Key Points
In the final analysis of RESONATE-2, survival and PFS benefits of ibrutinib treatment were sustained, with median PFS reached at 8.9 years.
Ibrutinib dose modifications were effective in mitigating AEs in most patients, facilitating long-term ibrutinib use.
Visual Abstract
With up to 10 years of follow-up, we report results from the final analysis of RESONATE- 2, a phase 3 study of first-line ibrutinib vs chlorambucil for the treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Patients aged ≥65 years with previously untreated CLL/SLL without del(17p) were randomly assigned to receive either single-agent ibrutinib (420 mg/d; n = 136) or chlorambucil (0.5-0.8 mg/kg; ≤12 cycles; n = 133). With a median follow-up of 9.6 years in the ibrutinib arm, the median progression-free survival (PFS) was 8.9 years (95% confidence interval [CI], 7.0 to not estimable [NE]) vs 1.3 years (95% CI, 0.9-1.6) for the chlorambucil arm. Among patients with unmutated immunoglobulin heavy chain variable (uIGHV), del (11q), mutated TP53, or complex karyotype, the median PFS was 8.4 years (95% CI, 6.8 to NE) with ibrutinib and 0.7 years (95% CI, 0.4-1.2) with chlorambucil. Median overall survival (OS) with ibrutinib was not reached. The most common adverse events (AEs) of any grade included diarrhea (52%), fatigue (41%), cough (39%), nausea (32%), arthralgia (31%), peripheral edema (31%), and hypertension (30%). During the entire study period, 34 of 136 patients (25%) had an ibrutinib dose reduction due to AEs; these AEs improved in 30 of 34 patients (88%). At study completion, 27% of patients remained on first-line ibrutinib treatment. This landmark RESONATE-2 study defines median PFS and demonstrates continued OS benefit of first-line ibrutinib treatment for patients with CLL/SLL, including those with high-risk genomic features. Sustained efficacy and tolerability of ibrutinib reemphasize the favorable benefit-risk profile. This trial was registered at www.ClinicalTrials.gov as NCT01722487/NCT01724346.
Introduction
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) is the most prevalent form of leukemia among older adults and is characterized by a clonal proliferation of mature B lymphocytes.1 Advanced age and associated comorbidities complicate treatment decisions, often preventing the utilization of aggressive therapies. Additionally, for the subset of patients with prognostic genomic alterations associated with inferior prognosis, such as tumor protein p53 (TP53) aberrations (TP53 mutations or del(17p)), del(11q), and unmutated immunoglobulin heavy chain variable (uIGHV), responses to chemotherapy and chemoimmunotherapy have been suboptimal.2,3
Ibrutinib, a once-daily Bruton tyrosine kinase inhibitor, is a transformative therapy for CLL/SLL and has demonstrated significant clinical benefits over nontargeted therapies.4 Approved globally for both previously untreated and relapsed/refractory CLL/SLL, ibrutinib treatment outcomes have resulted in a shift toward targeted therapies by offering improved progression-free survival (PFS)5-8 and overall survival (OS)5,6,9 compared with traditional chemotherapy and chemoimmunotherapy.
The landmark RESONATE-2 trial (PCYC-1115/PCYC-1116; ClinicalTrials.gov identifier: NCT01722487/NCT01724346) is an international, randomized, phase 3 study evaluating the efficacy and safety of first-line ibrutinib vs chlorambucil in patients with CLL/SLL aged ≥65 years. Initial findings demonstrated significant PFS and OS benefits with ibrutinib compared with chlorambucil in all patients, including those with high-risk genomic aberrations.10 These findings persisted across extended follow-up; for example, at 7 years, PFS was 59% with ibrutinib vs 9% with chlorambucil for all randomly assigned patients; 52% vs 0% for those with del(11q); and 58% vs 2% for those with uIGHV, respectively.11 Ibrutinib also demonstrated significant OS benefit vs chlorambucil (83% vs 68% at 5 years of follow-up); per study protocol, after 5 years, OS follow-up continued for the ibrutinib arm only and was 78% at year 7.5,11 Similar to the rates observed in the intent-to-treat population, OS rates at 5 years in patients with high-risk genomic aberrations (TP53 mutation, del(11q), and/or uIGHV) were higher in the ibrutinib arm (84%) than in the chlorambucil arm (62%).5 The long-term continued use and efficacy of ibrutinib made evaluation of ibrutinib's performance crucial to understanding its role in managing CLL/SLL in these older adults.
Here, we present results from the final analysis of the RESONATE-2 trial, encompassing up to 10 years of follow-up, with the median PFS for ibrutinib treatment having finally been reached. We provide comprehensive insights into the long-term efficacy and safety of ibrutinib treatment, with a particular focus on survival outcomes and the management of adverse events (AEs) in an older patient cohort, including those with high-risk genomic features. This analysis not only reemphasizes the pivotal role of ibrutinib in first-line CLL/SLL treatment, but also informs clinical practice on long-term therapeutic strategies for older adults with CLL/SLL.
Methods
RESONATE-2 is an open-label, multicenter, international, randomized phase 3 study comparing the efficacy and safety of ibrutinib vs chlorambucil in patients with previously untreated CLL/SLL. Details of RESONATE-2 have been previously described.10 Briefly, patients aged ≥65 years with previously untreated CLL/SLL without del(17p) were randomly assigned 1:1 to receive either (1) once-daily single-agent ibrutinib at 420 mg/d until progressive disease (PD) or unacceptable toxicity or (2) single-agent chlorambucil for up to 12 cycles, ranging from 0.5 mg/kg to 0.8 mg/kg, depending on tolerability. After confirmed PD, patients assigned to the chlorambucil arm were eligible for crossover to second-line treatment with ibrutinib. After a median follow-up of 5 years, the study protocol was amended to include up to 10 years of follow-up to assess PFS in both arms and OS in the ibrutinib arm only. Subsequently, patients with PD in the chlorambucil arm were exited from the study, and those who had crossed over to next-line ibrutinib had the opportunity to enroll in a long-term ibrutinib safety study.
This study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice and was approved by the institutional review boards of participating institutions. All patients provided written informed consent.
Abnormalities in chromosomes 11q, 12, 13q, and 17p were detected using standard CLL fluorescence in situ hybridization probes on either a bone marrow biopsy or a peripheral blood sample collected at baseline. Deletion of the short arm of chromosome 17, del(17p13.1), was defined as del(17p) in >20% of cells examined on any pretreatment fluorescence in situ hybridization or cytogenetic evaluation. Complex karyotype (CK), defined as ≥3 chromosomal aberrations, was performed by local laboratories and reported by the investigator according to the International System for Human Cytogenetic Nomenclature 2009. Mutational status of IGHV was determined using blood samples collected at baseline. DNA and RNA isolated from peripheral blood mononuclear cells were used to assess TP53 mutations using the FoundationOne Heme next-generation sequencing assay, performed centrally per European Research Initiative on Chronic Lymphocytic Leukemia (ERIC) guidelines.12
End points included PFS, OS, overall response rate (ORR), improvement in hematologic parameters, and safety. Responses were assessed per International Workshop on CLL 2008 criteria,13 as modified to allow for isolated treatment-related lymphocytosis, based on history, physical examination, laboratory testing, radiographic imaging, and bone marrow assessments (to confirm complete responses [CRs]), as previously described.10 Long-term response follow-up was based on investigator assessments. PFS and OS were analyzed according to the Kaplan-Meier method. Hazard ratios (HRs) were estimated using a stratified Cox regression model. Long-term safety data are reported for patients receiving ongoing treatment in the ibrutinib arm only; chlorambucil safety data have previously been reported.5
Results
Patients
A total of 269 patients were randomly assigned to receive single-agent ibrutinib (n = 136) or chlorambucil (n = 133) in the RESONATE-2 study (supplemental Figure 1, available on the Blood website). As previously described, patient baseline demographic and clinical characteristics were well balanced between the 2 arms (supplemental Table 1). Among patients with data available in the ibrutinib arm, 29 of 130 patients (22%) had del(11q), 58 of 98 patients (59%) had uIGHV, 11 of 130 patients (8%) had a TP53 mutation, and 6 of 93 patients (6%) had CK. Fewer patients in the chlorambucil arm had a TP53 mutation (3/95 [3%]), but risk characteristics were otherwise well balanced.
At study closure, with a median follow-up of 9.6 years (range, 0.0-10.2), ibrutinib treatment was ongoing in 37 patients (27%), and 98 patients (73%) had discontinued treatment (Table 1).
Duration of ibrutinib treatment
| . | Ibrutinib, n = 135∗ . |
|---|---|
| Duration of ibrutinib treatment, median (range), y | 6.2 (0.06-10.2) |
| Continuing ibrutinib at study closure, n (%) | 37 (27) |
| Reason for ibrutinib discontinuation, n (%) | |
| AE | 44 (33) |
| PD | 18 (13) |
| Death | 12 (9) |
| Withdrawal of consent for treatment by patient | 13 (10) |
| Investigator decision | 11 (8) |
| . | Ibrutinib, n = 135∗ . |
|---|---|
| Duration of ibrutinib treatment, median (range), y | 6.2 (0.06-10.2) |
| Continuing ibrutinib at study closure, n (%) | 37 (27) |
| Reason for ibrutinib discontinuation, n (%) | |
| AE | 44 (33) |
| PD | 18 (13) |
| Death | 12 (9) |
| Withdrawal of consent for treatment by patient | 13 (10) |
| Investigator decision | 11 (8) |
One additional patient was randomly assigned to the ibrutinib arm but did not receive any doses of ibrutinib.
Efficacy
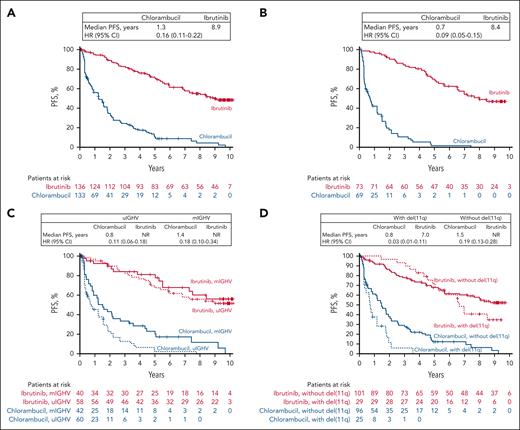
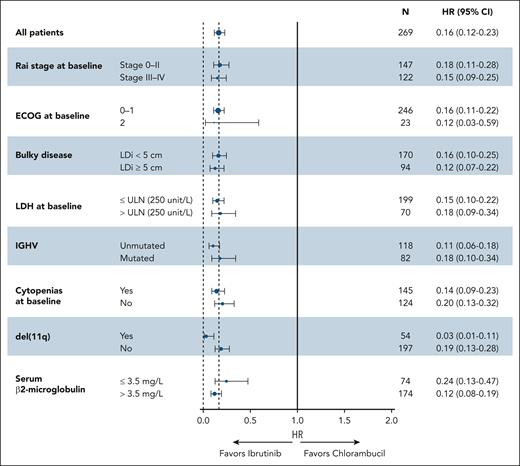
At the final analysis, the median PFS with ibrutinib was reached and was 8.9 years (95% confidence interval [CI], 7.0 to not estimable [NE]) vs 1.3 years (95% CI, 0.9-1.6) with chlorambucil. (Figure 1A). At 9 years, the PFS rate among all patients assigned to ibrutinib treatment was 50% (95% CI, 40-58). The PFS benefit observed with ibrutinib was sustained in subgroup analyses based on baseline clinical characteristics at the time of treatment randomization, including Rai stage, Eastern Cooperative Oncology Group performance status score, bulky disease, lactase dehydrogenase levels, IGHV mutational status, cytopenias, del(11q), and serum β2-microglobulin levels (Figure 2).
Investigator-assessed PFS. (A) All randomly assigned patients. (B) Patients with ≥1 high prognostic risk factor including mutated TP53, uIGHV, del(11q), and/or CK. (C) Patients with uIGHV or mIGHV. (D) Patients with or without del(11q). CK, complex karyotype; HR, hazard ratio; mIGHV, mutated immunoglobulin heavy chain variable; NR, not reached; PFS, progression-free survival; uIGHV, unmutated immunoglobulin heavy chain variable.
Investigator-assessed PFS. (A) All randomly assigned patients. (B) Patients with ≥1 high prognostic risk factor including mutated TP53, uIGHV, del(11q), and/or CK. (C) Patients with uIGHV or mIGHV. (D) Patients with or without del(11q). CK, complex karyotype; HR, hazard ratio; mIGHV, mutated immunoglobulin heavy chain variable; NR, not reached; PFS, progression-free survival; uIGHV, unmutated immunoglobulin heavy chain variable.
PFS by baseline characteristic subgroups. ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; IGHV, immunoglobulin heavy chain variable; LDH, lactate dehydrogenase; LDi, longest diameter; PFS, progression-free survival; ULN, upper limit of normal.
PFS by baseline characteristic subgroups. ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; IGHV, immunoglobulin heavy chain variable; LDH, lactate dehydrogenase; LDi, longest diameter; PFS, progression-free survival; ULN, upper limit of normal.
In patients with ≥1 high prognostic risk factor, including mutated TP53, uIGHV, del(11q), and/or CK, the median PFS for patients treated with ibrutinib was 8.4 years (95% CI, 6.8 to NE) and 0.7 years (95% CI, 0.4-1.2) with chlorambucil (Figure 1B); 9-year PFS rate in the ibrutinib arm was 47% (95% CI, 34-58).
Examined individually, IGHV mutational status was available in a total of 200 patients in this analysis (ibrutinib, n = 98; chlorambucil, n = 102). Ibrutinib provided significantly longer median PFS than chlorambucil in both patients with uIGHV (median PFS, not reached [95% CI, 5.5 years to NE] vs 0.8 years [95% CI, 0.4-1.5]) and mIGHV (mutated immunoglobulin heavy chain variable; median PFS, not reached [95% CI, 5.5 years to NE] vs 1.4 years [95% CI, 0.8-2.2]; Figure 1C). At 9 years, PFS rates for ibrutinib were 51% (95% CI, 37-64) and 56% (95% CI, 37-72) with uIGHV and mIGHV, respectively. The median PFS was significantly longer with ibrutinib among patients with del(11q) (median PFS, 7.0 years [95% CI, 5.3 to NE] vs 0.8 years [95% CI, 0.3-1.2] for chlorambucil) and without del(11q) (median PFS, not reached [95% CI, 7.0 years to NE] vs 1.5 years [95% CI, 1.2-2.0]; Figure 1D). At 9 years, PFS rates with ibrutinib were 35% (95% CI, 17-53) and 54% (95% CI, 43-64) with and without del(11q), respectively.
Exploratory univariate analysis of PFS with ibrutinib treatment showed no significant associations for the presence vs absence of the following baseline high-risk factors: uIGHV (HR, 1.17; 95% CI, 0.61-2.25), bulky disease (HR, 1.35; 95% CI, 0.81-2.25), serum β2-microglobulin ≥3.5 mg/L (HR, 1.20; 95% CI, 0.67-2.14), and mutated TP53/CK (HR, 0.97; 95% CI, 0.43-2.19; supplemental Figure 2). Due to the crossing of the Kaplan-Meier curves for ibrutinib-treated patients with vs without del(11q) (Figure 1D), the proportional hazards assumption is violated, and therefore, the univariate HR of 1.30 (95% CI, 0.74-2.29) should be interpreted with caution (supplemental Figure 2).
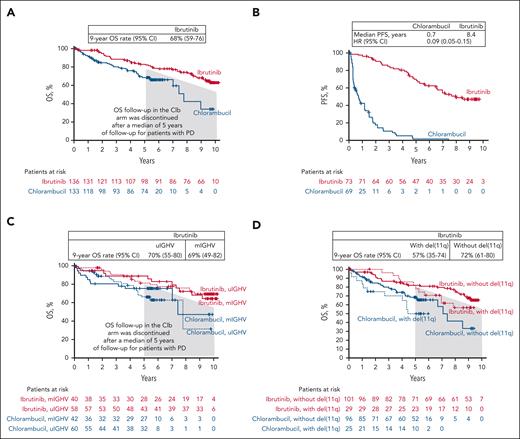
With up to 10 years of follow-up, the median OS was not reached in the ibrutinib arm (range, 0.10-122.8 months); the OS rate at 9 years was 68% (95% CI, 59-76; Figure 3A). In patients with at least 1 high-risk genomic factor, including mutated TP53/uIGHV/del(11q)/CK, who received ibrutinib, the median OS was also not reached, and the 9-year OS rate was 66% (95% CI, 53-76; Figure 3B). For those with IGHV mutational testing results available, the 9-year OS rates were 70% (95% CI, 55-80) and 69% (95% CI, 49-82) for those with uIGHV and mIGHV in the ibrutinib arm, respectively (Figure 3C). For patients with and without del(11q), the 9-year OS rates were 57% (95% CI, 35-74) and 72% (95% CI, 61-80), respectively (Figure 3D).
Overall survival (OS). (A) All randomized patients. (B) Patients with ≥1 high prognostic risk factor including mutated TP53, uIGHV, del(11q), and/or CK. (C) Patients with uIGHV or mIGHV. (D) Patients with or without del(11q). CK, complex karyotype; Clb, chlorambucil; mIGHV, mutated immunoglobulin heavy chain variable; PD, progressive disease; uIGHV, unmutated immunoglobulin heavy chain variable.
Overall survival (OS). (A) All randomized patients. (B) Patients with ≥1 high prognostic risk factor including mutated TP53, uIGHV, del(11q), and/or CK. (C) Patients with uIGHV or mIGHV. (D) Patients with or without del(11q). CK, complex karyotype; Clb, chlorambucil; mIGHV, mutated immunoglobulin heavy chain variable; PD, progressive disease; uIGHV, unmutated immunoglobulin heavy chain variable.
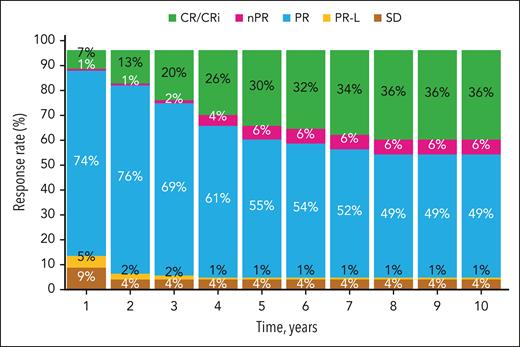
Over the study duration, the proportion of patients in the ibrutinib arm with a best response of CR or CR with incomplete bone marrow recovery (CRi) continued to increase during the first 7 years (Figure 4). During years 8 to 10, the cumulative CR/CRi rate remained stable at 36%. The median duration of CR was not reached (range, 0.0-9.5 years). Consistent with data from previous follow-up, the ORR was 91% in the ibrutinib arm and 37% in the chlorambucil arm.
Change in investigator-assessed responses over time. CR, complete response; CRi, complete response with incomplete bone marrow recovery; nPR, nodular partial response; PR, partial response; PRL, partial response with lymphocytosis; SD, stable disease.
Change in investigator-assessed responses over time. CR, complete response; CRi, complete response with incomplete bone marrow recovery; nPR, nodular partial response; PR, partial response; PRL, partial response with lymphocytosis; SD, stable disease.
Safety
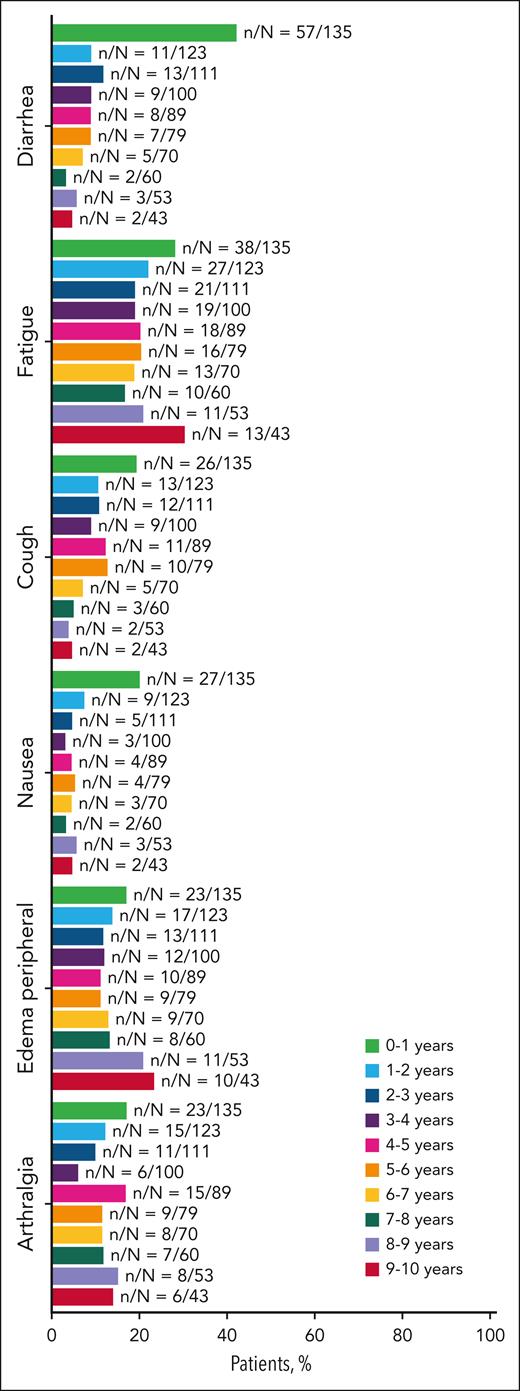
At the time of final analysis, the median duration of ibrutinib treatment was 6.2 years (range, 0.1-10.2). The most frequent AEs of any grade were diarrhea (n = 70 [52%]), fatigue (n = 55 [41%]), cough (n = 53 [39%]), and nausea (n = 43 [32%]); the prevalence of AEs over time is shown in Figure 5.
Summary of adverse events for patients treated with ibrutinib. Adverse events with prevalence ≥30% in all patients, excluding hypertension, are shown.
Summary of adverse events for patients treated with ibrutinib. Adverse events with prevalence ≥30% in all patients, excluding hypertension, are shown.
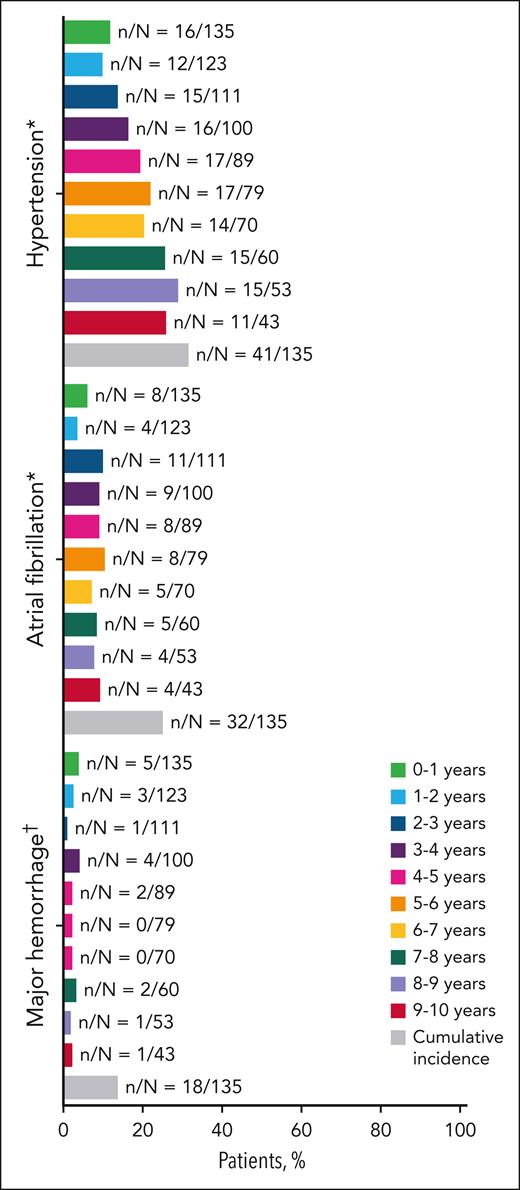
The prevalence of ibrutinib AEs of clinical interest during the additional follow-up years 8 to 9 and 9 to 10 was 28% and 26% for hypertension, 8% and 9% for atrial fibrillation, and 2% and 2% for major hemorrhage, respectively (Figure 6). In years 8 to 9 and 9 to 10, up to 21% and 28% of patients experienced grade ≥3 serious AEs, and 4% and 2% of patients experienced treatment-related serious AEs, respectively. The prevalence of AEs leading to death was 6% during years 8 to 9 and 0% during years 9 to 10. Overall, 25 AEs leading to death were noted in the ibrutinib arm, including pneumonia, cardiac failure, CLL, and death (supplemental Table 2), of which 2 events were considered related to ibrutinib (pneumonia and cardiac failure). Second malignancies reported during this long-term follow-up included nonmelanoma skin cancers (n = 32 [24%]), melanoma skin cancer (n = 2 [1%]), and nonskin cancers (n = 23 [17%]; supplemental Table 3).
Prevalence of adverse events of clinical interest over time. ∗Hypertension and atrial fibrillation per MedDRA preferred terms. †Major hemorrhage included serious or grade ≥3 hemorrhage, and central nervous system hemorrhage of any grade among bleeding events identified by Hemorrhage Standardized MedDRA Queries excluding laboratory terms.
Prevalence of adverse events of clinical interest over time. ∗Hypertension and atrial fibrillation per MedDRA preferred terms. †Major hemorrhage included serious or grade ≥3 hemorrhage, and central nervous system hemorrhage of any grade among bleeding events identified by Hemorrhage Standardized MedDRA Queries excluding laboratory terms.
COVID-19 treatment-emergent AEs (TEAEs) occurred in 24 patients (18%) receiving active ibrutinib treatment: 8 patients experienced grade 3 to 5 COVID-19 infection (grade 3 and 4, n = 7; grade 5, n = 1). Of these, 2 AEs caused ibrutinib discontinuation, including 1 with a fatal outcome. Both AEs were assessed as not related to ibrutinib by the investigator.
Ibrutinib discontinuations and dose management
With up to 10 years of follow-up, yearly mean ibrutinib dosing decreased from 397 mg/d in year 1 to 373 mg/d in year 10 of the study treatment (year 1 median, 417 mg/d [range, 148-421]; year 10 median, 415 mg/d [range, 122-420]). Over the course of the entire study, dose reductions to 280 mg/d or 140 mg/d (lasting ≥28 days) were used in 22% and 7% of patients, respectively.
A total of 34 patients (25%) had an ibrutinib dose reduction due to AEs at any time during the study. The proportion of patients experiencing an ibrutinib dose reduction due to an AE generally decreased over time, with 1 and 4 patients with AE-related dose reduction in years 8 to 9 and 9 to 10, respectively (supplemental Figure 3). Of the 34 patients who had AEs of any grade leading to dose reduction, 30 patients (88%) had these AEs resolved or resolving at the time of data cutoff.
Eighty-eight patients (65%) had an ibrutinib dose hold of ≥7 days due to AEs. Of those, 70 patients (80%) had all AEs leading to a dose hold resolved or resolving; 56 patients (64%) restarted ibrutinib at the initial dose of 420 mg/d, 23 patients (26%) restarted at a reduced dose, and 9 patients (10%) discontinued ibrutinib.
The reasons for ibrutinib treatment discontinuation were AEs in 44 patients (33%), PD in 18 patients (13%), death in 12 patients (9%), patient withdrawal of consent for treatment in 13 patients (10%), and investigator decision in 11 patients (8%) (Table 1). AEs leading to ibrutinib discontinuation in ≥2 patients are listed in supplemental Table 4, the most common being atrial fibrillation (n = 7) and pneumonia (n = 5). No increasing trend in the rate of ibrutinib discontinuation was observed over time; rates for years 8 to 9 and 9 to 10 were 21% and 12%, respectively (supplemental Figure 4).
Outcomes after ibrutinib discontinuation
Overall, baseline clinical and genomic characteristics of patients remaining on ibrutinib at study closure and those who discontinued ibrutinib earlier were largely similar (supplemental Table 5). Of the 61 PFS events observed in the ibrutinib arm through the final analysis, 33 were progression events. Eighteen of 33 progression events occurred in patients who discontinued ibrutinib due to PD, and 15 of 33 occurred in patients who discontinued ibrutinib due to reasons other than PD or death, either during ibrutinib treatment (n = 2), within 30 days after treatment discontinuation (n = 2), or >30 days after the last dose of ibrutinib (n = 11). At 9 years, the PFS rate was 35% among patients who discontinued ibrutinib due to AEs at any time during the study. Among 68 patients who discontinued ibrutinib at any time during the study due to any reason other than PD or death (including due to AEs), the 9-year PFS rate was 41%, with a median PFS of 8.4 years (95% CI, 5.3 to NE; supplemental Figure 5A). It is notable that 37 of 68 patients either experienced PFS events (n = 10) or stopped response assessments (n = 27) within 30 days of ibrutinib discontinuation. In the remaining 31 of 38 patients, the PFS rate at 12 months after treatment was 56% (95% CI, 36-72; supplemental Figure 5B).
After discontinuation of first-line ibrutinib, 24 patients (18%) were reported to receive 1 of 11 different second-line antineoplastic therapies. Of these, next-line treatments reported in ≥2 patients were venetoclax (n = 5); acalabrutinib (n = 4); bendamustine plus rituximab (n = 3); fludarabine, cyclophosphamide, and rituximab (n = 3); chlorambucil (n = 2); and chlorambucil plus obinutuzumab (n = 2; supplemental Table 6). Responses to the first subsequent systemic therapy for the 15 patients with available data are also included in supplemental Table 6.
Outcomes with next-line ibrutinib crossover treatment in chlorambucil-randomized patients after experiencing PD
A total of 78 patients (59%) in the chlorambucil arm crossed over to next-line ibrutinib treatment after experiencing PD, of whom 36 were on treatment until study arm closure, as described above. Reasons for discontinuation for the other 42 patients included AEs (n = 17), PD (n = 9), death (n = 4), and investigator or patient decision (n = 12). The median duration of next-line ibrutinib treatment was 26.9 months (range, 0.5-83.8). Assessed from the start of next-line ibrutinib treatment, the median PFS was 48.5 months (95% CI, 33.8 to NE). At 4 years after crossing over to next-line ibrutinib therapy, the PFS rate was 53% (95% CI, 39-65), with an ORR of 77%, including 12 patients with CR/CRi (15.4%). The median OS was not reached after crossing over to next-line ibrutinib therapy, and the 4-year OS rate was 68% (95% CI, 55-79). The safety profile of next-line ibrutinib treatment was unremarkable. The most common AEs during crossover ibrutinib treatment were diarrhea (37%), arthralgia (28%), upper respiratory tract infection (26%), cough (24%), and fatigue (24%; supplemental Table 7). AEs led to ibrutinib dose reductions in a total of 15 patients (19%), most commonly due to arthralgia or muscle spasms (n = 3 patients each), followed by diarrhea or fatigue (n = 2 patients each). No AEs leading to discontinuation occurred in more than 1 patient. Nine patients experienced TEAEs leading to death.
Discussion
These data from the RESONATE-2 study provide compelling evidence for the sustained long-term efficacy and safety of ibrutinib as a first-line treatment for patients with CLL/SLL. With its global approvals and use in >320 000 patients worldwide to date,14 ibrutinib has been established as a cornerstone in the management of disease in these patients. With up to 10 years of follow-up, this final analysis offers the most extensive data set available for any Bruton tyrosine kinase inhibitor in the first-line setting and demonstrates long-term value with a median PFS of 8.9 years and a median OS that was not reached with ibrutinib treatment. These findings highlight the durable clinical benefits of ibrutinib across various subgroups of patients, including those with high prognostic risk genomic or clinical factors.
The OS benefit for ibrutinib vs chlorambucil was previously established at the 5-year follow-up (HR, 0.45; 95% CI, 0.27-0.76).5 After a median follow-up of 5 years in a protocol amendment to extend study follow-up to 10 years, OS data were collected for the ibrutinib arm only, and the patients in the chlorambucil arm who had experienced PD were not followed further. In this study, the 9-year OS rate for the ibrutinib arm was 68%, compared with 83% at year 5, emphasizing the long-term survival advantage conferred by ibrutinib, even among patients with high-risk disease features. Moreover, the deepening of responses over time, with 27% of patients remaining on first-line ibrutinib at study closure, further validates the long-term efficacy of ibrutinib.
For patients with ≥1 high-risk genomic feature, such as TP53 mutations, del(11q), uIGHV, or CK, ibrutinib treatment confers a marked improvement in median PFS compared with chlorambucil treatment (median PFS, 8.4 years [95% CI, 6.8 to NE] vs 0.7 years [95% CI, 0.4-1.2] for chlorambucil).11 Examined in individual subgroups using univariate analyses, there were no statistically significant associations between PFS and the presence or absence of these and other risk factors in patients treated with ibrutinib. These findings are particularly important because these genomic features have historically been associated with poor outcomes in patients treated with conventional chemotherapy or chemoimmunotherapy.2,14 Although fixed-duration ibrutinib plus venetoclax, venetoclax plus obinutuzumab, and acalabrutinib plus venetoclax (with or without obinutuzumab) have demonstrated durable efficacy for patients with high-risk genomic features,15-18 the established long-term benefits may make single-agent ibrutinib a preferred treatment option for patients in high-risk populations.
In terms of safety, ibrutinib remains well tolerated, with no new safety signals emerging over the extended follow-up period. The incidence of discontinuations due to AEs was stable over time, highlighting the tolerability of ibrutinib and enabling patients to maximize the clinical benefits of long-term ibrutinib therapy. Furthermore, active dose management strategies, including dose reductions and dose holds, were effective in managing AEs, enabling most patients who underwent dose modifications to resolve AEs and continue treatment.
As demonstrated in the RESONATE study of ibrutinib vs ofatumumab for relapsed/refractory CLL/SLL,19 patients with relapsed/refractory disease after being randomly assignment to chlorambucil treatment experienced durable efficacy and had no new safety signals during second-line crossover ibrutinib treatment.
In conclusion, the results of this final analysis of the RESONATE-2 study reemphasize the role of ibrutinib as a first-line treatment for CLL/SLL, including those with high-risk genomic and clinical features. Its sustained efficacy, coupled with a manageable safety profile achievable through AE mitigation with dose modifications, make ibrutinib a highly effective option for sustaining long-term remission.
Acknowledgments
The authors thank the patients who participated in the study and their supportive families, as well as the investigators and clinical research staff from the study centers. Medical writing support was provided by Agnieszka Looney and Cindi A. Hoover, and was funded by AbbVie.
This study was sponsored by Pharmacyclics LLC, an AbbVie Company.
AbbVie participated in the study design, research, analysis, data collection, and interpretation of data and the review and approval of the manuscript.
Authorship
Contribution: All authors had access to relevant data and participated in the drafting, review, and approval of this publication.
Conflict-of-interest disclosure: J.A.B. received honoraria from Gilead, Janssen, Novartis, Pharmacyclics LLC, an AbbVie Company, and TG Therapeutics; reported a consulting or advisory role with BeiGene, Gilead, Janssen, Pharmacyclics LLC, an AbbVie Company, and TG Therapeutics; received research funding from AstraZeneca, BeiGene, and Pharmacyclics LLC, an AbbVie Company; served on the speakers bureau for BeiGene, Gilead, Janssen, Pharmacyclics LLC, an AbbVie Company, and TG Therapeutics; and received travel, accommodations, and expenses from Gilead, Janssen, Novartis, Pharmacyclics LLC, an AbbVie Company, and TG Therapeutics. P.M.B. reported a consulting or advisory role with AbbVie, AstraZeneca, Bristol Myers Squibb, Celgene, Genentech, Gilead, Janssen, MEI Pharma, Merck, MorphoSys, Seagen, Pharmacyclics LLC, an AbbVie Company, and TG Therapeutics; and received research funding from AstraZeneca and TG Therapeutics. T.R. received honoraria from AstraZeneca, BeiGene, and Janssen; reported a consulting or advisory role with Acerta Pharma, AstraZeneca, BeiGene, and Janssen; and received research funding from Acerta Pharma, AstraZeneca, BeiGene, and Janssen. C.O. received honoraria from AbbVie, AstraZeneca, BeiGene, Gilead, Incyte, Janssen, Merck, and Roche. A.T. reported consulting or advisory role with AbbVie, AstraZeneca, BeiGene, and Janssen; and served on the speakers bureau for AbbVie, AstraZeneca, BeiGene, and Janssen. P.E.M.P. received honoraria from AbbVie, AstraZeneca, BeiGene, and Janssen; reported a consulting or advisory role with AbbVie, AstraZeneca, BeiGene, and Lilly; and received research funding from AstraZeneca and BeiGene. H.M. received honoraria from BeiGene and Janssen; reported a consulting or advisory role with AbbVie and Janssen; and received travel, accommodations, or expenses from Janssen. E.S.-G., C.Z., A. Szoke, J.P.D., and L.N. reported employment with and stock ownership in AbbVie. P.G. received honoraria from AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Galapagos, Janssen, Loxo/Lilly, Merck, and Roche; reported a consulting or advisory role with AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Galapagos, Janssen, Loxo/Lilly, Merck, and Roche; and received research funding from AbbVie, AstraZeneca, Bristol Myers Squibb, and Janssen. T.J.K. received research funding from AbbVie, Breast Cancer Research Foundation, National Institutes of Health, Oncternal Therapeutics, Leukemia and Lymphoma Society, and VelosBio; and received travel or honoraria from AbbVie, Dava Oncology, European Research Initiative on chronic lymphocytic leukemia, Genentech, and Janssen. The remaining authors declare no competing financial interests.
Correspondence: Jan A. Burger, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 368, Houston, TX 77030; email: jaburger@mdanderson.org.
References
Author notes
AbbVie is committed to responsible data sharing regarding the clinical trials it sponsors. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided after review and approval of a research proposal and a statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time after approval in the United States and Europe and after publication of this article. The data will be accessible for 12 months, with possible extensions considered. Requests for access to individual participant data from clinical studies conducted by Pharmacyclics LLC, an AbbVie Company, can be submitted through the Yale Open Data Access Project site at http://yoda.yale.edu.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal