were subsequently frozen and thawed (twice) by dry ice and 37°C water bath, rotated 20 minutes at 4°C, and centrifuged at 14 000 rpm for 20 minutes. The clear supernatant was collected and the proteins were dialyzed for 4 hours against buffer C (dialysis buffer) (50 mM KCl, 25 mM HEPES pH 7.8, 10% glycerol, 1 mM PMSF, 10 μg/mL leupeptin, 20 μg/mL aprotinin, and 100 μM DTT). Equal amounts of nuclear proteins were incubated with radiolabeled DNA probe in a 20-μL reaction mixture containing 20 mM Tris (pH 7.5), 60 mM KCl, 2 mM ethylenediaminetetraacetic acid (EDTA), 0.5 mM DTT, 1 μg of poly(dI-dC), and 4% Ficoll. Nucleoprotein complexes were resolved by electrophoresis on 5% nondenaturing polyacrylamide gels in 0.5X Tris-borate–EDTA buffer at 12 V/cm for 2 hours at room temperature. Dried gels were exposed to Kodak XAR-5 film (Eastman) at −80°C with intensifying screens. Oligonucleotides were purchased from M-Medical (Florence, Italy) and were end-labeled using Klenow enzyme and α-[32P]deoxycytidine 5′-triphosphate: approximately 1 ng labeled DNA was used in a standard electrophoretic mobility shift assay (EMSA) reaction. Oligonucleotide sequences used in EMSA are from the Ig-kB site and are reported here: 5′-TGACAGAGGGGACTTTCCGAGAG-3′; 3′-CTCCCCTGAAAGGCTCTCCTAGT-5′. The NF-kB motif is underlined.
Western blot and interleukin-1 receptor–associated kinase assay
Fresh human monocytes (20 × 106 cells/sample) were preincubated overnight with IFNγ (500 U/mL) or with medium alone and then stimulated with 10 ng/mL LPS (45 minutes). Cells were then lysed in 1 mL lysis buffer (0.5% Nonidet P-40, 10% glycerol, 50 mM HEPES pH 7.9, 250 mM NaCl, 20 mM glycerophosphate, 5 mM p-nitrophenylphosphate, 1 mM EDTA, 1 mM Na orthovanadate, 5 mM dithioerythrol, 1X complete protease inhibitors [Roche]). IRAK was immunoprecipitated using 1 μg/sample anti-IRAK mAb, a kind gift of Tularik (San Francisco, CA). In vitro kinase assay was performed as described.24,28 29 Briefly, immunoprecipitated IRAK was collected and washed twice in kinase buffer (20 mM HEPES pH 6.5, 150 mM NaCl, 5 mM MgCl2, 5 mM MnCl2) and then incubated for 40 minutes in 30 μL of kinase buffer supplemented with 1 μCi (37 Bq) of γ-[32P]ATP per sample (Amersham Pharmacia Biotech) at 37°C. The reaction was stopped by addition of 3X Laemmli buffer followed by heating at 95°C for 10 minutes. Samples were resolved on a 7% SDS–polyacrylamide gel electrophoresis (PAGE) gel, after which the gel was dried and subjected to autoradiography at −80°C with intensifying screens. For Western blot, 100 μg/sample of total proteins was resolved by 10% SDS-PAGE gel, transferred to a nitrocellulose membrane (Schleicher and Schuell, Dassel, Germany), and blotted with the indicated antibody in TBS 5% nonfat milk, 0.05% Tween 20. Specific bands were detected using horseradish peroxidase (HRP)–labeled secondary reagents (Amersham Life Science) and the enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech).
ELISA
Human TNFα was detected using a sandwich ELISA as previously described.30 Determination of human IL-12 supernatants was conducted by using an ELISA kit purchased from Endogen (Woburn, MA).
Results
Effect of IFNγ on TLR4 expression
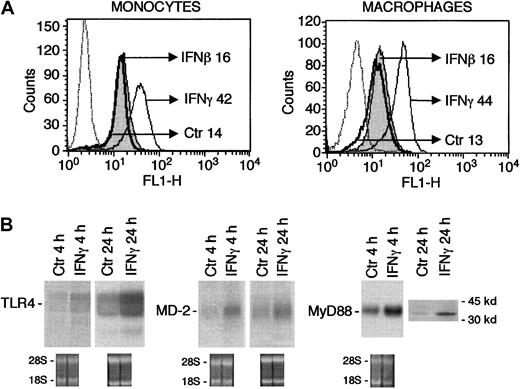
TLR4 expression was examined in human mononuclear phagocytes by Northern analysis and flow cytometry using a specific mAb and a rabbit antiserum with similar results. As shown in Figure1A, exposure of human monocytes for 24 hours to 500 U/mL IFNγ dramatically increased the surface expression of TLR4 with a mean channel of fluorescence (MCF) of 42 compared with 14 for cells cultured with medium alone. In a series of 7 experiments, IFNγ caused a 4.3-fold (± 1.3 SD) increase of MCF values for TLR4. In contrast, IFNβ (Figure 1A) and IFNα (not shown) had little effect. IFNγ was also effective in augmenting TLR4 expression in monocyte-derived mature macrophages (Figure 1A). IFNγ-induced augmented surface TLR4 was associated with increased steady-state levels of mRNA transcripts at 4 and 24 hours (Figure 1B). In a series of 5 experiments, densitometric analysis showed that IFNγ caused a 2.3-fold (± 0.5 SD) increase in transcript levels at 4 hours.
Stimulation of TLR4 expression in human mononuclear phagocytes by IFNγ.
(A) TLR4 surface expression in human monocytes and macrophages exposed to IFNγ or IFNβ (500 U/mL, 24 hours) compared with cells treated with medium alone (Ctr). MCF is indicated for each treatment. Dotted line indicates irrelevant control mAb. (B) Human monocytes were treated with IFNγ (500 U/mL) or with medium alone (Ctr) for 4 or 24 hours and analyzed for their TLR4, MD-2, and MyD88 mRNA content by Northern blotting. The lower part of the panel shows the ethidium bromide staining, after RNA transfer to the membrane. Western analysis is also shown for MyD88 (IFNγ, 500 U/mL, 24 hours).
Stimulation of TLR4 expression in human mononuclear phagocytes by IFNγ.
(A) TLR4 surface expression in human monocytes and macrophages exposed to IFNγ or IFNβ (500 U/mL, 24 hours) compared with cells treated with medium alone (Ctr). MCF is indicated for each treatment. Dotted line indicates irrelevant control mAb. (B) Human monocytes were treated with IFNγ (500 U/mL) or with medium alone (Ctr) for 4 or 24 hours and analyzed for their TLR4, MD-2, and MyD88 mRNA content by Northern blotting. The lower part of the panel shows the ethidium bromide staining, after RNA transfer to the membrane. Western analysis is also shown for MyD88 (IFNγ, 500 U/mL, 24 hours).
The LPS receptor complex involves, in addition to TLR4, CD14 and MD-2.31,27 The adapter protein MyD88 bridges the receptor complex to downstream signaling events.18 19 As shown in Figure 1B, IFNγ increased the expression of MD-2 (mRNA) and MyD88 (mRNA and protein) at 4 and 24 hours, whereas surface CD14 was not affected (not shown).
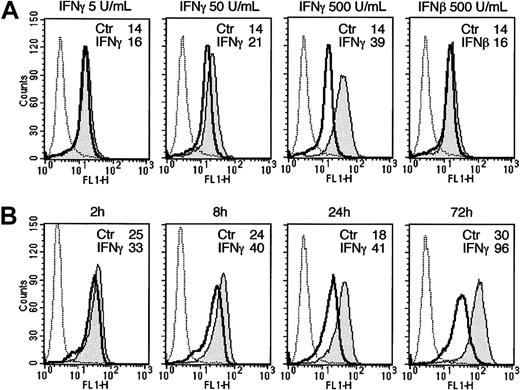
The IFNγ-mediated up-regulation of TLR4 was time- and dose-dependent: it was barely detectable at doses as low as 5 U/mL and peaked at 500 U/mL (Figure 2A); the level of the receptor was already slightly increased after 2 hours of treatment, reached a plateau at 24 hours, and was sustained throughout the 72-hour observation period (Figure 2B).
Dose- and time-dependent stimulation of TLR4 in human monocytes.
Human monocytes were incubated with increasing amounts of IFNγ or IFNβ at 500 U/mL for 24 hours (A) or with IFNγ at 500 U/mL for different periods of time (B) and analyzed for TLR4 surface expression by flow cytometry. MCF is indicated for each treatment. Dotted line indicates irrelevant control mAb.
Dose- and time-dependent stimulation of TLR4 in human monocytes.
Human monocytes were incubated with increasing amounts of IFNγ or IFNβ at 500 U/mL for 24 hours (A) or with IFNγ at 500 U/mL for different periods of time (B) and analyzed for TLR4 surface expression by flow cytometry. MCF is indicated for each treatment. Dotted line indicates irrelevant control mAb.
We tested a series of cytokines (eg, IL-1, TNFα, MCP-1) and other stimuli active on phagocytes for augmentation of TLR4 surface expression by flow cytometry. IFNγ was unique among 8 agents tested in its ability to markedly increase surface TLR4 on monocytes (data not shown).
Effect of LPS and IFNγ on TLR4
In agreement with our original observations,18 22 LPS increased TLR4 mRNA levels in human monocytes at 4 and 24 hours (insert in Figure 3A). In a series of 5 experiments, LPS at 100 ng/mL caused a 2.82-fold (± 0.76 SD) increase in TLR4 transcripts at 4 hours as assessed by densitometry. However, in contrast to transcript expression, LPS caused a reduction in TLR4 surface levels with MCFs of 15 and 7 for control and LPS-treated monocytes, respectively, in the experiment shown in Figure3A. In a series of 7 experiments, LPS caused a decrease of TLR4 MCF values to 58 ± 18% of control. The effect of LPS was observed with both an anti-TLR4 mAb and a polyclonal antiserum, and was time-dependent in that it was observed between 4 and 24 hours. Exposure to CHX did not substantially affect surface levels of TLR4 (Figure 3B). However, when CHX was combined with LPS, it dramatically amplified the LPS-induced down-regulation of surface TLR4 (Figure 3B).
Divergent effects of LPS on TLR4 mRNA and surface protein levels.
(A) Surface expression of TLR4 in human monocytes exposed to LPS (100 ng/mL) or medium alone (Ctr) for 24 hours. The insert shows TLR4 mRNA levels after 4 hours of treatment (see also references 20 and 24). (B) TLR4 surface expression in human monocytes exposed for 4 hours to LPS (100 ng/mL) or CHX (10 μg/mL), or to a combination of the 2. (C) TLR4 surface expression in human monocytes exposed for 24 hours to the combination of IFNγ (500 U/mL) and LPS (100 ng/mL). MCF is indicated for each treatment. Dotted line indicates irrelevant control mAb.
Divergent effects of LPS on TLR4 mRNA and surface protein levels.
(A) Surface expression of TLR4 in human monocytes exposed to LPS (100 ng/mL) or medium alone (Ctr) for 24 hours. The insert shows TLR4 mRNA levels after 4 hours of treatment (see also references 20 and 24). (B) TLR4 surface expression in human monocytes exposed for 4 hours to LPS (100 ng/mL) or CHX (10 μg/mL), or to a combination of the 2. (C) TLR4 surface expression in human monocytes exposed for 24 hours to the combination of IFNγ (500 U/mL) and LPS (100 ng/mL). MCF is indicated for each treatment. Dotted line indicates irrelevant control mAb.
The effect of combinations of IFNγ and LPS was then studied. As shown in Figure 3C, IFNγ counteracted the LPS-induced down-regulation of surface TLR4 at 24 hours, with MCFs of 8, 4, 56, and 15 for control, LPS, IFNγ, and IFNγ+LPS treated cells, respectively. In a series of 3 experiments, IFNγ cotreatment caused a 3.4-fold (± 1.1 SD) increase of TLR4 levels compared with LPS alone.
Priming by IFNγ for responsiveness to LPS
The results discussed above suggested that IFNγ primes mononuclear phagocytes to respond more efficiently to LPS by upregulating crucial components of the LPS signaling receptor complex (TLR4, MD-2, MyD88). It was therefore important to investigate downstream events under these conditions.
Activation of IRAK is the first event downstream of MyD88 recruitment in the TLR4 signaling pathway.18,19 As shown in Figure4A (one experiment of 2 performed), LPS-induced IRAK phosphorylation was markedly increased in IFNγ-primed cells as assessed on the basis of the relative intensity of the shifted band.28,29 37
IFNγ priming for signaling and responsiveness to LPS.
Human fresh monocytes were preincubated overnight with IFNγ or IFNα at 500 U/mL or with medium alone and then exposed to different concentrations of LPS as indicated in each lane. (A) After a treatment with LPS (10 ng/mL) for 45 minutes, cells were lysed and IRAK activation was determined. The figure shows autophosphorylation of IRAK (p-IRAK). (B) Cells were lysed after a 2-hour LPS treatment and nuclear extracts analyzed for NF-kB DNA binding activity by EMSA. CP: the 100 ng/mL LPS-treated extract in lane 6 was also incubated with32P-labeled NF-kB probe plus a 100-fold excess unlabeled NF-kB probe. (C) After exposure to LPS (100 ng/mL) for 6 hours, supernatants were analyzed for secreted TNFα (gray columns, i) and IL-12 (filled columns, ii). Error bars represent SDs of triplicate samples. Similar results were obtained in monocyte-derived macrophages.
IFNγ priming for signaling and responsiveness to LPS.
Human fresh monocytes were preincubated overnight with IFNγ or IFNα at 500 U/mL or with medium alone and then exposed to different concentrations of LPS as indicated in each lane. (A) After a treatment with LPS (10 ng/mL) for 45 minutes, cells were lysed and IRAK activation was determined. The figure shows autophosphorylation of IRAK (p-IRAK). (B) Cells were lysed after a 2-hour LPS treatment and nuclear extracts analyzed for NF-kB DNA binding activity by EMSA. CP: the 100 ng/mL LPS-treated extract in lane 6 was also incubated with32P-labeled NF-kB probe plus a 100-fold excess unlabeled NF-kB probe. (C) After exposure to LPS (100 ng/mL) for 6 hours, supernatants were analyzed for secreted TNFα (gray columns, i) and IL-12 (filled columns, ii). Error bars represent SDs of triplicate samples. Similar results were obtained in monocyte-derived macrophages.
NF-kB activation was then studied. As shown in Figure 4B, IFNγ dramatically (10-fold to 100-fold) augmented the sensitivity of monocytes to LPS. IFNγ-primed monocytes showed a strong response to 0.01 ng/mL LPS, a concentration inactive on resting cells which required 0.1 ng/mL to 1 ng/mL. The NF-kB DNA binding activity of IFNγ-primed monocytes was higher also at higher LPS concentrations, as assessed by densitometry.
Finally, as expected, priming with IFNγ dramatically increased the production of TNFα and IL-12 (Figure 4C, one representative experiment of 6 performed).
Discussion
The results presented here show that IFNγ augments mRNA and surface expression of TLR4, a crucial component of the LPS signaling receptor complex. Concomitantly, IFNγ also increased expression of the accessory component MD-2, which associates with TLR4 on the cell surface and may be a link between LPS and TLR4 itself.27Similarly, expression of the adapter protein MyD88 was also enhanced. CD14 is present in biologic fluids as well as on the cell surface. In membrane bound or soluble form recognizes LPS and is part of the LPS receptor complex: this presumably nonrate limiting molecule is not affected by IFNγ. Therefore, by up-regulating TLR4, MyD88, and possibly MD-2, IFNγ prepares mononuclear phagocytes for recognition of, and activation by, pathogens.
The effect of IFNγ on responsiveness to microbial moyeties such as LPS is dramatic,1-4 as illustrated in this study by NF-kB activation and cytokine production (Figure 4). Mechanisms other than up-regulation of crucial components of the LPS signaling receptor complex may play a role in up-regulation of responsiveness by IFNγ.
The human TLR4 gene has been cloned and its 5′-proximal promoter characterized.32 The transcription factors PU.1 and interferon consensus sequence-binding protein (ICS-BP) were found to participate in the basal expression of TLR4 in macrophages.32 In that study, IFNγ only slightly affected TLR4 expression by reverse transcriptase–polymerase chain reaction (RT-PCR) and the ICS-BP–PU.1 complex. Moreover, IFNγ did not significantly affect the activity of this 5′-proximal fragment.32 The specific cellular context (THP-1 cells) and fragment utilized may explain the apparent discrepancy with the strong TLR4 induction observed in primary monocytes and macrophages in the present study.
The effect of LPS on TLR4 has been the object of seemingly conflicting results.18,22,33,34 We and others have repeatedly found over time that LPS up-regulates TLR4 steady-state transcripts in human monocytes and neutrophils.18,22,24 In contrast, in the mouse LPS was found to decrease TLR4 levels.33,34 In the present study focused on human mononuclear phagocytes, we observed that LPS has divergent effects on TLR4 at the mRNA and surface protein levels, with up-regulation of the former and down-regulation of the latter. It has been recently described that LPS is internalized and transported to the Golgi apparatus.35 36 Therefore, up-regulation of transcript expression may represent a compensatory mechanism to partially counteract ligand-induced TLR4 down-regulation. Consistently with this view, inhibition of protein synthesis by CHX increased the LPS-induced reduction of surface TLR4.
The results reported here show that IFNγ primes mononuclear phagocytes to encounter with pathogens by up-regulating TLR4, MyD88, and possibly MD-2. As expected on the basis of these observations, IFNγ-primed monocytes exposed to LPS showed enhanced phosphorylation of IRAK, the first element downstream of MyD88,18,19,20,37 38 and increased NF-kB DNA binding activity. Accordingly, cytokine (TNFα and IL-12) induction was dramatically increased in IFNγ-primed monocytes.
Hence, these observations of IFNγ-mediated enhancement of TLR4 provide a molecular basis for the long-known1-4 priming and synergistic activity of IFNγ in the responsiveness of mononuclear phagocytes to microbial moyeties, LPS in particular.
Supported by European Commission grant QLG1-CT-1999-00549, MURST, Istituto Superiore di Sanita' programma nazionale ricerche AIDS, and Associazione Italiana per la Ricerca sul Cancro (AIRC). D.B. is supported by a fellowship from Fondazione Italiana per la Ricerca sul Cancro (FIRC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alberto Mantovani, Department of Immunology and Cell Biology, Mario Negri Institute, via Eritrea 62, Milano, I-20157, Italy; e-mail: mantovani@marionegri.it.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal