Key Points
Weekly InO 1.8 mg/m2 per cycle is associated with manageable toxicities and encouraging activity in patients with relapsed/refractory ALL.
Achievement of MRD negativity and disease burden was not correlated; InO may thus be effective regardless of baseline disease severity.
Abstract
This study evaluated the safety, antitumor activity, pharmacokinetics, and pharmacodynamics of inotuzumab ozogamicin (InO) for CD22-positive relapsed/refractory acute lymphoblastic leukemia. In phase 1, patients received InO 1.2 (n = 3), 1.6 (n = 12), or 1.8 (n = 9) mg/m2 per cycle on days 1, 8, and 15 over a 28-day cycle (≤6 cycles). The recommended phase 2 dose (RP2D) was confirmed (expansion cohort; n = 13); safety and activity of InO were assessed in patients receiving the RP2D in phase 2 (n = 35) and in all treated patients (n = 72). The RP2D was 1.8 mg/m2 per cycle (0.8 mg/m2 on day 1; 0.5 mg/m2 on days 8 and 15), with reduction to 1.6 mg/m2 per cycle after complete remission (CR) or CR with incomplete marrow recovery (CRi). Treatment-related toxicities were primarily cytopenias. Four patients experienced treatment-related venoocclusive disease/sinusoidal obstruction syndrome (VOD/SOS; 1 fatal). Two VOD/SOS events occurred during treatment without intervening transplant; of 24 patients proceeding to poststudy transplant, 2 experienced VOD/SOS after transplant. Forty-nine (68%) patients had CR/CRi, with 41 (84%) achieving minimal residual disease (MRD) negativity. Median progression-free survival was 3.9 (95% confidence interval, 2.9-5.4) months; median overall survival was 7.4 (5.7-9.2) months for all treated patients, with median 23.7 (range, 6.8-29.8) months of follow-up for all treated patients alive at data cutoff. Achievement of MRD negativity was associated with higher InO exposure. InO was well tolerated and demonstrated high single-agent activity and MRD-negativity rates. This trial was registered at www.clinicaltrials.gov as #NCT01363297.
Introduction
Acute lymphoblastic leukemia (ALL) is a heterogeneous malignancy with an estimated incidence of 6250 newly diagnosed cases in 2015.1,2 Although the majority (58%) of these cases were estimated to be among pediatric and young adult patients aged <20 years, 26% were among adults ≥45 years old.1,2
Current treatments for adults with newly diagnosed B-cell ALL yield complete remission (CR) rates ranging from 60% to 90%3-12 ; however, many patients eventually relapse and only ∼30% to 50% maintain extended disease-free survival for ≥3 years.6-10 Moreover, overall survival (OS) rate at 5 years after relapse has been reported to be only 7%.13 For B-cell ALL that has relapsed or is refractory to standard chemotherapy, CR rates decrease with successive salvage treatments (eg, ∼30% to 40% in first and ∼10% to 25% in second or later salvage).3,7,14 Because CR is required for ensuing allogeneic stem cell transplant (SCT), few (5% to 30%) patients in second or later salvage are eligible for allogeneic SCT, which remains the only potentially curative option and the primary objective of treatment after relapse.3,13-16
Salvage therapies for B-cell ALL using cytotoxic chemotherapy combinations possess limited efficacy and are frequently associated with significant toxicities,16,17 which are further exacerbated by dose intensification or by the addition of other agents.18 Targeted antibody-drug conjugate therapies can minimize exposure of normal tissues to a conjugated cytotoxic agent.19 Lineage-specific antigens expressed on the surface of leukemic cells function as targets for antibody-drug conjugate treatments and are used as diagnostic markers and for classifying immunologic subtypes.20 CD22, a B-cell–restricted sialic acid–recognizing immunoglobulin superfamily lectin (Siglec),21 is expressed on leukemic blasts in >90% patients with B-cell ALL22-25 and is not shed into the extracellular compartment.26,27 CD22 has therefore become an important therapeutic target in B-cell ALL and other B-cell malignancies.28-30
Inotuzumab ozogamicin (InO; CMC-544) is a humanized CD22 monoclonal antibody conjugated to calicheamicin, a cytotoxic antibiotic.31-33 After binding to CD22, InO is rapidly internalized into lysosomes, where calicheamicin is released to bind the minor DNA groove, leading to double-strand cleavage and subsequent apoptosis.31,33-37 A previous single-institution phase 2 study (N = 90) demonstrated that InO, administered on either a single-dose monthly or a weekly schedule, is active and tolerable in patients with relapsed/refractory ALL (overall response rate, 58%; median duration of remission [DOR], 7 months; minimal residual disease [MRD]–negativity rate among responders, 72%; median survival, 6.2 months).18,38
This phase 1/2 multisite study consisted of a dose-escalation phase 1 part that determined the recommended phase 2 dose (RP2D) for evaluation of the safety and antitumor activity of InO administered on a weekly schedule to patients with CD22-positive relapsed/refractory B-cell ALL. The study is also the first to undertake exploratory pharmacokinetic (PK), pharmacodynamic, and pharmacogenomic (PG) analyses to examine the following potential correlations: (1) InO PK with peripheral blood B-lymphocyte depletion and regeneration; (2) bone marrow (BM) blasts with B-lymphocyte depletion and regeneration; (3) InO PK and BM blast counts with achievement of MRD negativity; and (4) InO treatment outcomes with expression of genes, including those involved in DNA damage response, apoptosis, B-cell antigen expression, glutathione metabolism, drug transport, and the PI3K/mTOR pathway.
Methods
Patients
Eligible patients were adults (≥18 years) diagnosed with CD22-positive ALL (≥20% blasts CD22-positive based on local immunophenotyping and histopathologic evaluation) that was refractory (disease progression/no response during most recent prior therapy) or relapsed (response to most recent prior therapy with subsequent relapse). Patients enrolled in phase 2 were in second or later salvage. Patients with lymphoblastic lymphoma with BM involvement with ≥5% lymphoblasts were eligible. Patients with peripheral absolute lymphoblast count ≥25 000/µL were included if treated with hydroxyurea or steroids within 2 weeks of the first dose to reduce white blood cell count to ≤25 000/µL; those with Philadelphia chromosome–positive ALL must have failed standard treatment with ≥1 tyrosine kinase inhibitor. Other key eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status 0-3; adequate liver and renal function; no isolated extramedullary relapse or active central nervous system leukemia; and no prior allogeneic hematopoietic SCT (HSCT) ≤4 months before first dose. Concurrent therapy for central nervous system prophylaxis was permitted.
The protocol was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice and was approved by Institutional Review Boards at each participating institution. Participants provided written informed consent before initiation of study-related activities.
Study design
This study was a prospective, open-label, phase 1/2 study performed at 8 sites in the United States. In phase 1, patients received InO (1-hour intravenous infusion) in the following dose cohorts (Table 1): total dose 1.2 mg/m2 per cycle (0.8 mg/m2 [day 1]; 0.4 mg/m2 [day 15]); 1.6 mg/m2 per cycle (0.8 mg/m2 [day 1]; 0.4 mg/m2 [days 8 and 15]); 1.8 mg/m2 per cycle (0.8 mg/m2 [day 1]; 0.5 mg/m2 [days 8 and 15]). Cycles were repeated every 28 days (contingent upon meeting dose-delay/redosing criteria [supplemental Materials]); treatment continued for ≤6 cycles or until disease progression, consent withdrawal, or prolonged/excessive toxicity. Patients with suitable donors could undergo allogeneic HSCT after InO treatment at the investigator’s discretion.
To determine the RP2D, 24 patients were enrolled into 3-patient cohorts using a traditional 3+3 design for the first 2 cohorts, and the adaptive dose-finding method of Thall and Cook39,40 using EffTox41 for the subsequent cohorts. EffTox takes into account toxicity (dose-limiting toxicity [DLT]) and efficacy (response better than progressive disease [PD]; see supplemental Materials for cohort assignment decision rules and candidate InO doses). The phase 1 expansion cohort included 13 patients who received the RP2D (1.8 mg/m2 per cycle); with dose reduction to 1.6 mg/m2 per cycle for patients achieving a CR or CR with incomplete marrow recovery (CRi). Thirty-five additional patients received InO at the RP2D in phase 2.
Safety and efficacy assessments
Safety was assessed from physical examinations, vital signs, 12-lead electrocardiograms, laboratory evaluations, and adverse events (AEs), graded using National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0, and monitored throughout the study and for ≥28 days after the last dose inclusive of the end-of-treatment visit. All treatment-related AEs were monitored until resolution, return to baseline, or stabilization. Venoocclusive disease/sinusoidal obstruction syndrome (VOD/SOS) was reported for up to 2 years after the first dose (see supplemental Materials for VOD/SOS definition).
The primary phase 2 endpoint was CR/CRi; key secondary endpoints included MRD negativity and cytogenetics in responders, DOR, OS, progression-free survival (PFS), PK, pharmacodynamics, and PG. BM aspirates (and/or biopsies if clinically indicated) and disease assessments were performed at screening, on day 21 (±7 days) of each cycle, at the end-of-treatment visit, during follow-up visits (every 12 weeks after the last disease assessment for 1 year from first dose and every 24 weeks in year 2 until PD, initiation of poststudy anti–cancer therapy, or until achievement of 2 years of follow-up), and as clinically indicated (see supplemental Materials for definitions used for response and PD status assessments). DOR was defined as the time from first CR/CRi to relapse after CR/CRi, treatment discontinuation due to health deterioration, or death, whichever occurred first. MRD negativity was defined as <0.01% leukemic blasts per total nucleated mononuclear BM cells, analyzed centrally using multiparametric flow cytometry. Patients were followed for survival for up to 2 years.
PK, pharmacodynamic, and PG assessments
Serum InO and unconjugated calicheamicin concentrations were determined from blood samples drawn during days 1 and 15 of cycles 1 and 2 (at 0, 1, and 3 hours), and day 1 of cycle 4 (at 0 and 1 hour). Samples were analyzed using a validated liquid chromatography coupled to tandem mass spectrometry procedure (Pharmaceutical Product Development Laboratories, Richmond, VA; lower limit of quantitation [LLOQ] = 1 ng/mL for InO; LLOQ = 50 pg/mL for unconjugated calicheamicin).
Circulating B-lymphocyte count was determined in blood samples collected on days 1, 8, 15, and 21 of cycles 1 through 6, at the end of treatment, and for 4 to 6 weeks after the last dose. CD22 expression on circulating CD19+ B-lymphocytes was determined in blood samples collected predose, at the end of infusion on days 1 and 15 of cycles 1 and 2, and on day 1 of cycle 4.
For assessments of InO PK and pharmacodynamic effects on the achievement of MRD negativity (defined as above), BM aspirates were collected at screening, day 28 of cycles 1 through 6, and at the end of treatment. Dose-normalized maximum (Cmax, dn) and minimum (Cmin, dn) serum InO concentrations were determined from the PK samples collected on days 1 and 15 of each cycle.
To examine correlations between clinical outcome and gene expression, peripheral whole blood samples were collected in PAXgene tubes (QIAGEN, Valencia, CA) on days 1 and 15 of cycle 1 (each at predose and postdose time points). Messenger RNA (mRNA) levels were determined using custom 96-gene format TaqMan Low Density Array cards (Thermo Fisher Scientific, Waltham, MA). Reference genes used were ACTB, B2M, GAPDH, and PGK1; mRNA levels for each target gene were reported as a normalized value, 2−ΔCt, where ΔCt = Ct target gene − Ct reference genes, averaged.
Statistical analysis
The estimated maximum sample size for the phase 1 dose-escalation part was 36 patients. However, this could not be precisely determined a priori due to the outcome-adaptive nature of the Bayesian procedure used for cohort assignment; 24 patients were ultimately enrolled. An interim futility analysis (IA) was to be performed based on 10 evaluable patients receiving the RP2D in the phase 1 dose escalation part: the trial was to be stopped if none achieved CR/CRi; otherwise, 12 additional patients were to be enrolled in a dose expansion cohort (13 patients were ultimately enrolled). For phase 2, a maximum sample size of 35 patients was estimated based on a 2-stage design using swogstat.42 In an IA of the first 12 patients in stage 1, an additional 23 patients were to be enrolled in stage 2 if ≥2 of these 12 patients achieved CR/CRi (see supplemental Materials for details of hypotheses).
All enrolled patients received treatment; safety and antitumor activity were assessed in all treated patients. DOR, PFS, and OS distributions were summarized using the Kaplan-Meier method. PK and pharmacodynamic analyses were based on patients with ≥1 quantifiable InO and/or unconjugated calicheamicin concentration or PK parameter of interest. PG analyses were based on enrolled patients who had received ≥1 InO dose and with ≥1 sample with both a baseline and a posttreatment assessment.
Results
Patients
A total of 72 patients received ≥1 dose of InO (phase 1 dose escalation, n = 24; phase 1 dose expansion, n = 13; phase 2, n = 35; supplemental Figure 1). The median age was 45 (range, 20-79) years; 22% had Ph+ disease, and 32% had received prestudy SCT; 78% had received ≥2 salvage treatments; 21% had complex cytogenetics (≥5 chromosomal aberrations); 60% had first CR duration <12 months; and 74% had ECOG performance status score 0-1 (Table 2).
Patients completed median 3 (range, 1-6) treatment cycles. Median treatment duration was 2.1 (range, 0.02-9.5) months; median follow-up was 12.1 (range, 6.8-12.8) months for patients in phase 2 alive at the time of data cutoff and 23.7 (range, 6.8-29.8) months for all treated patients alive at the time of data cutoff. As of the data cutoff date (30 January 2015), 96% of patients had discontinued treatment, most commonly due to PD/relapse (42%), proceeding to HSCT (25%), or AEs (18%); 18 patients were alive.
RP2D determination and confirmation
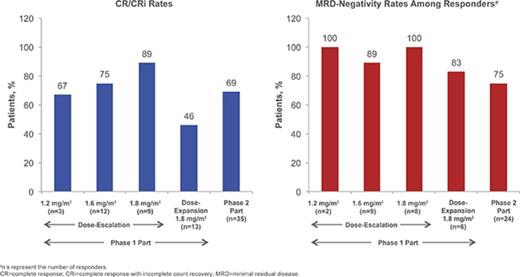
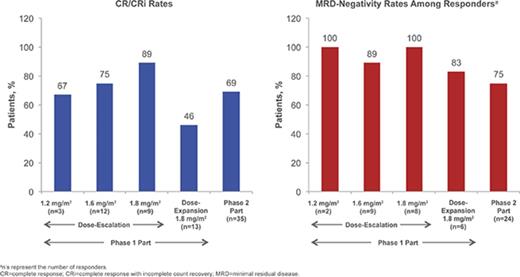
Details of patient enrollment and associated DLTs in the phase 1 dose-escalation portion are presented in supplemental Materials; briefly, no DLTs occurred in the 1.2- and 1.6-mg/m2 per cycle cohorts and 1 DLT occurred in the 1.8-mg/m2 per cycle cohort (grade 4 elevated lipase). For the 1.2-, 1.6-, and 1.8-mg/m2 per cycle cohorts, respectively, the CR/CRi rates were 67% (n = 2/3 [95% confidence interval (CI) 9% to 99%]), 75% (n = 9/12 [43% to 95%]), and 89% (n = 8/9 [52% to 100%]), of whom 2, 8, and 8 patients achieved MRD negativity (overall: 95% [n = 18/19]). Based on considerations of CR/CRi, MRD-negativity rates, and DLTs, the RP2D was determined to be 1.8 mg/m2 per cycle (0.8 mg/m2 [day 1]; 0.5 mg/m2 [days 8 and 15]) as a starting dose. Subsequent dose reduction to 1.6 mg/m2 per cycle (0.8 mg/m2 [day 1]; 0.4 mg/m2 [days 8 and 15]) was recommended upon achievement of CR/CRi, based on the previous observations: (1) InO exposure increases with subsequent doses following remission,43 (2) tumor burden or blast count may be related to exposure, (3) dose modifications are common later in therapy, and (4) 7/9 (78%) patients in the 1.8 mg/m2 per cycle cohort experienced AEs leading to dose delay.
In a futility IA of 10 evaluable patients receiving the RP2D in the phase 1 dose-escalation part (see supplemental Materials), ≥1 achieved CR/CRi (n = 7). Per protocol, 13 additional patients were enrolled in the dose-expansion cohort to confirm the RP2D, among whom no DLTs were observed. Six (46% [95% CI, 19% to 75%]) of these patients achieved CR/CRi, of whom 5 (83%) achieved MRD negativity. In a futility IA of the first 12 evaluable patients receiving the RP2D in stage 1 of phase 2, ≥2 achieved CR/CRi; per protocol, 23 patients were enrolled in stage 2 without interruption, for a total of 35 patients in phase 2 of the study.
Safety and tolerability
Among all treated patients (n = 72), the most frequent treatment-related treatment-emergent AEs (TEAEs; ≥15% of patients) were thrombocytopenia (any grade, 36%; grade ≥3, 33%), neutropenia (28%; 25%), aspartate aminotransferase (AST) increased (26%; 3%), nausea (21%; 0%), vomiting (17%; 1%), fatigue (15%; 0%), and febrile neutropenia (15%; 13%; Table 3). The incidence of all-causality TEAEs followed a similar pattern (supplemental Table 1). The most common serious TEAE was febrile neutropenia (22%).
Treatment-related hepatic TEAEs included elevated AST (any grade, 26%; grade ≥3, 3%), γ-glutamyltransferase (GGT: 13%; 1%), alanine aminotransferase (ALT; 11%; 3%), and hyperbilirubinemia (10%; 0%). Four VOD/SOS cases were reported (1 during phase 1 dose expansion [1.8 mg/m2] and 3 during phase 2), 2 confirmed by biopsy; all were considered treatment related and 1 was fatal. One VOD/SOS event occurred during treatment (patient recovered with sequelae of ascites and was able to proceed to allogeneic HSCT without further liver toxicity); another occurred during follow-up without intervening HSCT, shortly after starting maintenance therapy with ponatinib and continued until death due to pneumonia. Three of the 4 patients with VOD/SOS had received defibrotide (2 recovered; 1 had VOD/SOS ongoing at the time of death due to PD). Of 24 patients who proceeded to HSCT, 2 experienced VOD/SOS after poststudy HSCT. These 2 patients received pretransplant conditioning with cyclophosphamide plus total body irradiation (TBI) and etoposide plus fractionated TBI.
Of all treated patients, 12 (17%) permanently discontinued treatment due to TEAEs, including ascites (n = 2) and liver-related TEAEs (elevated ALT, AST, alkaline phosphatase [AP], GGT, and VOD [n = 1 each]; supplemental Table 2). Seven patients (10%) reduced dose due to TEAEs, most commonly thrombocytopenia (n = 3) and neutropenia (n = 3); 37 had dose delays due to TEAEs, most commonly thrombocytopenia (n = 10), AST increased (n = 9), neutropenia (n = 10), and ALT increased (n = 6; supplemental Table 2).
Fifty-four deaths (75%) occurred, including 12 during treatment and up to 42 days after last dose and 42 during follow-up. Causes of death during treatment included PD (n = 9), septic shock, pneumonia, and subdural hematoma (n = 1 each).
Efficacy
Of the 35 patients receiving the RP2D in phase 2, 24 (69%) achieved CR/CRi (1-sided P < .0001 for the null hypothesis: CR/CRi rate ≤20%); therefore, the primary phase 2 endpoint (CR/CRi) was met (Table 4). Potential prognostic factors (supplemental Table 3) were also assessed.
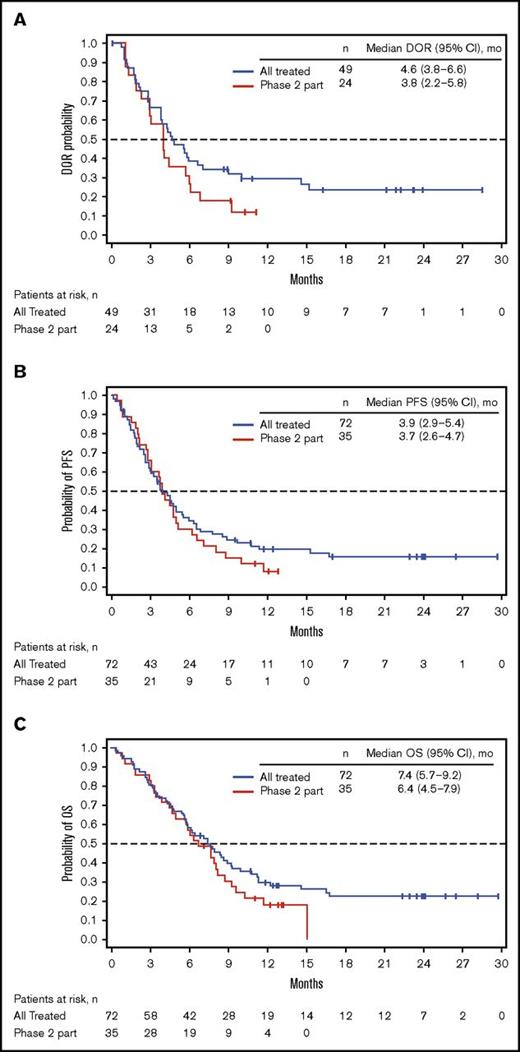
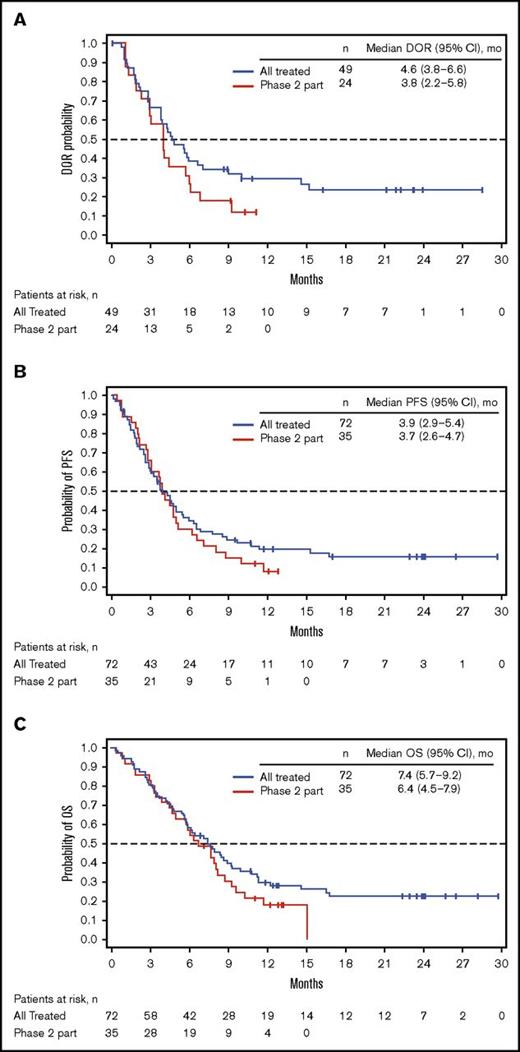
Among all patients treated (n = 72), 49 (68%) achieved CR/CRi (CR, n = 23; CRi, n = 26; Table 4), with a median time to CR/CRi of 27.0 (range, 15-91) days. The median DOR was 4.6 (95% CI, 3.8-6.6) months (Figure 1A). Ten patients who initially had CRi later achieved CR during treatment. Of the 49 patients with CR/CRi, 41 (84%) achieved MRD negativity (median time to MRD negativity, 29.0 [range, 21-141] days); 18 (37%) relapsed during treatment or within 30 days of the last dose. Median DOR and MRD-negativity rates among patients achieving CRi, CR, and CR/CRi were similar (Table 4).
Kaplan-Meier distributions. (A) DOR. (B) PFS. (C) OS. See supplemental Materials for DOR, PFS, and OS definitions. PR, partial response.
Kaplan-Meier distributions. (A) DOR. (B) PFS. (C) OS. See supplemental Materials for DOR, PFS, and OS definitions. PR, partial response.
For all treated patients, median PFS and OS were 3.9 (95% CI, 2.9-5.4) and 7.4 (95% CI, 5.7-9.2) months; the Kaplan-Meier estimated probabilities of PFS and OS at 12 months were 0.20 (95% CI, 0.10-0.30) and 0.30 (95% CI, 0.20-0.40), respectively (Figure 1B-C). The effect of BM blast count or salvage status on PFS is shown in supplemental Table 4.
In all treated patients, 24 (33%) proceeded to poststudy HSCT (median time to HSCT, 45.5 [range, 20-148] days), nearly all of whom (n = 22/24 [92%]) had achieved CR/CRi before HSCT. Of the 2 patients who proceeded to HSCT without achieving CR/CRi, 1 had a partial response and 1 had disease resistant to InO treatment and initiated new (non-InO) induction therapy before HSCT. Before HSCT, most patients (n = 15/24 [63%]) had received fludarabine- and/or TBI-based conditioning regimens (n = 17/24 [71%]); only 1 had received dual alkylator conditioning (cyclophosphamide, thiotepa, and fludarabine). Of the 24 patients who had poststudy HSCT, 12 subsequently died (2 died due to relapse/PD). Seven of the 12 patients died ≤100 days after poststudy HSCT (1 died due to relapse/PD). Other causes of death after poststudy HSCT were sepsis (n = 4), complications of HSCT, gut graft-versus-host disease/liver dysfunction, hepatic failure/SOS, respiratory failure, Klebsiella pneumoniae/liver graft-versus-host disease, and unknown (each n = 1). During the study, 4 patients had extramedullary disease that regressed, and 4 of the patients had extramedullary disease that remained stable or increased in size. The 27 patients who achieved CR/CRi and did not have poststudy HSCT received median 4 (range, 1-6) treatment cycles; 5 (19%) of these patients were aged ≥65 years; 15 (56%) received prestudy SCT; 21 (78%) received ≥2 salvage treatments; 16 (59%) experienced PD/relapse <1 month of discontinuing treatment; 3 (11%) died <1 month of discontinuing treatment (1 due to relapse/PD); and 11 (41%) initiated a new induction systemic therapy during follow-up.
Pharmacokinetics
Mean peak serum InO concentrations were observed at or near the end of infusion; these generally increased with cycle number (supplemental Table 5). Unconjugated calicheamicin concentrations were below the LLOQ for most patients and time points.
Peripheral blood B-lymphocyte depletion and regeneration
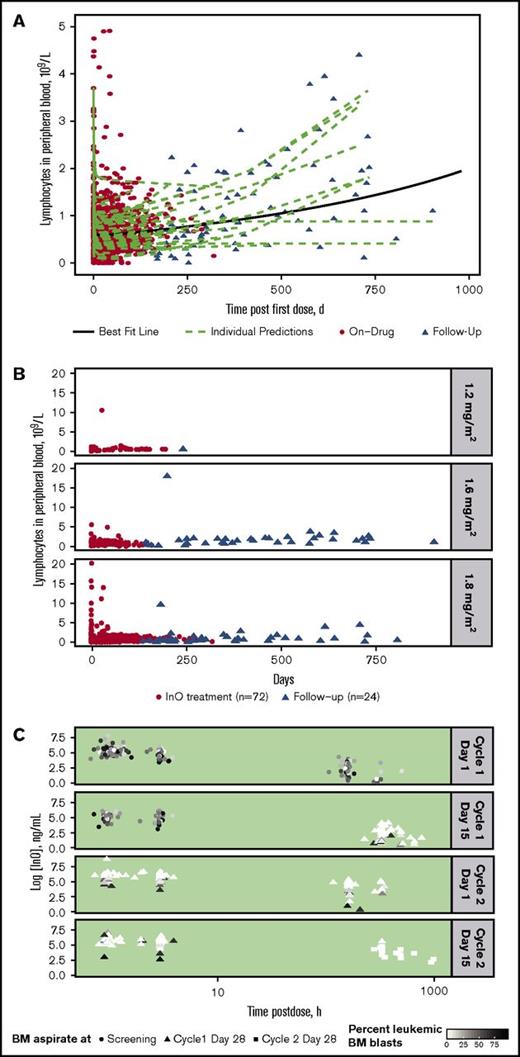
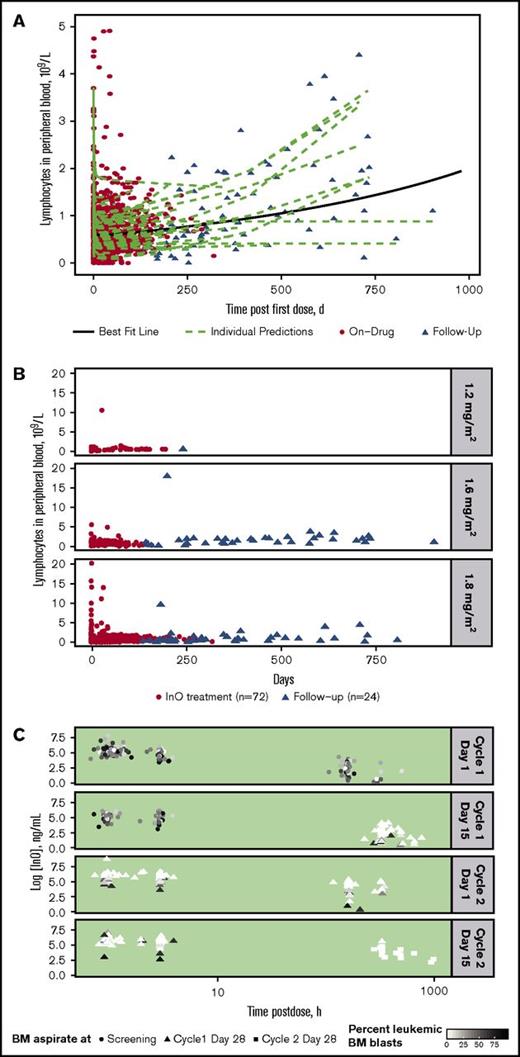
For all treated patients (n = 72), InO treatment was accompanied by a consistently rapid depletion followed by a slow regeneration of B-lymphocytes in peripheral blood during follow-up, with substantial interpatient differences in regeneration rate (Figure 2A). A similar rapid depletion followed by slow regeneration of B-lymphocytes was apparent regardless of InO dose, although few patients received the lower doses (Figure 2B).
Peripheral blood B-lymphocyte depletion and regeneration. (A) B-lymphocyte concentration in blood was determined vs time for individual patients during InO treatment (red circles; n = 72) and during follow-up (triangles; n = 24). Data were fitted to a linear mixed-effects model (green dashed lines = individual model predictions; solid black line = best fit line for overall mean effect of time on B-lymphocyte depletion and regeneration). (B) B-lymphocyte concentration profiles vs time by InO dose (1.2, 1.6, and 1.8 mg/m2 per cycle) for individual patients. (C) InO elimination vs time by percent of BM blasts for individual patients. The 4 panels show serum InO concentration vs time data for InO dosing at cycle 1 day 1 and 15 and cycle 2 days 1 and 15. BM aspirates were collected at screening (circles), cycle 1 day 28 (triangles), and cycle 2 day 28 (squares). Symbol colors are scaled by percent leukemic blasts in BM.
Peripheral blood B-lymphocyte depletion and regeneration. (A) B-lymphocyte concentration in blood was determined vs time for individual patients during InO treatment (red circles; n = 72) and during follow-up (triangles; n = 24). Data were fitted to a linear mixed-effects model (green dashed lines = individual model predictions; solid black line = best fit line for overall mean effect of time on B-lymphocyte depletion and regeneration). (B) B-lymphocyte concentration profiles vs time by InO dose (1.2, 1.6, and 1.8 mg/m2 per cycle) for individual patients. (C) InO elimination vs time by percent of BM blasts for individual patients. The 4 panels show serum InO concentration vs time data for InO dosing at cycle 1 day 1 and 15 and cycle 2 days 1 and 15. BM aspirates were collected at screening (circles), cycle 1 day 28 (triangles), and cycle 2 day 28 (squares). Symbol colors are scaled by percent leukemic blasts in BM.
InO elimination according to percentage of leukemic blasts in BM (relative to total leukocytes) separated by dosing events is shown for individual patients in Figure 2C. As shown by the color gradient of the points (darker = higher percent blasts; lighter = lower percent blasts), the percentage of leukemic blasts in BM decreased over time and with each cycle, with very low percentages observed at later time points. In general, the InO serum elimination rate constant decreased with time on treatment (∼50% at C4D1 compared with C1D1 [data not shown]); this decrease tended to be less in patients who had relatively higher proportions of leukemic BM blasts, suggesting a direct relationship between the extent of InO elimination and BM blast level.
BM blasts and MRD negativity
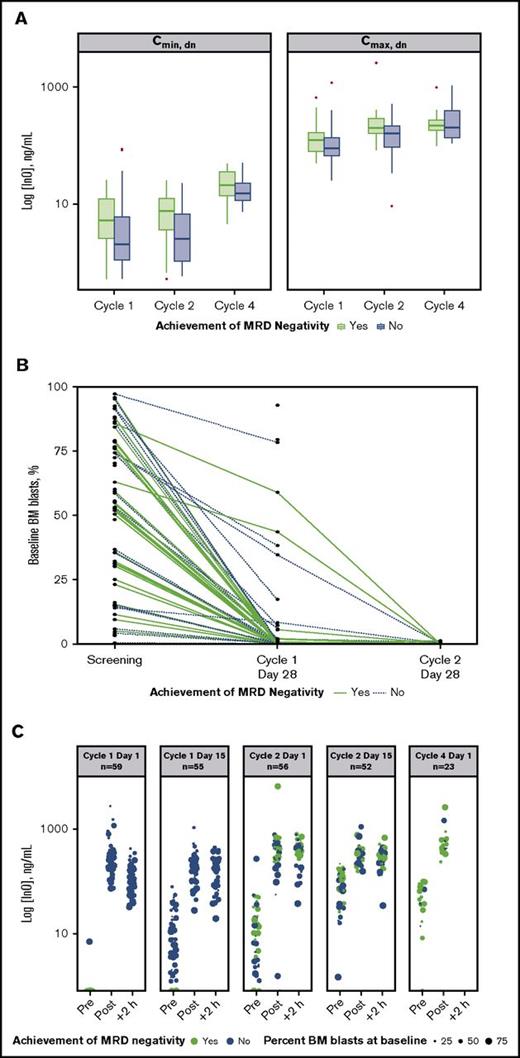
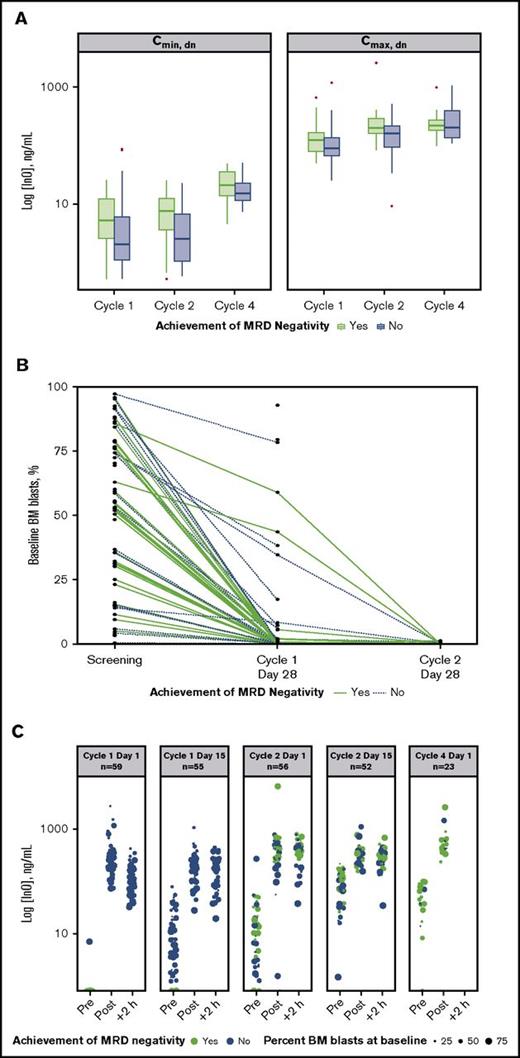
An analysis of dose-normalized Cmax and Cmin by MRD-negativity status indicated that patients achieving MRD negativity tended to have higher peak and trough InO concentrations throughout treatment vs those who did not (Figure 3A). In contrast, achievement of MRD negativity appeared to be unrelated to baseline disease burden (percentage baseline BM blasts). Evidence supporting this is provided by plots of baseline BM blast percentages determined for individual patients at each protocol-specified BM aspirate collection time (Figure 3B), which show that line profiles for patients achieving MRD negativity (green lines) originated across a range of baseline BM blast percentages. This lack of a relationship between the percentage of BM blasts at baseline and achievement of MRD negativity was also apparent from the serum InO concentration time course during cycles 1 through 4 (Figure 3C).
Relationship between InO PK parameters and MRD status. (A) Box plots of dose normalized (dn) values of median Cmax and Cmin by cycle (1 to 4) and by achievement of MRD negativity (yes vs no). Boxes correspond to the 25% and 75% percentiles; whiskers extend to the most extreme data point that is no more than 1.5 times the length of the box away from the box. Red points represent potential outliers and correspond to individual values >1.5 times the interquartile range above or below the respective quartile. (B) Relationship between percentage BM blasts at baseline and achieving MRD negativity for individual patients. Green and blue plots correspond to patients achieving and not achieving MRD negativity, respectively. (C) The 5 panels show serum InO concentration vs time by MRD-negativity status for dosing at cycle 1 day 1 and 15, cycle 2 days 1 and 15, and cycle 4 day 1. Symbol sizes represent percent leukemic blasts in BM.
Relationship between InO PK parameters and MRD status. (A) Box plots of dose normalized (dn) values of median Cmax and Cmin by cycle (1 to 4) and by achievement of MRD negativity (yes vs no). Boxes correspond to the 25% and 75% percentiles; whiskers extend to the most extreme data point that is no more than 1.5 times the length of the box away from the box. Red points represent potential outliers and correspond to individual values >1.5 times the interquartile range above or below the respective quartile. (B) Relationship between percentage BM blasts at baseline and achieving MRD negativity for individual patients. Green and blue plots correspond to patients achieving and not achieving MRD negativity, respectively. (C) The 5 panels show serum InO concentration vs time by MRD-negativity status for dosing at cycle 1 day 1 and 15, cycle 2 days 1 and 15, and cycle 4 day 1. Symbol sizes represent percent leukemic blasts in BM.
Baseline mRNA levels in peripheral whole blood and relationship with clinical outcome
Baseline levels of several mRNAs were significantly higher in patients who subsequently achieved CR/CRi and MRD negativity vs those who did not achieve CR/CRi, including mRNAs encoded by genes implicated in stress response/drug resistance (ABCG2, median sevenfold higher [P = .0071]; BCL2L1, median 11-fold higher [P = .0055]; FASLG, median fourfold higher [P = .036]). No relationship was apparent between baseline peripheral blood CD22 mRNA levels and achieving CR/CRi and MRD negativity.
Peripheral blood CD22 mRNA levels were decreased by day 15 relative to baseline (median, 97% decrease; P < .0001), consistent with selective ablation of CD22-positive leukemic blasts by InO (supplemental Figure 2). CD22 mRNA levels in patients who subsequently achieved CR/CRi and MRD negativity were lower by day 15 vs those who did not achieve CR/CRi (P = .01). Comparable reductions and trends were evident for several other mRNAs encoding B-cell lineage markers, including PAX5, VPREB1, and EBF1 (data not shown). Conversely, mRNA levels for several genes implicated in stress response, drug resistance, and apoptotic regulation were two- to fivefold higher by day 15 relative to baseline (ABCG2, BCL2L1, FASLG; all P < .0001). P values for these PG analyses were not adjusted for the potential for false discovery due to the large number of samples assessed; hence, these findings are considered preliminary.
Discussion
In this phase 1/2 trial of single-agent InO for relapsed/refractory CD22-positive ALL, the RP2D was 1.8 mg/m2 per cycle (0.8 mg/m2 [day 1]; 0.5 mg/m2 [days 8 and 15]) as a starting dose, with subsequent dose reduction to 1.6 mg/m2 per cycle upon achievement of CR/CRi. Toxicities associated with treatment were primarily cytopenias and liver-related toxicities. Encouraging clinical activity was observed, with 68% of all treated patients achieving CR/CRi and 33% of patients able to proceed to poststudy HSCT. Nevertheless, remission was often transitory with 18 (37%) patients relapsing during treatment or within 30 days of the last dose; consolidation with HSCT for suitable patients with readily available donors may extend the DOR. The short-lived remission might appear at odds with the observed high MRD-negativity rate (84%) among responders, which has been shown to be prognostic of prolonged remission following induction therapy for de novo ALL.44 However, the distribution of MRD markers used at initial diagnosis can change at relapse due to clonal evolution,45 suggesting that MRD-negativity rate in second or subsequent remission may have decreased prognostic value in DOR compared with that in the de novo ALL setting.
The observation that >50% of patients with CR/CRi achieved a CRi as opposed to a CR raises the question whether the CR rate may have been improved by extending the time allowed for platelet recovery; notably, 10 patients who initially achieved CRi later achieved CR during treatment. However, the extent to which platelets would have recovered given more time is unclear because most patients achieving CR/CRi proceeded to additional therapies such as HSCT to consolidate the response to InO.
The reported CR/CRi rate is higher compared with that observed in a previous phase 1/2 study (68% vs 58%)18 and compared with conventional chemotherapy (ranging from 30% to 40% after first salvage and 10% to 20% after later salvage).3,13,30 Other novel therapies have also yielded higher response rates compared with standard chemotherapy. Blinatumomab, a bispecific CD19-directed CD3 T-cell engager,30 has demonstrated relatively high remission rates, with 69% and 43% of patients with early and refractory relapses achieving CR or CR with partial hematologic recovery in 2 phase 2 trials.46,47 The median OS in these 2 blinatumomab trials were 9.8 (95% CI, 8.5-14.9) and 6.1 (95% CI, 4.2-7.5) months (median follow-up, 12.1 and 9.8 months) vs 7.4 (95% CI, 5.7-9.2) months reported here for InO.46,47 Chimeric antigen receptor–modified T cells targeting CD19 have also demonstrated high remission rates (60% to 90%) and MRD-negativity rates among responders (80% to 100%) in pediatric and young adult patients with relapsed/refractory ALL.48-50 InO can be administered in the outpatient setting as a short (1 hour) weekly infusion, which is a particular advantage over other therapies that require hospital admission (eg, intensive chemotherapy) or infusion over prolonged durations (eg, 4 weeks of continuous infusion for blinatumomab).51
The safety profile of single-agent InO in this study is consistent with that reported previously, and no new unexpected toxicities were observed.18 Four patients developed VOD/SOS, none of whom had received prestudy HSCT. Two patients experienced VOD/SOS during therapy or follow-up but without an intervening HSCT and 2 developed VOD/SOS after poststudy HSCT. In a previous study, 36 of 90 patients receiving single-agent InO (40%) proceeded to allogeneic SCT, of whom 6 (17%) experienced VOD/SOS after transplantation.18 The VOD/SOS rate was higher in patients who had undergone SCT after receiving a dual alkylator conditioning regimen compared with those who had received a single alkylator regimen (n = 5/13 vs 1/21). In another study of 79 patients receiving single-agent InO for relapsed/refractory non-Hodgkin lymphoma, which excluded patients with prior allogeneic SCT, 1 developed VOD/SOS.43 Taken together, these data suggest that patients receiving InO without transplantation may be at a lower risk for developing VOD/SOS and that the risk of VOD/SOS may be higher in patients subsequently proceeding to SCT. In particular, patients who received conditioning with double-alkylating agents (eg, high-dose busulfan and cyclophosphamide) may be at especially higher risk of VOD/SOS.52 Lower doses of InO combined with chemotherapy are also being examined in patients with relapsed/refractory ALL (Southwestern Oncology Group S1312 trial [NCT01925131]) to assess whether this regimen will be associated with increased efficacy and/or decreased toxicity.
InO treatment was accompanied by a rapid decrease in B-lymphocyte count, followed by slow and variable regeneration. Although CD22 expression is not a significant determinant of InO exposure, separate analyses (not shown) demonstrated that the number of doses administered did influence elimination rate, an effect thought to be associated in part with target-mediated drug clearance. Notably, patients achieving MRD negativity tended to have higher serum InO concentrations. Changes in mRNA profiles in blood consistent with the mechanism of action of InO were evident, including decreased CD22 mRNA. By day 15, CD22 mRNA levels in patients who subsequently achieved CR/CRi and MRD negativity were lower vs those who did not achieve CR/CRi (P = .01). The results corroborate the proposed mechanism of action of InO, although these exploratory exposure and pharmacodynamic/PG analyses require confirmation by further studies. Moreover, measurement of CD22 mRNA in peripheral whole blood may warrant further exploration as a simple and sensitive way to assess circulating disease burden.
In conclusion, single-agent InO administered in a weekly regimen at an initial cumulative dose of 1.8 mg/m2 per cycle is associated with manageable toxicities and encouraging clinical activity in this multiple relapsed/refractory population. Patients achieving MRD negativity tended to have higher peak and trough InO concentrations throughout treatment. In contrast, no correlation was observed between achievement of MRD negativity and baseline disease burden, suggesting that InO treatment might be effective regardless of baseline disease severity. Further exploration of InO for the treatment of CD22-positive relapsed/refractory ALL in patients having undergone 1 and 2 salvage treatments is currently ongoing.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Craig Davis, Sherry Li, and Jean-Claude Marshall (employees of Pfizer Inc) for their contributions to pharmacogenomics assay planning, design, execution, and data analysis, and Hui Zhang (employee of Pfizer Inc) for her statistical contributions to the study.
This study was sponsored by Pfizer Inc. Editorial support was provided by Simon J. Slater and Kevin O’Regan, of Complete Healthcare Communications, LLC, and was funded by Pfizer Inc. K.L. was employed as a clinician through inVentiv Health, who were paid contractors to Pfizer Inc for the development of this manuscript.
Authorship
Contribution: R.A., J.B., A.S.A., D.J.D., E.V., and H.M.K. designed the study; C.A.S., M.L., A.S.A., W.S., D.J.D., A.S.S., A.S., and H.M.K. executed the study; A.D.L., M.L., A.S.A., W.S., D.J.D., A.S.S., A.S., and H.M.K. supervised the data acquisition; R.A., A.D.L., C.A.S., M.L., J.B., A.S.A., W.S., D.J.D., A.S.S., A.S., E.V., K.L., H.M.K., and L.F. oversaw the data analysis or the data interpretation; and R.A., A.D.L., C.A.S., M.L., J.B., A.S.A., W.S., D.J.D., A.S.S., A.S., E.V., K.L., H.M.K., and L.F. prepared the manuscript.
Conflict-of-interest disclosure: D.J.D. served as a consultant for Pfizer, Novartis, Baxalta, BMS, and Amgen. W.S. received honoraria from ADC Therapeutics, Amgen, Gilead Sciences, Sigma-Tau and received royalties for a chapter in Up to Date; A.S.S. received honoraria, research funding, travel, accommodations, and expenses, served as a consultant for Amgen and Stemline Therapeutics, and received research funding, travel, accommodations, expenses, and served as a consultant for Amgen, Pharmacyclics, Seattle Genetics, and Stemline Therapeutics. M.L. has received research funding and served as a consultant for Pfizer. C.A.S. has received research funding from Pfizer and Amgen and served as a consultant for Pfizer. E.V., K.L., R.A., J.B., A.D.L., and L.F. are employees and stockholders of Pfizer. H.M.K. has received research funding from Pfizer, Amgen, Astex, Novartis, and BMS. A.S.A. has received research funding and served as a consultant for Pfizer. A.S. declares no competing financial interests.
Correspondence: Daniel J. DeAngelo, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Dana Building, Room 2050, Boston, MA 02215; e-mail: daniel_deangelo@dfci.harvard.edu.