Key Points
Three distinct neutrophil subsets were identified, with immunosuppressive CD10+TREM1+CXCR2+ neutrophils dominating the TME.
CXCR2 blockade suppresses neutrophil activity and enhances antitumor immune responses to standard treatments in MM models.
Visual Abstract
Understanding the roles of myeloid cells in the tumor microenvironment (TME) has emerged as a promising strategy to identify novel targets to counteract the immunosuppressive barriers protecting multiple myeloma (MM). Neutrophils are a new cancer research focus due to their potential to reduce the efficacy of immune-based therapies. This study aimed to deepen understanding of neutrophil function in MM by analyzing freshly isolated myeloid cells from paired focal lesions (FLs) and bone marrow using single-cell RNA sequencing, immunofluorescence imaging, and functional assays. We describe 3 distinct CXCR2+ mature neutrophil subsets: TREM1+CD10+, RETN+LCN2+, and TNFAIP3+CXCL8+, each exhibiting unique phenotypes within the TME. Notably, the TREM1+CD10+ subset was highly prevalent, particularly in FLs, demonstrating potent immunosuppressive effects on T cells. This subset’s gene signature was correlated with shorter overall survival (OS) in a large data set of patients with MM, underscoring its clinical significance. Targeted inhibition of neutrophil activity through CXCR2 blockade, alone or combined with standard anti-MM therapies, significantly reduced tumor burden, improving OS in preclinical MM models. These insights into neutrophil-mediated immunosuppression in MM provide valuable knowledge regarding mechanisms driving immune evasion, and reveal new therapeutic approaches to enhance the efficacy of MM treatment.
Introduction
Multiple myeloma (MM) exhibits characteristics of solid and hematologic cancers, with bone marrow (BM) infiltration and focal lesions (FLs) presenting as osteolytic lesions (OLs), paramedullary or extramedullary disease (EMD).1-3 Over 80% of patients with MM are diagnosed with OLs, associated with severe complications, including fractures and spinal cord compression.4-6 OLs and EMD occur with advanced MM,7,8 although pathogenic mechanisms are not fully understood. Current immunomodulatory therapies rely on functional immune cells; however, immune cell dysregulation is common in the MM tumor microenvironment (TME), typically involving altered cytokine release, disrupted cellular signaling, and changes in immune cell populations.9,10 Malignant plasma cells (PCs) exacerbate immunosuppression by increasing regulatory T cells, myeloid-derived suppressor cells, dysfunctional natural killer cells, and tumor-associated macrophages; fostering angiogenesis, chemotherapy resistance, and tumor progression.11,12
Chemoimmunotherapy, checkpoint inhibition, and adoptive cellular therapies have become standard treatments for selected solid and hematologic malignancies. Despite these advances, many cancers develop resistance by evading immune destruction through several immunosuppressive mechanisms.13 While the roles of various immune cells in this process are increasingly well characterized, the specific contributions of neutrophils to cancer-induced immunosuppression remain poorly understood.14 Emerging evidence suggests that neutrophils can promote cancer progression through diverse mechanisms, such as neutrophil extracellular trap formation, secretion of inflammatory and immunosuppressive cytokines, and direct T-cell inhibition.15-19 A key pathway implicated in the recruitment of immunosuppressive neutrophils to the TME is the CXCL1-CXCR2 signaling axis, which has been shown to facilitate tumor progression.20,21 Furthermore, large-scale initiatives such as ImmGenMaps provide valuable insights into the TME cellular landscape.22 In murine models of pancreatic cancer, distinct neutrophil subpopulations have been identified, with at least 1 subtype shown to support tumor growth.23 MM neutrophil phenotype and function remain poorly understood, as fragility limits analysis of stored BM samples, leaving their role in FLs and BM of patients largely unexplored. Using single-cell transcriptomics and functional assays on fresh FLs and BM samples, we identified notable myeloid heterogeneity, with an enrichment of CXCR2+TREM1+CD10+ mature, immunosuppressive neutrophils in MM FLs. Functional assays confirmed their suppressive activity. In preclinical MM models, CXCR2 inhibition improved survival, highlighting mature neutrophils as potential therapeutic targets and biomarkers for disease progression.
Materials and methods
Patient and healthy samples
Thirteen patients underwent biopsy for OLs (n = 11) and EMD (n = 2). BM from healthy donors (HBM), aged 50-70 years, was freshly collected via iliac crest aspiration. For patients with MM, BM and FL samples were obtained from random BM biopsies at sites without OLs, and from active FLs via computed tomography-guided biopsy, respectively, at Roswell Park. The study was approved by the Roswell Park Institutional Review Board (protocol I 66418). Written, informed consent was obtained before all sampling. Ammonium-chloride-potassium lysing buffer removed red blood cells. CD11b+CD3–CD56–CD138– myeloid cells were isolated by SONY flow sorter in complete RPMI 1640 medium supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin with an RNase inhibitor (10 μg/mL). Cells were immediately processed for single-cell RNA sequencing (scRNA-seq) for transcriptomic integrity.
Preclinical MM models
The Vk∗MYC myeloma clone, Vk12653, from VkMYC transgenic mice,24 was used in both C57BL/6 models. Recipients received IV injections of Vk12653 (1 × 106 CD138+CD19− cells) 2 weeks before autologous (syngeneic) hematopoietic stem cell transplant (auto-HSCT), establishing the cancer. Auto-HSCT was performed as described.25 Serum samples were collected biweekly. M-band levels were measured using the Helena QuickGel manual serum protein electrophoresis system: Helena QuickGel was stained with amido black, and scanned via an onboard densitometer. The γ (gamma) and albumin fractions were quantified to calculate the γ/albumin ratio (M-band). Mice were monitored daily to 120 days after auto-HSCT. Those developing hindlimb paralysis or clinical scores ≥626 were euthanized. All murine experiments were approved under Institutional Animal Care and Use Committee protocol 1143 at Roswell Park Comprehensive Cancer Center.
Results
High-throughput scRNA-seq identifies altered neutrophil subsets in MM FLs
To investigate the full myeloid cell repertoire within the TME of patients with MM, we collected fresh BM aspirates from patients with MM with clinical need for biopsy, and 3 HBM. Capturing the range of neutrophil gene expression and phenotypes, patient samples were obtained concurrently from FLs and intact pelvic BM by imaging (supplemental Figure 1A, available on the Blood website). We analyzed ∼120 237 myeloid cells with 10× Genomics Chromium V3 5ʹ chemistry (see “Materials and methods”). Myeloid cells were isolated by flow cytometry, sorted based on side scatter/forward scatter profiles, and identified as CD3−CD56−CD138−CD11b+ cells (supplemental Figure 1B). Paired FL and BM aspirates were collected from 12 patients with MM (1 had BM only; supplemental Figure 1C). Natural killer cell populations were excluded due to the focus of this study. After quality control, we assessed CD11b+CD3−CD56−CD138− sorted cells (n = 105 192) from the MM TME: BM (n = 49 420) cells and FLs (n = 45 331) from patients who were newly diagnosed with MM (NDMM, n = 6), and from patients with relapsed/refractory MM (RRMM, n = 7). We obtained 10 086 cells from HBM (n = 3). Cell quantity, recovery rates, gene expression per cell, and patient demographics are shown in supplemental Tables 1 and 2 (the column A numbers refer to Figure 1C numbers; the colors correspond to the 3 groups: HBM [green], NDMM [blue], and RRMM [red]).
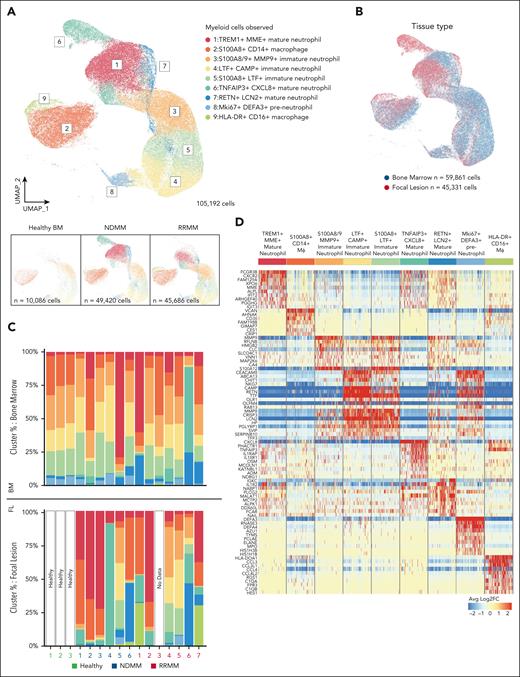
CD11b+ myeloid cells from BM and FLs from patients with MM, and HBM. (A) Samples from patients with NDMM (n = 6), RRMM (n = 7) (BM and FLs), and from HBM (n = 3) are shown in the UMAP representation of 105 192 CD11b+ cells captured with 10× V3 5ʹ scRNA-seq (see “Materials and methods” for complete details). Nine clusters observed were: (1) TREM+MME+ mature neutrophils, (2) S100A8+CD14+ macrophages (Mϕ), (3) S100A8/9+MMP9+ immature neutrophils, (4) LTF+CAMP+ immature neutrophils, (5) S100A8+LTF+ immature neutrophils, (6) TNFAIP3+CXCL8+ mature neutrophils, (7) RETN+LCN2+ mature neutrophils, (8) Mki67+DEFA3+ preneutrophils, and (9) HLA-DR+CD16+ macrophages. Bottom panels represent cell contributions from HBM (left), NDMM (middle), and RRMM (right). (B) UMAP representation of CD11b+ cells observed. The contribution of BM-derived cells (n = 59 861 cells) is shown in blue. The contribution of FL-derived cells (n = 45 331 cells) is shown in red. (C) Scaled bar graph representation of each cell type contribution (y-axis) from healthy donors and patients with MM (x-axis; n = 16). Color convention as per panel A. Top panel, cell type distribution from BM cells; bottom panel, cell type distribution from FLs (1 sample without cells). (D) Scaled normalized heat map of top 10 transcripts associated with each cluster. Sample downsized to 5000 cells per cluster. Blue represents downregulated genes, and red represents upregulated (log2 fold-change) genes in cell types shown at top.
CD11b+ myeloid cells from BM and FLs from patients with MM, and HBM. (A) Samples from patients with NDMM (n = 6), RRMM (n = 7) (BM and FLs), and from HBM (n = 3) are shown in the UMAP representation of 105 192 CD11b+ cells captured with 10× V3 5ʹ scRNA-seq (see “Materials and methods” for complete details). Nine clusters observed were: (1) TREM+MME+ mature neutrophils, (2) S100A8+CD14+ macrophages (Mϕ), (3) S100A8/9+MMP9+ immature neutrophils, (4) LTF+CAMP+ immature neutrophils, (5) S100A8+LTF+ immature neutrophils, (6) TNFAIP3+CXCL8+ mature neutrophils, (7) RETN+LCN2+ mature neutrophils, (8) Mki67+DEFA3+ preneutrophils, and (9) HLA-DR+CD16+ macrophages. Bottom panels represent cell contributions from HBM (left), NDMM (middle), and RRMM (right). (B) UMAP representation of CD11b+ cells observed. The contribution of BM-derived cells (n = 59 861 cells) is shown in blue. The contribution of FL-derived cells (n = 45 331 cells) is shown in red. (C) Scaled bar graph representation of each cell type contribution (y-axis) from healthy donors and patients with MM (x-axis; n = 16). Color convention as per panel A. Top panel, cell type distribution from BM cells; bottom panel, cell type distribution from FLs (1 sample without cells). (D) Scaled normalized heat map of top 10 transcripts associated with each cluster. Sample downsized to 5000 cells per cluster. Blue represents downregulated genes, and red represents upregulated (log2 fold-change) genes in cell types shown at top.
We identified 9 major myeloid cell subtypes (Figure 1A; supplemental Table 3). To explore FL myeloid cell heterogeneity, we utilized uniform manifold approximation and projection (UMAP), projecting cells in 2 dimensions, clustering them using approximate nearest neighbor analysis with differential gene expression profiling. Cells were normalized by modeling RNA expression as regularized negative binomial distributions with SCTransform. Dimensionality reduction was derived by using principal components from the SCTransform-normalized data. There were 2 FL tumor-associated macrophages populations, expressing S100A8+ CD14+ and HLA-DR+ CD16+, respectively, and 7 neutrophil clusters in FLs and BM with differences in locational frequencies. These included neutrophils that were TREM1+ MME+ (mature), S100A8/9+ MMP9+ (immature), LTF+ CAMP+ (immature), S100A8+ LTF+ (immature), TNFAIP3+ CXCL8+ (mature), RETN+ LCN2+ (mature), and Mki67+ DEFA3+ (preneutrophils; Figure 1A-D). All abbreviations are listed in supplemental Table 4.
Comparing our results with HBM neutrophil findings,27 no new developmental clusters or trajectories were observed in healthy neutrophil subsets (Figure 1A-B,D). However, MM tumor-associated neutrophils (TANs) acquired distinct transcriptional profiles in FLs/BM, forming 3 mature, neutrophil clusters (RETN+LCN2+, TREM1+MME+, and TNFAIP3+CXCL8+).
Neutrophils from patients with MM exhibit altered transcriptional states
TANs demonstrated a heterogeneous myeloid cell phenotype. To understand this heterogeneity, we revisualized, and reclustered them using UMAP, leading to cluster renumbering. TREM1+MME+, TNFAIP3+CXCL8+, and RETN+LCN2+ mature neutrophil populations were enriched in FLs compared with paired BM biopsies (Figure 2A-B). In the UMAP analysis, MM TANs clustered by development from preneutrophils (Mki67+DEFA3+) to 3 immature groups (LTF+CAMP+) to (S100A8+LTF+) to (S100A8/9+MMP9+) to 3 mature groups (RETN+LCN2+) to (TREM1+MME+) to (TNFAIP3+CXCL8+) (Figure 2A; supplemental Figure 2A,C). We focused on these populations. To explore links between neutrophil clusters in MM FLs and BM TME, we employed an RNA velocity approach.28 This technique estimates the rate of gene expression changes over time, preserving differentiation trajectories. The RNA velocity analysis revealed trajectory progression from Mki67+DEFA3+ preneutrophils to TNFAIP3+MME+ mature neutrophils, with immature neutrophils developing into 2 distinct mature subsets: TREM1+MME+ and TNFAIP3+CXCL8+ (Figure 2A-B). This implies that mature neutrophils arise from immature precursors in the MM TME. RETN+LCN2+ neutrophils have an intermediate score between immature and mature (Figure 2C). They may represent a transitional admixture of immature (S1008/9+MMP9+) and mature (TREM1+MME+) neutrophils, and we observed velocity vectors originating from immature and mature neutrophil subsets terminating in the RETN+LCN2+ cluster (Figure 2A). Our findings support previous studies demonstrating mature and immature neutrophils infiltrating the tumor TME.29 This analysis also indicates that RETN+LCN2+ and TREM1+MME+ subsets remain capable of further differentiation/maturation into TNFAIP3+CXCL8+ neutrophils, supporting their transitional nature.
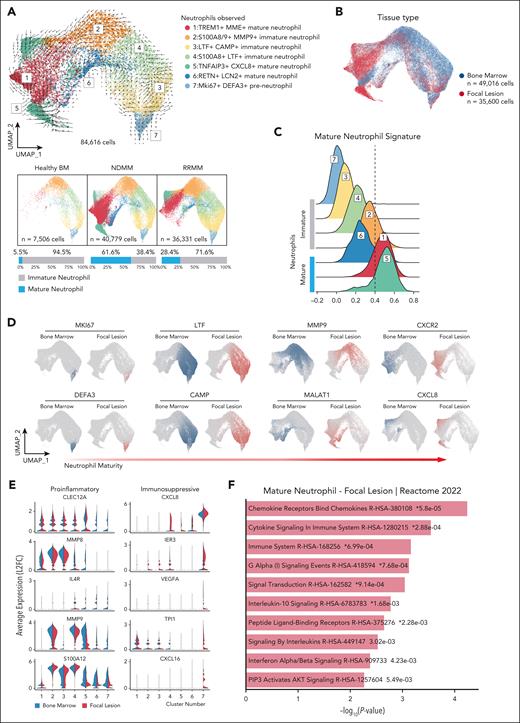
Neutrophils associated with FLs acquire a protumor phenotype. (A) RNA velocity of the reanalyzed neutrophil data set excluding macrophages. UMAP embeddings from 84 616 cells. Color convention as per Figure 1A. The visual representation of neutrophil maturation from HBM (left), NDMM (middle), and RRMM (right). Scaled bar graphs below HBM and patient-specific UMAP represent scaled contribution of immature (gray) and mature (blue) neutrophils. (B) UMAP representation of neutrophils observed. The contribution of BM cells (n = 49 016 cells) is shown in blue. The contribution of FL cells (n = 35 600 cells) is shown in red. (C) Clusters scored against mature neutrophil signature (see “Materials and methods” for details). High scores represent transcripts within clusters most like mature neutrophils. (D) Highly variable features associated with neutrophil maturity in single cell data. Data are organized by early to late neutrophil development. Blue indicates BM cells, and red indicates FL cells. (E) Differentially expressed transcripts in neutrophils between BM (left, blue) and FLs (right, red). Features were selected to emphasize proinflammatory or immunosuppressive phenotypes. (F) Paired gene ontogeny analysis of mature neutrophils from FLs vs neutrophils from HBM.
Neutrophils associated with FLs acquire a protumor phenotype. (A) RNA velocity of the reanalyzed neutrophil data set excluding macrophages. UMAP embeddings from 84 616 cells. Color convention as per Figure 1A. The visual representation of neutrophil maturation from HBM (left), NDMM (middle), and RRMM (right). Scaled bar graphs below HBM and patient-specific UMAP represent scaled contribution of immature (gray) and mature (blue) neutrophils. (B) UMAP representation of neutrophils observed. The contribution of BM cells (n = 49 016 cells) is shown in blue. The contribution of FL cells (n = 35 600 cells) is shown in red. (C) Clusters scored against mature neutrophil signature (see “Materials and methods” for details). High scores represent transcripts within clusters most like mature neutrophils. (D) Highly variable features associated with neutrophil maturity in single cell data. Data are organized by early to late neutrophil development. Blue indicates BM cells, and red indicates FL cells. (E) Differentially expressed transcripts in neutrophils between BM (left, blue) and FLs (right, red). Features were selected to emphasize proinflammatory or immunosuppressive phenotypes. (F) Paired gene ontogeny analysis of mature neutrophils from FLs vs neutrophils from HBM.
We evaluated maturational and functional states of mature neutrophils. Using a modified neutrophil maturation gene signature,23,30 we scored clusters by maturation. Neutrophils expressing TREM1+MME+ scored as mature, and RETN+LCN2+ as intermediate, reflecting the mixed immature and mature states (cluster 6, Figure 2C). TNFAIP3+CXCL8+ neutrophils (cluster 5), expressed the highest maturation score, likely representing terminally differentiated neutrophils.
To identify preneutrophils, we used a previously published expression signature.31 Our analysis captured the complete lineage, from preneutrophils (Mki67+),32 to immature (MMP9+) and mature (CXCR2+), the latter represented by clusters 1, 5, and 6 (Figures 1A and 2D; supplemental Figure 3A). As an orthogonal approach analyzing developmental trajectories, we used monocle 3,33 generating minimum spanning trees across the neutrophil data set continuum. Utilizing the preneutrophil signature, we determined the root node for random walks (supplemental Figure 3B). Seeing an increase in mature neutrophil clusters in FLs, we analyzed mature neutrophil differences between HBM and FLs, finding divergent mature neutrophil expression profiles. We compared neutrophil gene expression between paired MM FLs and BM. Inflammatory mediators including S100A12, MMP8, and MMP9 increased in MM BM neutrophils, while immunosuppressive and protumor genes including CXCL8, VEGFA, and TPI1 increased in FL neutrophils (Figure 2E; supplemental Figure 3E). Biological process enrichment analysis demonstrated that mature FL neutrophils enriched for processes related to cytokine response and proinflammatory cytokine production (Figure 2F). BM neutrophils enriched in neutrophil degranulation and innate immune response pathways (supplemental Figure 3F). This implies conserved, innate neutrophil activity in BM neutrophils compared with proinflammatory functions in mature FL neutrophils.
To explore the biological plausibility of a conserved tumor-associated inflammasome across cancer types, we compared our MM data set with a recent study on neutrophil heterogeneity in pancreatic cancer.23 That study showed that the solid tumor TME can elicit an innate immune-driven inflammatory response with early neutrophil recruitment and subsequent polarization into tumor-promoting phenotypes. Based on this model, we hypothesize that similar inflammatory mechanisms may operate in MM (supplemental Figure 3C-D). In murine pancreatic cancer, scRNA-seq identified 3 primary TAN populations: T1, T2, transitional states differentiating into T3, which is a terminally differentiated neutrophil population. As shown in supplemental Figure 3C-D for the 3 subsets, the T1 signature is enriched in TREM1+MME+ and TNFAIP3+CXCL8+ mature neutrophils. The T2 signature is enriched in immature subsets. The T3 signature predominates in the TNFAIP3+CXCL8+ subset. Notably, the TNFAIP3+CXCL8+ mature neutrophil subset is distinct in patients with MM, and rare in HBM. The RETN+LCN2+ mature neutrophil population has not been previously described in MM or in pancreatic cancer models.23 This indicates that in the MM TME, TANs undergo additional differentiation, leading to emergence of new subsets with distinct transcriptional signatures.
CD10 and TNFAIP3 distinguish 3 distinct subpopulations of mature, CXCR2+ TANs in patients with MM
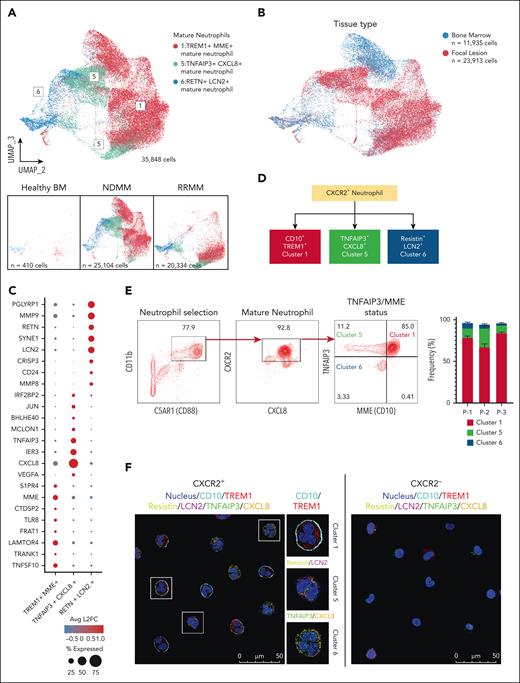
After demonstrating that distinct, mature TAN subsets accumulate in MM BM, especially in FLs, we evaluated them for differential gene expression and increased protein expression of neutrophil trajectory markers. We reclustered the CXCR2+ mature neutrophils (Figure 2 numbering) into 3 groups using highly variable features within the data set, and projected in low-dimensional space using UMAP (Figure 3A). Cluster 5 (TNFAIP3+CXCL8+) mature TANs concentrated in FLs. Cluster 1 (TREM1+MME+) and cluster 6 (RETN+LCN2+) were in MM BM and FLs (Figure 3B). During differential expression analysis of the 3 mature TAN clusters, we identified intracellular and extracellular protein differences in expression, allowing for distinct population identification (Figure 3C; supplemental Figure 4D). Gene ontogeny enrichment analysis revealed the TREM1+MME+ subset is enriched for the NF-κB pathway and inflammatory responses, seen in activated states (supplemental Figure 4A). Predominant pathways in TNFAIP3+CXCL8+ neutrophils are metabolic reprogramming towards glycolysis and increased intrinsic apoptotic signaling, features of long-lived tumor-infiltrating neutrophils23 (supplemental Figure 4B). The RETN+LCN2+ subset enriched in pathways related to cytoplasmic transition and RNA processing, features of transitioning neutrophils32 (supplemental Figure 4C). This suggests that mature, CXCR2+ neutrophils undergo continuous maturation in the TME, demonstrating dynamic development progression (Figure 3D). We employed multicolor flow cytometry to test our hypothesis. Live CD138–CD11b+ cells from FLs from 3 patients with MM were screened for the following differentially expressed markers (Figure 3E), including C5AR1 (CD88), (pan neutrophil), CXCR2, (mature neutrophil), and TNFAIP3 and MME (CD10), discriminating between subsets. Flow cytometry confirmed CD10 and TNFAIP3 expression in cluster 1. The RETN+LCN2+ subset (cluster 6) showed no to low expression of CD10 and TNFAIP3. The TNFAIP3+CXCL8+ subset (cluster 5) also showed low or no expression of CD10. The flow cytometry analyses aligned with scRNA-seq data, confirming cluster 1 (CD10+TNFAIP+) represented the predominant mature neutrophil subpopulation in the 3 patients (Figure 3E). To orthogonally validate the scRNA-seq data, we used confocal microscopy with specific marker staining to visualize all 3 subsets. We sorted C5AR1+CXCR2+ and C5AR1+CXCR2– neutrophils from FLs. We stained immature and mature neutrophils based on specific differentially expressed markers identified in the scRNA-seq analysis (Figure 3D). These included CD10, TREM1, RETN, LCN2, CXCL8, and TNFAIP3. We detected the 3 distinct subsets of CXCR2+ mature neutrophils in patient samples. The RETN+LCN2+ cells were immature, as evidenced by nuclear hypolobulation morphologically by confocal immunofluorescence staining (Figure 3F). In contrast, the TREM1+CD10+ and CXCL8+TNFAIP3+ neutrophils exhibited nuclear multilobulation, a mature phenotype. The expression of CXCR2, CD10, and TNFAIP3 allows for phenotypic division of MM mature neutrophils into 3 populations, aligning with transcriptomic analyses. To confirm the expression of signature genes for immature neutrophils described in Figure 2E, we stained CXCR2– neutrophils for MMP9 and S100A12. Confocal microscopy confirmed that CXCR2– neutrophils express MMP9 and S100A12 proteins, supporting the scRNA-seq data (supplemental Figure 5). This novel finding demonstrates diverse populations of CXCR2+ mature neutrophils in patients with MM, highlighting the importance of transcriptomic and protein-based approaches to characterize MM TANs.
Mature neutrophil representation at sites of FLs. (A) The mature neutrophils were reclustered and represented using highly variable features. UMAP is plotted in the second and third dimension for visual optimization. Bottom panels represent cell contributions from HBM (left), NDMM (middle), and RRMM (right). (B) UMAP representation of reclustered mature neutrophils observed. In blue, the contribution of cells derived from BM (n = 11 935 cells). In red, the contribution of cells from sites of FLs (n = 23 913 cells). (C) Dot plot depicting the top 8 transcripts from each mature neutrophil cluster. The radius of each circle represents the percentage of cells expressing feature within cluster, and color represents average log2 fold-change. (D) Schematic of CXCR2+ neutrophil developmental trajectories in patients with MM. (E) The fluorescence-activated cell sorter gating strategy involved an initial selection of CD11b+ neutrophils and C5AR1+ cells, followed by further selection based on CXCR2+ and CXCL8+ expression. Subsequently, the 3 CXCR2+ neutrophil clusters were identified using CD10 and TNFAIP3 gates. The analysis quantified the frequency of these 3 neutrophil subsets sampled from the BM of 3 individual patients. (F) Confocal immunofluorescence images of CXCR2+ neutrophils with markers associated with subset features identified in scRNA-seq (right) and CXCR2– neutrophils with decreased marker expression (right). The data presented in panels E-F are representative of findings from 3 different patients.
Mature neutrophil representation at sites of FLs. (A) The mature neutrophils were reclustered and represented using highly variable features. UMAP is plotted in the second and third dimension for visual optimization. Bottom panels represent cell contributions from HBM (left), NDMM (middle), and RRMM (right). (B) UMAP representation of reclustered mature neutrophils observed. In blue, the contribution of cells derived from BM (n = 11 935 cells). In red, the contribution of cells from sites of FLs (n = 23 913 cells). (C) Dot plot depicting the top 8 transcripts from each mature neutrophil cluster. The radius of each circle represents the percentage of cells expressing feature within cluster, and color represents average log2 fold-change. (D) Schematic of CXCR2+ neutrophil developmental trajectories in patients with MM. (E) The fluorescence-activated cell sorter gating strategy involved an initial selection of CD11b+ neutrophils and C5AR1+ cells, followed by further selection based on CXCR2+ and CXCL8+ expression. Subsequently, the 3 CXCR2+ neutrophil clusters were identified using CD10 and TNFAIP3 gates. The analysis quantified the frequency of these 3 neutrophil subsets sampled from the BM of 3 individual patients. (F) Confocal immunofluorescence images of CXCR2+ neutrophils with markers associated with subset features identified in scRNA-seq (right) and CXCR2– neutrophils with decreased marker expression (right). The data presented in panels E-F are representative of findings from 3 different patients.
Immunofluorescence imaging reveals CXCR2+ neutrophil infiltration with increasing malignant PC accumulation
To study the accumulation and spatial distribution of CXCR2+ mature neutrophils and their association with disease burden, we used Vectra Polaris multiplex immunofluorescence to evaluate paired FL and BM biopsies. MM forms focal clusters with patchy tumor accumulation,34,35 which we observed (Figure 4).
Visualization of heterogeneity of CXCR2+ TAN infiltration in MM BM and FLs. Vectra Polaris staining was applied to formalin-fixed, paraffin-embedded sections from 12 patient samples (BM, n = 12; paired FL, n = 12). Representative Vectra images from 3 individual patient samples ([A-C, top rows] original magnification ×10; [A-C, bottom rows] original magnification ×30). Differential marker and cell distribution of CD138+ tumor cells and CD16+ cells. (A) The absence of CXCR2+ cells in BM with <1% malignant PCs (CD138+). (B) Accumulation of CXCR2+ neutrophils along with an increase of CD138+ cells in BM. (C) Extensive intratumoral infiltration of CXCR2+ neutrophils with increased CD138+ cells. (D-F) Representative visualizations of the 3 mature TAN populations: (1) CXCR2+CD10+TNFAIP3+ cells (D, arrow), (2) CXCR2+CD10+TNFAIP3– cells (E, arrow), and (3) CXCR2+CD10–TNFAIP3– cells (F, arrow). These data highlight the TAN heterogeneity and diversity within neutrophil populations in MM, emphasizing distinct phenotypic and functional subsets that may contribute variably to the TME.
Visualization of heterogeneity of CXCR2+ TAN infiltration in MM BM and FLs. Vectra Polaris staining was applied to formalin-fixed, paraffin-embedded sections from 12 patient samples (BM, n = 12; paired FL, n = 12). Representative Vectra images from 3 individual patient samples ([A-C, top rows] original magnification ×10; [A-C, bottom rows] original magnification ×30). Differential marker and cell distribution of CD138+ tumor cells and CD16+ cells. (A) The absence of CXCR2+ cells in BM with <1% malignant PCs (CD138+). (B) Accumulation of CXCR2+ neutrophils along with an increase of CD138+ cells in BM. (C) Extensive intratumoral infiltration of CXCR2+ neutrophils with increased CD138+ cells. (D-F) Representative visualizations of the 3 mature TAN populations: (1) CXCR2+CD10+TNFAIP3+ cells (D, arrow), (2) CXCR2+CD10+TNFAIP3– cells (E, arrow), and (3) CXCR2+CD10–TNFAIP3– cells (F, arrow). These data highlight the TAN heterogeneity and diversity within neutrophil populations in MM, emphasizing distinct phenotypic and functional subsets that may contribute variably to the TME.
With minimal malignant PC burden BM (<1% CD138+ cells), there was no CXCR2+ neutrophil accumulation (representative sample, Figure 4A). With higher BM tumor burden, CXCR2+ neutrophil numbers increased near malignant PCs (representative sample, Figure 4B). In FLs with extensive malignant PC infiltration, CXCR2+ neutrophil accumulation was more pronounced (representative sample, Figure 4C). These images support the scRNA-seq data, demonstrating increasing mature TANs with increasing tumor. MM biopsy samples were evaluated by multiplex immunofluorescence histopathology, and detected the 3 TAN subsets: (1) CXCR2+CD10+TNFAIP3+ (Figure 4D), (2) CXCR2+CD10+TNFAIP3– (Figure 4E), and (3) CXCR2+CD10–TNFAIP3– (Figure 4F). The histopathology supports increased, distinct CXCR2+ TAN populations in FLs and BM, illustrating the neutrophil heterogeneity within the MM TME.
Accumulation of CXCR2+ TANs particularly in FLs, led us to evaluate their role in MM disease progression through cytokine release and antitumor immune response suppression.
FL mature TANs have potent immunosuppressive activity compared with BM TANs
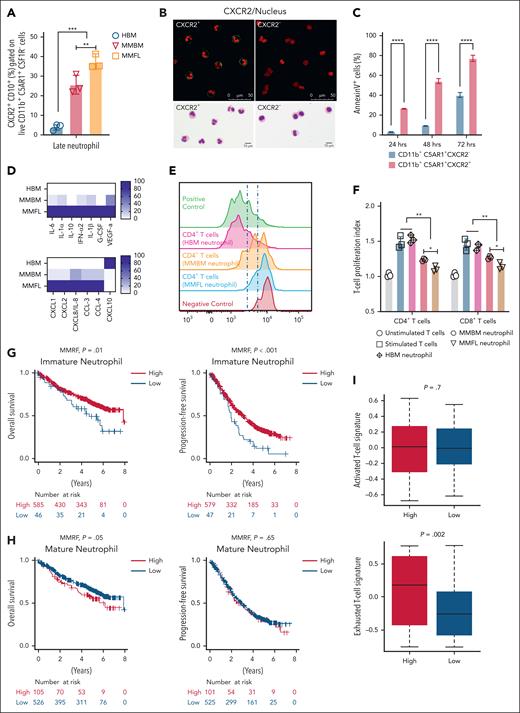
We analyzed CXCR2+ neutrophils, focusing on the CXCR2+TREM1+CD10+ TAN subset, using multicolor flow cytometry on FL, BM, and HBM samples. Staining for maturation markers showed a significant increase in CXCR2+ neutrophils in MM FLs and BM compared with HBM (supplemental Figure 6). Flow cytometry analysis demonstrated that CXCR2+ neutrophils were significantly increased in MM FLs and BM when compared with HBM (Figure 5A). There were more CXCR2+ neutrophils in FLs compared with paired BM (Figure 5A). We sorted CXCR2+ neutrophils from FLs and BM. Confocal immunofluorescence microscopy and Wright Giemsa staining confirmed that CXCR2+ neutrophils were mature, with nuclear multilobulation compared with immature hyposegmentation of CXCR2– neutrophils (Figure 5B). In vitro culture of the 2 populations with annexin V viability staining demonstrated that CXCR2+ cells were shorter-lived at 24 (70%-80%), 48 (50%-60%), and 72 hours (20%-30%) when compared with CXCR2– cells at 24 (90%-95%), 48 (90%-95%), and 72 hours (70% to 80%), respectively (Figure 5C).
CXCR2+CD10+ neutrophils are inflammatory and immunosuppressive. (A) The percentage of CXCR2+CD10+ neutrophils in BM and FLs from patients with MM (MM BM and MM FLs, respectively) and HBM. CD138–CD3–CD56–CSF1R–CD11b+CD10+CXCR2+C5AR1+ were characterized as mature neutrophils. CXCR2+CD10+ neutrophils accumulated in MM BM and MM FL samples. (B) Confocal microscopy and Wright Giemsa staining of CXCR2+ and CXCR2– neutrophils from MM microenvironment; CXCR2+ neutrophils were mature with increased nuclear segmentation (lobulation), and CXCR2– neutrophils were immature cells with nuclear hypolobulation. CXCR2 was marked by fluorescein isothiocyanate anti-CXCR2 (green), while the nucleus was stained with NucSpot 750/780 (red). These imaging results further emphasize the high purity of the CXCR2+ neutrophil sorting, underscoring the precision in identifying distinct neutrophil populations within the MM microenvironment. (C) CD11b+C5AR1+CXCR2– (immature) and CD11b+C5AR1+CXCR2+ (mature) neutrophils were isolated from MM BM and MM FLs. Sorted neutrophils were cultured in complete media for 24, 48, and 72 hours. Cells were harvested and stained for Annexin V at each time point. CXCR2+ cells had significantly shorter survival compared with CXCR2– cells. (D) CD138–CD3–CD56–CSF1R–CD11b+CD10+CXCR2+C5AR1+ neutrophils isolated from HBM (n = 3), MM BM (n = 3), and MM FLs (n = 3). Sorted neutrophils were cultured in complete media for 24 hours, and supernatants were collected (see “Materials and methods” for details). Multiplex enzyme-linked immunosorbent assay protein measurement was performed on collected supernatants. Several markers of inflammation were elevated in MM FLs compared with MM BM and HBM. CXCL10 was elevated in HBM. (E-F) HBM, MM BM, and MM FL neutrophils were cocultured (1:4 ratio of neutrophils to T cells) with healthy carboxyfluorescein succinimidyl ester–stained T cells activated with CD28/CD3 antibodies in the presence of IL-2. On day 4, T-cell activation was analyzed using CFSE dilution by flow cytometry. Proliferation index, with a value of 1.0 representing baseline (no proliferation), serves as the reference point. (E) Representative flow cytometry histograms of T-cell proliferation. (F) FL neutrophils showed significantly higher immunosuppression of T-cell proliferation compared with BM neutrophils from the same (paired) patient with MM. (G) Kaplan-Meier curves of OS (left) and PFS (right) for patients with MM from the MMRF CoMMpass data set stratified by the “immature neutrophil” signature. (H) OS (left) and PFS (right) curves for patients with MM from the same data set stratified by the “mature neutrophil” signature. (I) T-cell activation and exhaustion signatures were calculated from the MMRF CoMMpass data set, and compared based on the ratio of “high mature neutrophil” and “low mature neutrophil” signatures. An exhausted T-cell signature was significantly associated with the “high mature neutrophil” signature. One-way analysis of variance with Tukey multiple comparison tests was used to test significant differences between groups in panels A,C. One-way analysis of variance with Kruskal-Wallis multiple comparisons test was used to compare means between groups in panel F; statistics were derived from BM and FLs from 3 HBM and 3 patients with MM. Experiments were performed in triplicate in panels D,F. ∗P < .05; ∗∗P < .01 (in all experiments). Data are presented as mean ± standard deviation.
CXCR2+CD10+ neutrophils are inflammatory and immunosuppressive. (A) The percentage of CXCR2+CD10+ neutrophils in BM and FLs from patients with MM (MM BM and MM FLs, respectively) and HBM. CD138–CD3–CD56–CSF1R–CD11b+CD10+CXCR2+C5AR1+ were characterized as mature neutrophils. CXCR2+CD10+ neutrophils accumulated in MM BM and MM FL samples. (B) Confocal microscopy and Wright Giemsa staining of CXCR2+ and CXCR2– neutrophils from MM microenvironment; CXCR2+ neutrophils were mature with increased nuclear segmentation (lobulation), and CXCR2– neutrophils were immature cells with nuclear hypolobulation. CXCR2 was marked by fluorescein isothiocyanate anti-CXCR2 (green), while the nucleus was stained with NucSpot 750/780 (red). These imaging results further emphasize the high purity of the CXCR2+ neutrophil sorting, underscoring the precision in identifying distinct neutrophil populations within the MM microenvironment. (C) CD11b+C5AR1+CXCR2– (immature) and CD11b+C5AR1+CXCR2+ (mature) neutrophils were isolated from MM BM and MM FLs. Sorted neutrophils were cultured in complete media for 24, 48, and 72 hours. Cells were harvested and stained for Annexin V at each time point. CXCR2+ cells had significantly shorter survival compared with CXCR2– cells. (D) CD138–CD3–CD56–CSF1R–CD11b+CD10+CXCR2+C5AR1+ neutrophils isolated from HBM (n = 3), MM BM (n = 3), and MM FLs (n = 3). Sorted neutrophils were cultured in complete media for 24 hours, and supernatants were collected (see “Materials and methods” for details). Multiplex enzyme-linked immunosorbent assay protein measurement was performed on collected supernatants. Several markers of inflammation were elevated in MM FLs compared with MM BM and HBM. CXCL10 was elevated in HBM. (E-F) HBM, MM BM, and MM FL neutrophils were cocultured (1:4 ratio of neutrophils to T cells) with healthy carboxyfluorescein succinimidyl ester–stained T cells activated with CD28/CD3 antibodies in the presence of IL-2. On day 4, T-cell activation was analyzed using CFSE dilution by flow cytometry. Proliferation index, with a value of 1.0 representing baseline (no proliferation), serves as the reference point. (E) Representative flow cytometry histograms of T-cell proliferation. (F) FL neutrophils showed significantly higher immunosuppression of T-cell proliferation compared with BM neutrophils from the same (paired) patient with MM. (G) Kaplan-Meier curves of OS (left) and PFS (right) for patients with MM from the MMRF CoMMpass data set stratified by the “immature neutrophil” signature. (H) OS (left) and PFS (right) curves for patients with MM from the same data set stratified by the “mature neutrophil” signature. (I) T-cell activation and exhaustion signatures were calculated from the MMRF CoMMpass data set, and compared based on the ratio of “high mature neutrophil” and “low mature neutrophil” signatures. An exhausted T-cell signature was significantly associated with the “high mature neutrophil” signature. One-way analysis of variance with Tukey multiple comparison tests was used to test significant differences between groups in panels A,C. One-way analysis of variance with Kruskal-Wallis multiple comparisons test was used to compare means between groups in panel F; statistics were derived from BM and FLs from 3 HBM and 3 patients with MM. Experiments were performed in triplicate in panels D,F. ∗P < .05; ∗∗P < .01 (in all experiments). Data are presented as mean ± standard deviation.
We measured the secretome of CXCR2+CD10+ neutrophils from paired FL and BM aspirates of 3 patients with MM and 3 HBMs (Figure 5D; supplemental Figure 7) using multiplex enzyme-linked immunosorbent assay on 24-hour cultured supernatants from sorted cells. FL CXCR2+CD10+ neutrophils produced significantly higher levels of chemokines involving neutrophil chemotaxis and migration, including CXCL8 family members (CXCL1, CXCL2, and CXCL8), and proinflammatory cytokines (CCL3 and CCL4; Figure 5D; supplemental Figure 7). FL TANs released more inflammatory and immunosuppressive cytokines; interleukin 6 (IL-6), IL-1α , IL-10, interferon α2 (IFN-α2), IL-1β, vascular endothelial growth factor, and granulocyte-colony stimulating factor, compared with MM BM and HBM (Figure 5D; supplemental Figure 7). CXCL10 (IP-10), an antitumor cytokine, was downregulated in supernatants from neutrophils isolated from MM FLs and BM, and upregulated in neutrophils from BM (Figure 5D; supplemental Figure 7). CCL-20, epidermal growth factor, granzyme B, IFN-γ, IL-13, IL-17, and Flt-3L were undetectable.
Investigating effects on activated T-cell proliferation, we cocultured neutrophils from HBM, MM FLs and MM BM with CD3/CD28-activated allogeneic T cells with IL-2. CXCR2+CD10+ FL TANs significantly suppressed CD4+ and CD8+ T-cell proliferation compared with MM BM and HBM neutrophils (Figure 5E-F).
We examined neutrophil gene expression and correlation with outcome for patients with MM. Based on the scRNA-seq analysis, we developed 2 distinct gene signatures from MM BM and FL neutrophils, denoted “immature” and “mature,” based on low and high gene expression (see “Materials and methods”). These were divided into “immature neutrophil” high and low, and “mature neutrophil” high and low groups, respectively, using an optimal cut-off. To examine TAN gene expression profiles, and correlation with overall and progression-free survival (OS/PFS) of patients with MM, we utilized the MMRF CoMMpass patient database (https://portal.gdc.cancer.gov/projects/MMRF-COMMPASS). This database includes RNA sequencing of TME cells from unfractionated diagnostic BM aspirates in NDMM, and links these data to patient outcomes. We found that a high “immature” neutrophil gene signature was significantly associated with improved OS and PFS (Figure 5G). A high “mature” neutrophil gene signature was associated with inferior OS without PFS difference (Figure 5H). We examined the “mature” neutrophil signatures with T-cell activation and exhaustion gene signatures in the data set (see “Materials and methods”). Patients with NDMM with a high “mature” neutrophil signature had a significantly increased exhausted T-cell signature, and not an activated one (Figure 5I). These findings are consistent with a shift to an aberrant mature neutrophil signature in MM, especially in FLs, correlating antitumor response suppression with MM tumor progression.
CXCR2 blockade alone inhibits MM and synergistically increases the efficacy of BTZ/DEX
Standard MM treatment often contains dexamethasone (DEX) and bortezomib (BTZ), which provide antitumor effects and enhance T-cell function.36,37 Given the immunosuppressive role of CXCR2+ neutrophils in the MM TME, we hypothesized that CXCR2 blockade could enhance the efficacy of BTZ/DEX therapy. To assess the role of CXCR2+ neutrophils in MM progression and therapy response, we selectively targeted them in vivo using the VK∗MYC murine MM model.38,39 We first confirmed CXCR2+Ly6G+ neutrophil accumulation during tumor progression (Figure 6A; supplemental Figure 8A). VK∗MYC cells were injected into C57BL/6 mice, and treatment began once the M-spike/albumin ratio exceeded 0.28, equivalent to ∼10 g/L serum M protein in patients with MM (supplemental Figure 8B). CXCR2 inhibition was then used to evaluate effects on MM progression and BTZ/DEX response. CXCR2 inhibitor (CXCR2-IN) treatment significantly reduced tumor burden, and improved OS compared with phosphate-buffered saline controls. Similar effects were seen with BTZ/DEX treatment. Combining CXCR2-IN and BTZ/DEX synergistically enhanced tumor killing and OS (Figure 6B). To investigate the effects of combination therapy on immune cell dynamics within the TME, we performed scRNA-seq on sorted CD45+ immune cells from 4 treatment groups. Our analysis revealed that the combination of the CXCR2-IN with BTZ/DEX, but not the CXCR2-IN alone, increased the proportions of T cells and dendritic cells (Figure 6C). Importantly, both scRNA-seq and flow cytometry data showed that these treatments did not deplete neutrophil subpopulations. CXCR2+ neutrophils remained detectable after treatment (Figure 6D; supplemental Figure 8C), consistent with reports that SX-682, a CXCR1/2 inhibitor in clinical development, does not cause severe neutropenia.40
CXCR2 blockade promotes MM control in murine MM models. Vk12653 murine MM cell lines were injected into recipients for different in vivo tumor models (see “Materials and methods” for full details). We used the following models: the NDMM model (tumor cell injection without irradiation, n = 40 from 2 experiments), the RRMM model (tumor cell injection followed by total body lethal irradiation, 1000 cGy, and syngeneic [autologous] stem cell transplant, n = 40 from 2 experiments), the CD8+ T-cell depletion mouse model (tumor cell injection without irradiation, n = 40 from 1 experiment), and the NSG mouse model (tumor cell injection without irradiation, n = 30 from 1 experiment). Progression of MM was confirmed by weekly serum protein electrophoresis measurement of the monoclonal protein (M-spike) to albumin ratio in the NDMM model. In the RRMM model, the mice were classified as MM-relapsed or MM-remission based on the M-spike to albumin ratio. Recipient BM was harvested and stained with appropriate antibodies. Results were analyzed via flow cytometry. (A) Quantification of frequency and number of CXCR2+ neutrophil clusters from the samples of CBM (no tumor injection), MTB, and HTB, the latter 2 based on serum protein electrophoresis measurements. Cells gated from CD11b+Ly6G+Ly6C– population. (B) Quantitation of M-spike development, and survival curve in the NDMM model. (C) UMAP representation of immune cells (excluding macrophages and neutrophils) observed in various experimental arms in the NDMM model. Cell type distribution highlighted below UMAP to show changes in cell kinetics upon treatment. (D) Concatenated mature neutrophils show distinct developmental stages observed in humans with little variance among experimental conditions: EIN represent = 22.39%, σ = 2.06%, LIN represent = 22.87%, σ = 1.36%, EMN represent = 28.23%, σ = 0.66%, LMN represent = 26.5%, σ = 2.96%. Neutrophils were scored with the same signatures as Figure 2. (E) Quantitation of M-spike development, and survival curve in T-cell depletion mouse model. (F) Quantitation of M-spike development, and survival curve in NSG mouse model. (G) Quantitation of M-spike development, survival curve, and CD8+ T-cell cytokine expression of IFN-γ and TNF-α. Data represent mean ± standard error of the mean. The 2-way analysis of variance was used to analyze statistical significance among tumor growth in different groups. Survival data are presented as percent survival (log-rank Mantel-Cox test). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. CBM, control bone marrow; EIN, early immature neutrophils; EMN, early mature neutrophils; HTB, high tumor burden bone marrow; LIN, late immature neutrophils; LMN, late mature neutrophils; MTB, moderate tumor burden bone marrow; ns, not significant; PBS, phosphate buffered saline; TNF-α, tumor necrosis factor-α.
CXCR2 blockade promotes MM control in murine MM models. Vk12653 murine MM cell lines were injected into recipients for different in vivo tumor models (see “Materials and methods” for full details). We used the following models: the NDMM model (tumor cell injection without irradiation, n = 40 from 2 experiments), the RRMM model (tumor cell injection followed by total body lethal irradiation, 1000 cGy, and syngeneic [autologous] stem cell transplant, n = 40 from 2 experiments), the CD8+ T-cell depletion mouse model (tumor cell injection without irradiation, n = 40 from 1 experiment), and the NSG mouse model (tumor cell injection without irradiation, n = 30 from 1 experiment). Progression of MM was confirmed by weekly serum protein electrophoresis measurement of the monoclonal protein (M-spike) to albumin ratio in the NDMM model. In the RRMM model, the mice were classified as MM-relapsed or MM-remission based on the M-spike to albumin ratio. Recipient BM was harvested and stained with appropriate antibodies. Results were analyzed via flow cytometry. (A) Quantification of frequency and number of CXCR2+ neutrophil clusters from the samples of CBM (no tumor injection), MTB, and HTB, the latter 2 based on serum protein electrophoresis measurements. Cells gated from CD11b+Ly6G+Ly6C– population. (B) Quantitation of M-spike development, and survival curve in the NDMM model. (C) UMAP representation of immune cells (excluding macrophages and neutrophils) observed in various experimental arms in the NDMM model. Cell type distribution highlighted below UMAP to show changes in cell kinetics upon treatment. (D) Concatenated mature neutrophils show distinct developmental stages observed in humans with little variance among experimental conditions: EIN represent = 22.39%, σ = 2.06%, LIN represent = 22.87%, σ = 1.36%, EMN represent = 28.23%, σ = 0.66%, LMN represent = 26.5%, σ = 2.96%. Neutrophils were scored with the same signatures as Figure 2. (E) Quantitation of M-spike development, and survival curve in T-cell depletion mouse model. (F) Quantitation of M-spike development, and survival curve in NSG mouse model. (G) Quantitation of M-spike development, survival curve, and CD8+ T-cell cytokine expression of IFN-γ and TNF-α. Data represent mean ± standard error of the mean. The 2-way analysis of variance was used to analyze statistical significance among tumor growth in different groups. Survival data are presented as percent survival (log-rank Mantel-Cox test). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. CBM, control bone marrow; EIN, early immature neutrophils; EMN, early mature neutrophils; HTB, high tumor burden bone marrow; LIN, late immature neutrophils; LMN, late mature neutrophils; MTB, moderate tumor burden bone marrow; ns, not significant; PBS, phosphate buffered saline; TNF-α, tumor necrosis factor-α.
To determine whether the therapeutic benefit of this combination relies on adaptive immunity, we utilized both a CD8+ T-cell depletion model and NOD scid gamma (NSG) immunodeficient mice. In the CD8+ T-cell–depleted cohort, the antitumor effects of both SX-682 and the triple combination therapy were abrogated, with no significant differences among the treatment arms (supplemental Figure 8E). Conversely, in the isotype control group, SX-682 retained its efficacy, consistent with observations seen with CXCR2-IN treatment (Figure 6E). Similarly, in NSG mice that lack functional adaptive immunity, treatment with CXCR2 inhibitors (CXCR2-IN-1 and SX-682) failed to impact tumor burden, as reflected by unchanged M-spike levels and overall survival (Figure 6F; supplemental Figure 8F). Collectively, these findings demonstrate that the therapeutic efficacy of CXCR2 inhibition requires an intact adaptive immune response.
In the RRMM model, VK∗MYC cells were injected before total body irradiation (anti-VK∗MYC treatment), followed by auto-HSCT.25 Recipient plasma was monitored for disease progression, and treatment was started. CXCR2-IN monotherapy significantly reduced tumor burden and improved OS (Figure 6G). With triple therapy, there was synergistic, improved OS. Studying immune effects, isolated CD8+ T cells were examined by flow cytometry intracellularly for the antitumor cytokines IFN-γ+ and tumor necrosis factor-α+. Both increased with CXCR2-IN or BTZ/DEX treatment, with marked increases with triple therapy (Figure 6G).
These findings underscore the pivotal role of CXCR2+ TANs to suppress anti-MM immunity and reduce tumor burden.
Discussion
Neutrophils are abundant immune cells in the BM,41 representing >50% of leukocytes in the TME, and ≤70% in BM. BM neutrophils are typically immature. They mature in peripheral blood and infiltrate inflammatory sites,32 but this study discovered a maturation continuum from immature to a predominant population of mature TREM1+MME+CXCR2+ TANs within FLs in MM, suggesting an alternate development and maturation trajectory in FLs. They likely are influenced by malignant PCs through the CXCL8-CXCR2 pathway,42,43 or other TME elements including mesenchymal stem cells,27 attracting them from peripheral blood. We found that FL TANs express and release substantial amounts of CXCL8 (IL-8), favoring the inflammatory chemokine hypothesis for CXCR2+ neutrophil recruitment. This aligns with murine pancreatic cancer scRNA-seq analyses, where mature neutrophils with proangiogenic and proinflammatory signatures are primary drivers of tumor growth.23 Of note, we elucidated mechanisms of how CXCR2 blockade alone or combined with chemotherapy decreases MM tumor burden, and underscoring the contribution of an intact adaptive immune response in the preclinical model.
CXCR2+ mature neutrophils are considered a homogeneous population in the circulation or tissues.44 We demonstrate that MM CXCR2+ neutrophils are heterogeneous, with 3 distinct subsets at different maturation stages. The RETN+LCN2+ subset has a low maturation score but expresses CXCR2, indicating incomplete maturation, as seen in infectious diseases.45,46 Previously, S100A8/S100A9+ immature neutrophils were found to promote megakaryocyte expansion and enhance MM progression.47 We observed a consistent S100A8/S100A9+ neutrophils presence across samples, regardless of disease status. Further studies are needed to determine their association with MM progression in patients.
FL and BM neutrophils exhibit distinct transcriptomic and functional profiles within their respective TMEs. Despite these differences, FL neutrophils follow a consistent maturation trajectory, suggesting that progenitor alterations arise within the TME. The MM TME appears to drive the development of protumor TANs, as recruited neutrophils adapt by promoting angiogenesis, immunosuppression, and malignant PC growth. FL CXCR2+ TANs secrete high levels of chemoattractant cytokines (CXCL1, CXCL2, CXCL8), reinforcing neutrophil recruitment via a positive feedback loop. Although CXCL1 and IL-1β (through BM mesenchymal stem cells) contribute to neutrophil infiltration,27,48 the mechanisms guiding CXCR2+ neutrophil recruitment and their role in MM progression remain unclear. Spatial transcriptomics has aided analysis of immune-tumor interactions in solid tumors; application to BM formalin-fixed paraffin-embedded slides is limited by the inability to accurately detect neutrophils.49
The FL neutrophil phenotype appeared independent of tumor burden, stage, location (OL vs EMD), FL number, or cytogenetic abnormalities, likely because patients were selected based on positron emission tomography-computed tomography evidence of active MM, ensuring clinical consistency. Due to our use of fresh BM biopsies with immediate cell sorting and scRNA-seq, we could not retrospectively determine postsorting PC percentages, limiting our ability to select patients based on percentage of PCs for analysis. However, FL neutrophil genotype correlated with MMRF CoMMpass patient outcomes. While the high “immature” gene signature was associated with OS and PFS, the high “mature” gene signature associated with inferior OS only. The lack of PFS association may reflect improved induction therapies. The inferior OS could be due to prolonged immune dysfunction. A better association may be found in fresh samples, as the CoMMpass data set samples are frozen.
This study provides valuable insights into neutrophil maturation in FLs of patients with MM, highlighting potential therapeutic targets for modulating the TME. Furthermore, our data indicate that targeting the neutrophil tumor TME to boost immune activity may offer a promising novel therapeutic approach. Understanding interactions between malignant PCs and neutrophils within the TME will be crucial for developing more effective antitumor immune treatments.
Acknowledgments
The authors thank Maximilian Merz, Kimberly Celotto, Kristina Slemmer, Amy R. McCracken, Mafoudh Saleh, Minhui Chen, Li Feng, and the Bone Marrow Laboratory team in the Department of Pathology for their support during the study. The authors also thank Marc Montminy and Mohamad Hussein for their helpful comments and insight regarding the manuscript.
This work is supported by National Heart, Lung, and Blood Institute, National Institutes of Health (NIH) grant R00HL155792, V Foundation grant T2024-017, and the Roswell Park Alliance Foundation (H.M.); an Institutional Educational Research Grant from the Celgene Corporation; and a donation from Brendan and Elise McCarthy through the Roswell Park Alliance (P.L.M.). M. Samur, N.C.M., and K.C.A. are supported by NIH Research Program Project grant P01-155258 and National Cancer Institute (NCI), NIH grant 5P50 CA100707; N.C.M. is supported by Department of Veterans Affairs Merit Review Award I01BX001584-01; and M. Samur, N.C.M., K.C.A., and J.R. are supported by The Paula and Rodger Riney Foundation. P.S. is supported by NCI, NIH Research Specialist Award R50CA283805. The Genomics, Biostatistics & Bioinformatics, and Flow & Image Cytometry Shared Resources are supported by NCI, NIH Cancer Center Support grant P30CA16056.
Authorship
Contribution: J.H., P.L.M., and H.M. were involved in the conception and design of the study; Q.Y., S.D., P.S., E.K., J.C., A.B., R.A., H.H., I.L., M. Schaefer, S.P., P.L.M., J.H., and H.M. were involved in the acquisition of data (acquired and managed patients, provided facilities, flow cytometry, suppression assay, etc); J.R., R.L., E.K., K.C.A., N.C.M., M. Samur, P.L.M., J.H., and H.M. were involved in the analysis and interpretation of data (eg, statistical analysis, biostatistics, computational analysis); and all authors were involved in the writing, review, and/or revision of the manuscript.
Conflict-of-interest disclosure: P.L.M. reports advisory board membership/consulting for, and honoraria from, BlueBird Bio, Bristol-Myers Squibb, Celgene, Fate Therapeutics, Janssen, Juno, Karyopharm, Magenta Therapeutics, Sanofi, and Takeda. J.H. reports advisory board membership/consulting for Johnson & Johnson, Regeneron, Prothena, Sebia, The Binding Site, GlaxoSmithKline, Bristol-Myers Squibb, Amgen, BeiGene, Angitia, and Pfizer. M. Samur is a consultant to AbbVie and K36 and is on the advisory board of Neuberg Center for Genomic Medicine. Q.Y., P.L.M., J.H., and H.M. hold a provisional patent titled “Discovering Anti-CXCR2 Inhibitor Alone or in Combination with Standard of Care for the Treatment of Multiple Myeloma–RP23-023/809466-01 US Provisional Application 63/546,962.” The remaining authors declare no competing financial interests.
Correspondence: Hemn Mohammadpour, Department of Cell Stress Biology, Center for Genetics and Pharmacology, Roswell Park Comprehensive Cancer Center, Elm St and Carlton St, Buffalo, NY 14263; email: hemn.mohammadpour@roswellpark.org.
References
Author notes
J.R. and Q.Y. contributed equally to this work.
M. Samur, P.L.M., J.H., and H.M. jointly supervised this work.
The raw and processed sequencing data (single-cell RNA sequencing) generated in this study have been deposited in the Gene Expression Omnibus database, and can accessed through the link: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE261171.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


![Visualization of heterogeneity of CXCR2+ TAN infiltration in MM BM and FLs. Vectra Polaris staining was applied to formalin-fixed, paraffin-embedded sections from 12 patient samples (BM, n = 12; paired FL, n = 12). Representative Vectra images from 3 individual patient samples ([A-C, top rows] original magnification ×10; [A-C, bottom rows] original magnification ×30). Differential marker and cell distribution of CD138+ tumor cells and CD16+ cells. (A) The absence of CXCR2+ cells in BM with <1% malignant PCs (CD138+). (B) Accumulation of CXCR2+ neutrophils along with an increase of CD138+ cells in BM. (C) Extensive intratumoral infiltration of CXCR2+ neutrophils with increased CD138+ cells. (D-F) Representative visualizations of the 3 mature TAN populations: (1) CXCR2+CD10+TNFAIP3+ cells (D, arrow), (2) CXCR2+CD10+TNFAIP3– cells (E, arrow), and (3) CXCR2+CD10–TNFAIP3– cells (F, arrow). These data highlight the TAN heterogeneity and diversity within neutrophil populations in MM, emphasizing distinct phenotypic and functional subsets that may contribute variably to the TME.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/146/20/10.1182_blood.2025028963/2/m_blood_bld-2025-028963-gr4.jpeg?Expires=1766100971&Signature=z8rNtfSg2s04yYPpNFK0mKmNqGBCY~74NPM7ICe50o4zzjZjT3fakEwnxm2dNblIF-QST3q2lI1M4tf9uTRIm2aUZF61fqGNosjWNFH1GlLpFLxJ1u0ICbCyOclDZefMop6Z7ncD4QQMGr72u0TvvZcrqLJz-aEERkBusey5YXys02CVndsmgaeFGdoA4j4vMDxXYr3hZX8CF91DFeocRIi0a0LEtnUrFw6vjE9Tj3UocA11GPqjdl9zrZcj53-tQaljv~GDYeKc6BCbZk8N65RHXBpLI3VzNdo9Ha4t6jH20ns04yuQs~clto5QZ6SWnDe76dY6C40zGBC2vpOndQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![CXCR2 blockade promotes MM control in murine MM models. Vk12653 murine MM cell lines were injected into recipients for different in vivo tumor models (see “Materials and methods” for full details). We used the following models: the NDMM model (tumor cell injection without irradiation, n = 40 from 2 experiments), the RRMM model (tumor cell injection followed by total body lethal irradiation, 1000 cGy, and syngeneic [autologous] stem cell transplant, n = 40 from 2 experiments), the CD8+ T-cell depletion mouse model (tumor cell injection without irradiation, n = 40 from 1 experiment), and the NSG mouse model (tumor cell injection without irradiation, n = 30 from 1 experiment). Progression of MM was confirmed by weekly serum protein electrophoresis measurement of the monoclonal protein (M-spike) to albumin ratio in the NDMM model. In the RRMM model, the mice were classified as MM-relapsed or MM-remission based on the M-spike to albumin ratio. Recipient BM was harvested and stained with appropriate antibodies. Results were analyzed via flow cytometry. (A) Quantification of frequency and number of CXCR2+ neutrophil clusters from the samples of CBM (no tumor injection), MTB, and HTB, the latter 2 based on serum protein electrophoresis measurements. Cells gated from CD11b+Ly6G+Ly6C– population. (B) Quantitation of M-spike development, and survival curve in the NDMM model. (C) UMAP representation of immune cells (excluding macrophages and neutrophils) observed in various experimental arms in the NDMM model. Cell type distribution highlighted below UMAP to show changes in cell kinetics upon treatment. (D) Concatenated mature neutrophils show distinct developmental stages observed in humans with little variance among experimental conditions: EIN represent x¯ = 22.39%, σ = 2.06%, LIN represent x¯ = 22.87%, σ = 1.36%, EMN represent x¯ = 28.23%, σ = 0.66%, LMN represent x¯ = 26.5%, σ = 2.96%. Neutrophils were scored with the same signatures as Figure 2. (E) Quantitation of M-spike development, and survival curve in T-cell depletion mouse model. (F) Quantitation of M-spike development, and survival curve in NSG mouse model. (G) Quantitation of M-spike development, survival curve, and CD8+ T-cell cytokine expression of IFN-γ and TNF-α. Data represent mean ± standard error of the mean. The 2-way analysis of variance was used to analyze statistical significance among tumor growth in different groups. Survival data are presented as percent survival (log-rank Mantel-Cox test). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. CBM, control bone marrow; EIN, early immature neutrophils; EMN, early mature neutrophils; HTB, high tumor burden bone marrow; LIN, late immature neutrophils; LMN, late mature neutrophils; MTB, moderate tumor burden bone marrow; ns, not significant; PBS, phosphate buffered saline; TNF-α, tumor necrosis factor-α.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/146/20/10.1182_blood.2025028963/2/m_blood_bld-2025-028963-gr6g.jpeg?Expires=1766100971&Signature=vjgCnTAqp3saYywLYVcGohenTk9PSCgO4fQ-3Dmb0tdwmVDpOjVpFePr1o8v6SeSS2YWWnmsCEo1B5GjYzFmugpeGQS3d9gTQcZKIhDn9ALLEOWaHy~CZc2U0mj9QF2Y-9~Hk3J608Ufwc~Uanz8f5oVfxj7Xk~47wsFMZ0bIOrraqOuI6CDSjhdhAwTjgNmp2FaIIVqYn8eeiChX-VwF3KWpHPQ3CK1YBjO9dztgwItRCp42TyrILPQ4LiQyj6bOcqy-6IFcZCan~j85DnkPKwoT6B2t0cxJaS6Enn8AAugB7Rbfcgtzbny0soexzQ1htw9vbD~v0IA~wDykTimZg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal