Key Points
Lower-risk MDS patients, who may share features with clonal hematopoiesis of indeterminate potential, have a high proportion of CVD deaths.
Patients who survive the initial period after an MDS diagnosis may merit more extensive cardiovascular monitoring and intervention.
Abstract
Myelodysplastic syndromes (MDS) are clonal hematopoietic stem cell disorders associated with progression to leukemia and poor survival. Clonal hematopoiesis in people without an MDS diagnosis carries an increased risk of cardiovascular death. Many clonally restricted mutations are shared between patients with MDS and those with non-MDS clonal hematopoiesis; therefore, we evaluated the risk of cardiovascular death among patients with MDS. We evaluated adults with MDS in the Surveillance, Epidemiology, and End Results database of the National Cancer Institute and compared them with the general population living in the same states. We grouped histological subtypes of MDS into lower-, intermediate-, and higher-risk disease. The primary outcomes were overall survival and primary cause of death (COD) as reported to state registries. A total of 21 372 patients with MDS between 2001 and 2011 died during follow-up with a known COD. The rate of death due to cardiovascular disease (CVD) was 4613 per 100 000 person-years, compared with 2091 in the age- and-sex-adjusted US population (standardized mortality ratio, 2.21). At 24 months, the cumulative incidence of death attributed to MDS or leukemia was 23% vs 8% for CVD. Among those alive at 60 months, 27% eventually died of CVD compared with 29% from MDS or leukemia; those with lower-risk disease who survived >60 months had more deaths attributed to cardiovascular causes (30%; 95% confidence interval [CI], 26.7-33.2%) than MDS itself (24%; 95% CI, 21.4-27.5%). Patients with MDS are more likely to die of cardiovascular causes than the general population. Modifying cardiovascular risk factors, especially among those with lower-risk disease, may be warranted for MDS-related clinical care.
Introduction
Myelodysplastic syndromes (MDS) are malignancies of myeloid progenitors or hematopoietic stem cells with a clinical presentation characterized by bone marrow failure and clonal cytopenias.1 MDS is predominantly a disease of older adults, with a median age at diagnosis of ∼70 years, and is associated with poor overall survival (median, ∼3 years with considerable heterogeneity).2-4 Patients with MDS may die of complications directly related to cytopenias, such as infection or bleeding, or progression to acute myeloid leukemia (AML); however, because many are elderly, they may die of another concurrent condition, either independent of or exacerbated by MDS.5-7 Therefore, to improve survival in MDS patients, it is important to consider other potential contributors to patient mortality.
MDS-associated somatic mutations can be identified in the blood of older persons who do not fulfill World Health Organization criteria for an MDS and have normal blood counts. This association has been characterized as clonal hematopoiesis of indeterminate potential (CHIP).8-10 Patients with CHIP have an increased risk of developing a hematologic malignancy (rate of progression 0.5-1%/year),11-14 but they also have an increased risk of fatal cardiovascular events and all-cause mortality for unclear reasons.13 CHIP patients appear to specifically have an increased risk of atherosclerotic heart disease.15,16 It is not known whether this cardiovascular risk is specific to CHIP, or whether patients with similar mutations and with an MDS diagnosis also carry this increased risk.
A major limitation in the care of patients with MDS, particularly those with lower-risk disease, is the lack of effective and tolerable therapies. Indeed, it has been over a decade since a new therapy has been approved by the US Food and Drug Administration for the treatment of MDS.17 Identifying modifiable risks factors for death along the course of an MDS diagnosis may inform future interventions, particularly among patients with a lower-risk MDS who will often live with MDS for years after diagnosis.
The Surveillance, Epidemiology, and End Results (SEER) database rigorously collects incident cancer cases, patient outcomes, and causes of death (COD) for ∼28% of the US population. We used these data to identify patients with a new MDS diagnosis between 2001 and 2011 to characterize the rates and timing of specific COD after an MDS diagnosis, with particular attention to cardiovascular deaths. We hypothesized that deaths due to cardiovascular causes would be more prevalent than in the general population, and that they might surpass those due to MDS as patients live longer from diagnosis.
Methods
We identified incident MDS cases using the November 2015 submission of the National Cancer Institute SEER 18 registries research database (1973-2011), released in April 2016. SEER is meant to be broadly representative of population demographics, income, and education; it includes cancer registries in Atlanta and rural Georgia, Connecticut, Detroit, Hawaii, Iowa, New Mexico, California, Seattle-Puget Sound, Alaska, Kentucky, Louisiana, and New Jersey. Along with cancer registry data, SEER provides US mortality data as reported by the National Center for Health Statistics and the Centers for Disease Control (www.cdc.gov/nchs).18 This study was considered exempt by the Dana-Farber/Harvard Cancer Center institutional review board.
We identified patients ≥15 years of age at the time of an MDS diagnosis for inclusion in this study. Cases were included if they had a pathologically confirmed diagnosis, had active follow-up, had a known age at diagnosis, and if MDS was the first cancer diagnosis in SEER. We excluded patients diagnosed at autopsy or with 0 days of follow-up after diagnosis. We identified patients diagnosed with MDS between 2001 and 2011 to allow for ≥2 years of follow-up. Those patients who died during the follow-up period were then analyzed to characterize causes and timing of death.
MDS subtypes are histologically confirmed and reported to SEER according to International Classification of Diseases for Oncology, 3rd edition codes and include refractory anemia (RA, 9980), refractory neutropenia (RN, 9991), refractory thrombocytopenia (RT, 9992), refractory anemia with ring sideroblasts (RARS, 9982), refractory cytopenia with multilineage dysplasia (RCMD, 9985), myelodysplastic syndrome with isolated del(5q) [MDS with del(5q), 9986], refractory anemia with excess blasts (RAEB, 9983, which includes both RAEB-1 and RAEB-2), and myelodysplastic syndrome, unclassifiable (MDS-U, 9989). For the purposes of this study, therapy-related MDS was not included. We grouped these subtypes of MDS, based on overall survival, into lower-risk disease (RA, RT, RN, and RARS), intermediate-risk disease [MDS-U, MDS with del(5q), and RCMD], and higher-risk disease (RAEB).19,20
To evaluate the risk of specific COD among patients who died during the period of the study, the COD was recorded using the SEER COD site recode (available in SEER as the “codpubkm” code). Death attributed to an MDS itself was defined as patients whose COD was MDS or AML. SEER determines the COD according to state death certificates, which are coded by the state health department in each region. For this analysis, we grouped SEER recoded COD according to common etiologies (Table 1). We then also identified US population mortality statistics, using COD site recodes also reported by SEER in SEER*Stat, within the same SEER registry regions during the study period. This comprised the population event rate for comparisons.
Statistical analysis
The incidence of a given COD among MDS patients was estimated using the cumulative incidence method; for each COD we investigated, all other CODs were considered to be competing risks.21 COD was also explored according to each MDS risk group. The time to event was calculated as the time, in complete months, from the date of diagnosis to the date of the event (death from a specific cause). The cumulative incidence was estimated with the cuminc utility in the “cmprsk” package22 in R23 and tested using the Gray test. Additionally, the subset of patients with an event, defined as a recorded death in SEER, and a known COD were explored. The time to death was categorized into discrete time intervals (≤12 months, >12-36 months, >36-60 months, and >60 months), and the proportion of patients dying from MDS/leukemia, cardiovascular disease (CVD), and all other CODs were summarized for each interval. The incidence of cardiovascular deaths in the US population residing in SEER counties between 2001 and 2013 was calculated using SEER*stat and adjusted according to the age and sex distribution of MDS patients in the study. The US population rates were at the county level and did not exclude patients with MDS who lived in those counties. Standardized mortality ratios (SMRs) were calculated using Stata version 9.1 (StataCorp, College Station, TX).
Results
A total of 29 267 patients were diagnosed with MDS between 2001 and 2011 (Table 2), with a median age at diagnosis of 76 years (range, 15-109 years), and 54% of the patients were male. There was a greater proportion of male patients among those with higher-risk disease (60%) compared with intermediate- (54%) and lower-risk MDS (52%) (P < .001). The median age at diagnosis was also younger among patients with higher-risk disease (73 years; interquartile range [IQR], 64-81 years) compared with intermediate-risk disease (77 years; IQR, 68-83 years) and lower-risk disease (76 years; IQR, 67-82 years) (P < .001). During the follow-up period, a total of 21 598 (73.8%) patients died, of which 21 372 had a documented COD, and this group comprised the patient set for the subsequent COD analysis. The patients who died during follow-up included 3355 (89.3%) of patients with higher-risk disease, 14 226 (73.7%) of patients with intermediate-risk disease, and 4017 (64.8%) of patients with lower-risk disease. The median follow-up was 84 months (95% confidence interval [CI], 83-85 months).
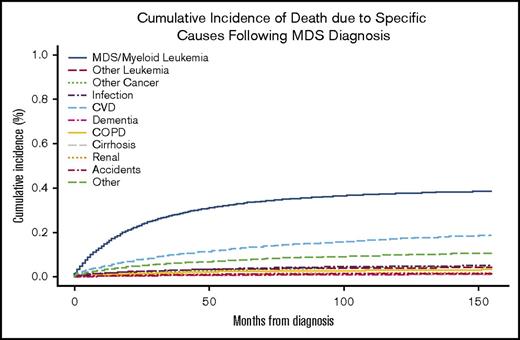
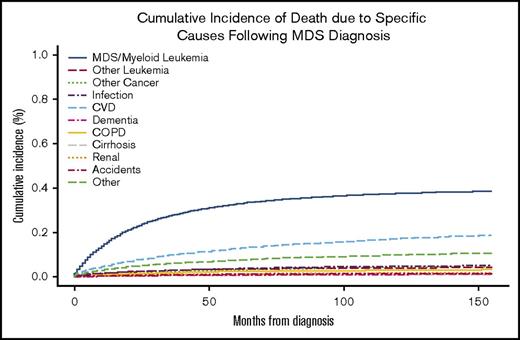
The most common COD for patients with newly diagnosed MDS was MDS or AML, followed by CVD (Figure 1). At 24 months, the overall cumulative incidence of death due to MDS/leukemia and CVD was 23.1% (95% CI, 22.7-23.6%) and 7.7% (95% CI, 7.4-8.0%), respectively (Table 3). The rate of death attributed to CVD in the MDS population was 4613 deaths per 100 000 person-years compared with 2091 in the general US SEER population adjusted for age and sex (standardized mortality ratio, 2.21; 95% CI, 2.09-2.32). Similarly, the SMR for each risk group, standardized for the age and sex distribution of the specific risk group, was increased relative to the US SEER population for lower-risk (SMR, 2.08; 95% CI, 1.97-2.20), intermediate-risk (SMR, 2.20; 95% CI, 2.09-2.32) and higher-risk MDS patients (SMR, 2.62; 95% CI, 2.48-2.77).
Cumulative incidence of specific COD. For each COD, death due to another cause was considered a competing risk.
Cumulative incidence of specific COD. For each COD, death due to another cause was considered a competing risk.
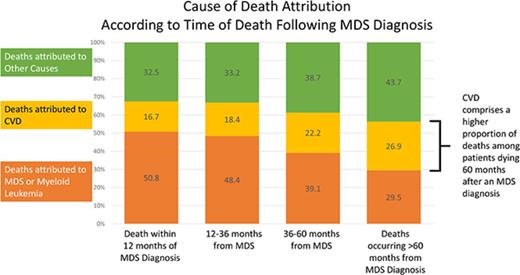
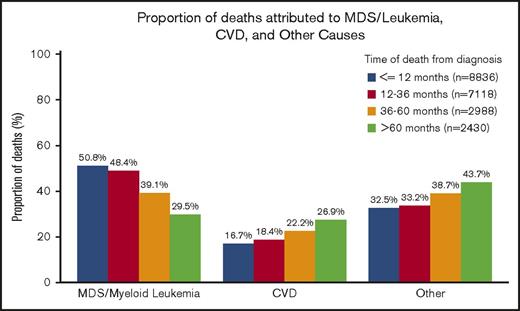
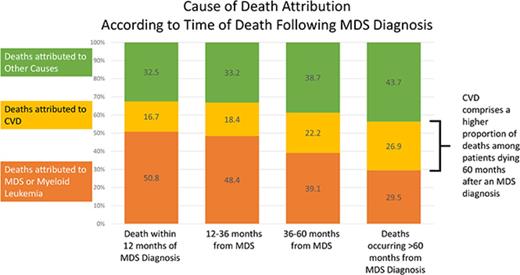
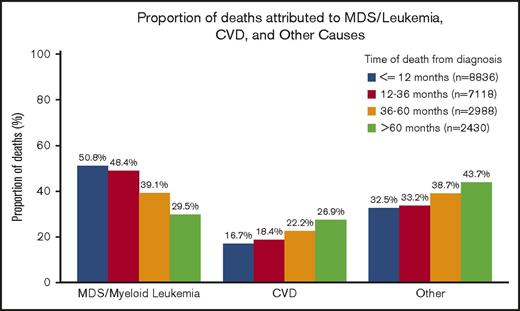
Of the 21 372 patients with a documented COD, 41.2% died within 12 months of diagnosis, 33.4% died between 12 and 36 months, 14.0% died between 36 and 60 months, and 11.4% died >60 months from diagnosis. Of the patients who died within 12 months of an MDS diagnosis, the COD was attributed to MDS/leukemia for 50.8%, whereas CVD was the attributed cause for 16.7% of deaths. However, for patients who survived >60 months beyond the initial diagnosis, the subsequent risk of death due to CVD (26.9%) approached the risk of death due to MDS/leukemia (29.5%) (Figure 2).
Specific COD according to survival time. Of the patients with a recorded death during follow-up, the deaths were categorized into 4 time categories (within 12 months from diagnosis, 12-36 months from diagnosis, 36-60 months from diagnosis, and >60 months from diagnosis). Within the respective survival categories, the proportion of patients with death due to CVD appeared to increase, whereas the proportion of patients with death due to MDS/leukemia appeared to decrease.
Specific COD according to survival time. Of the patients with a recorded death during follow-up, the deaths were categorized into 4 time categories (within 12 months from diagnosis, 12-36 months from diagnosis, 36-60 months from diagnosis, and >60 months from diagnosis). Within the respective survival categories, the proportion of patients with death due to CVD appeared to increase, whereas the proportion of patients with death due to MDS/leukemia appeared to decrease.
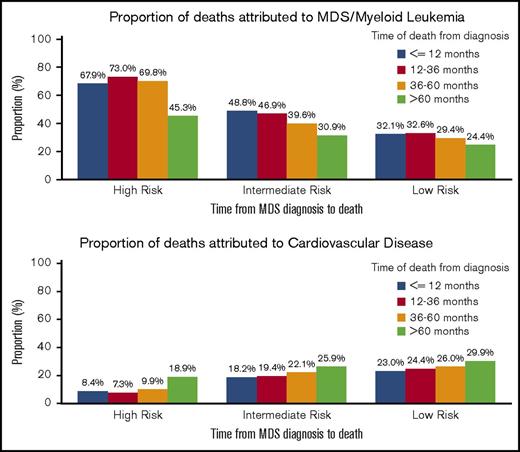
The cumulative incidence of death attributable to CVD compared to MDS varied significantly between low-, intermediate-, and higher-risk MDS subtypes. For patients with higher-risk MDS, the cumulative incidence of death attributed to MDS/leukemia was 34.7% (95% CI, 33.2-36.2%) at 12 months and 50.4% (95% CI, 48.8-52.0%) by 24 months. The cumulative incidence of death attributed to CVD in this subgroup was 4.3% (95% CI, 3.7-5.0%) and 5.7% (95% CI, 5.0-6.5%) at 12 months and 24 months, respectively. In contrast, among lower-risk patients, the risk of death attributed to MDS/leukemia was more similar to that attributed to CVD both at 12 months (6.0% vs 4.3%) and at 24 months (9.9% vs 7.2%) after the initial MDS diagnosis. Intermediate-risk patients had a cumulative incidence of death from MDS or leukemia between the rates seen for lower- and higher-risk patients, but this group also had a higher cumulative incidence of CVD deaths (Table 3).
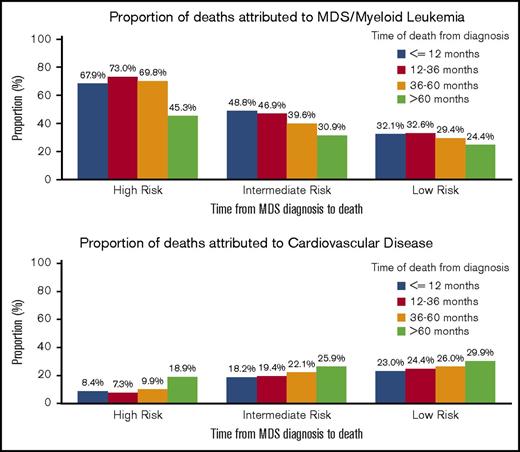
Of the patients with a known COD during the study period, the rate of death attributed to CVD appeared to increase in each increasing interval of time from diagnosis to death for all risk groups, whereas the rate of death attributed to MDS/leukemia appeared to decrease (Figure 3). For those patients with a diagnosis of a lower-risk MDS who survived >60 months, a similar percentage had deaths attributed to CVD (29.9%; 95% CI, 26.7-33.2%) as compared with an MDS (24.4%; 95% CI, 21.4-27.5%) (supplemental Table 1).
Specific COD according to survival time and risk group. Of the patients with a recorded death during follow-up, the deaths were categorized into 4 time categories (within 12 months from diagnosis, 12-36 months from diagnosis, 36-60 months from diagnosis, and >60 months from diagnosis). For all risk groups, within the survival categories, the proportion of patients with death due to CVD appeared to increase, whereas the proportion of patients with death due to MDS/leukemia appeared to decrease.
Specific COD according to survival time and risk group. Of the patients with a recorded death during follow-up, the deaths were categorized into 4 time categories (within 12 months from diagnosis, 12-36 months from diagnosis, 36-60 months from diagnosis, and >60 months from diagnosis). For all risk groups, within the survival categories, the proportion of patients with death due to CVD appeared to increase, whereas the proportion of patients with death due to MDS/leukemia appeared to decrease.
We explored the potential impact of specific MDS subtypes on the outcomes of patients with a known COD by evaluating the cardiovascular outcomes of patients with MDS with del(5q) and those with RARS (enriched for mutations in SF3B1).24-26 Patients with MDS with del(5q) more frequently died with COD attributed to MDS/leukemia (55%, 270 of 487 patient deaths) than did patients with RARS (34%, 550 of 1611 patient deaths) or other MDS patients (47%, 8993 of 19 274 patient deaths; supplemental Figure 1). In contrast, patients with RARS who died within 12 months of diagnosis had a similar rate of death attributed to MDS (34%) as those who died >60 months from diagnosis (32%), whereas the proportion of deaths attributed to CVD increased from 23% to 29% in the time intervals of survival after diagnosis (supplemental Table 2).
Discussion
We interrogated the SEER registry to identify pathologically defined MDS cases and correlate these outcomes with CODs reported centrally to cancer registries in SEER regions. We confirmed the high rates of death directly attributed to an MDS and leukemia in this population, particularly among those with higher-risk disease, but also identified the time-dependent importance of other COD, particularly CVD. For those patients who survived 5 years after a diagnosis of MDS, the magnitude of the risk of death attributed to CVD approximated that of MDS, suggesting an area of potential intervention in this patient population.
There are relatively few treatments for patients with MDS, and many patients with lower-risk disease have mild cytopenias and are observed without specific therapy for a prolonged period. Identifying areas of modifiable risk, such as CVD, may improve outcomes for this patient population. Controlling blood pressure, aspirin therapy (when safe for a patient’s platelet count), weight loss, effective management of anemia, and statin therapy may all be important considerations for patients with a lower-risk MDS.
Our analysis clarifies the magnitude of CVD for patients with MDS compared with the general population and illustrates the timing of cardiovascular events. A recent analysis of 7212 primarily untreated MDS patients found that revised International Prognostic Scoring System risk groups equilibrated after 3.5 years, such that the hazard of death or transformation to leukemia was equivalent between groups.27 We found that for those patients who were alive 5 years after their diagnosis, the cumulative risk of death attributed to CVD was similar to that of death from an MDS or leukemia. In fact, for patients with lower-risk MDS who were alive at 5 years, we found that the risk of death attributed to CVD was equal to and trending toward being greater than the deaths attributed directly to MDS.
Similar to previous studies, we also found that approximately half of the deaths among MDS patients were attributed to the disease itself by clinicians certifying death6,28 and even greater when including deaths due to infection or bleeding, which are recognized as frequent complications of MDS.29-34 Previous analyses have considered cohorts of patients with varying follow-up that may not account for time-dependent risks in MDS. Moreover, referral patterns may bias outcomes due to patient selection for those with higher-risk disease, particularly within larger referral hospitals.35 We chose to analyze a population-based cohort of patients with recorded COD, across a range of MDS disease severity, using the cumulative incidence of individual CODs to characterize specific risks over time.
The MDS population is, in general, an older population with a higher risk of CVD at baseline. However, we found that patients with MDS had a markedly increased rate of cardiovascular mortality compared with the age-matched US population, with an SMR for death attributed to CVD of 2.21. The mechanism behind this relationship is not known, and some have hypothesized that it may be related to the effects of chronic anemia19 or iron overload.36,37 A systemic inflammatory state may be seen in MDS38 and has also been associated with poor cardiovascular outcomes.39,40 Erythropoiesis-stimulating agents have been associated with increased cardiovascular events in some hemodialysis patients, although this association has not been seen in patients with MDS.41 A previous analysis of Medicare claims data similarly reported that cardiovascular event rates were higher in patients with MDS compared with the Medicare population (odds ratio, 2.10) and were more prevalent among transfusion-dependent patients;42 however, this study did not have the same follow-up, nor did it attempt to characterize time-varying risks. There are also a number of limitations to the use of claims data as the basis of identifying MDS diagnoses;43 therefore, we focused on histologically verified cases supplied by SEER. Nonetheless, the overall burden of CVD in the Goldberg et al42 analysis is consistent with our current findings.
Recurrent somatic mutations associated with myeloid malignancies, in particular DNMT3A, TET2, and ASXL1, are associated with an increased rate of incident coronary heart disease and ischemic stroke among patients with CHIP.13,15 This may relate to an interaction between the clonal cells and the endothelium or to a shared predisposition to both problems; for instance, a germ line DNA repair defect that both accelerates atherogenesis and increases the risk of clonal hematopoiesis. One murine model of TET2-deficient clonal hematopoiesis implicates macrophage TET2 deficiency in accelerated atherogenesis and CVD,16,44 whereas another recent case control analysis revealed higher rates of atherosclerotic heart disease associated with mutations in DNMT3A, TET2, ASXL1, and JAK2 among CHIP patients.15 Although clonal hematopoiesis does not always result in a clinical presentation of MDS,8,11,12 our data showing a steadily increasing incidence of cardiovascular and cerebrovascular events from the time of MDS diagnosis may suggest a common underlying pathophysiology. Although the SEER database does not contain comprehensive molecular data, we noted that among patients with RARS, which is enriched for SF3B1 mutations and characterized by slower rates of progression, the proportion of deaths attributed to MDS was relatively steady with time, whereas the proportion of deaths attributed to CVD increased as patients lived longer from their time of diagnosis. Because mutational status is not reported in SEER, any such associations are speculative, but merit future investigation.
We recognize several limitations to our analytic approach. First, the clinicians’ attribution of death in patients with multisystemic illness may be problematic and incomplete, and each patient has only 1 COD recorded in SEER. This analysis relies on death certificates reported to cancer registries in the SEER regions and does not include medical record review. However, with ∼30 000 patients, a record review to reassign CODs would be impractical. Moreover, the vast majority of cancer patient deaths are attributed to their cancer diagnosis45 ; the estimate of noncancer CODs therefore may underestimate the true clinical burden of these comorbid conditions. The fact that so many patients still had CVD as the attributed COD, while also carrying a cancer diagnosis, supports the role of CVD as a significant challenge in this patient population. Second, SEER does not provide hemoglobin or absolute neutrophil counts, ferritin levels, or disease status to clarify whether deaths due to infection were related to severe cytopenias, or whether anemia or iron overload were likely to have contributed to cardiac deaths. Transfusion status is also not provided, and it is likely that the effective management of long-standing anemia could impact cardiovascular risk. Because of these factors, we identified the CODs as reported, but cannot strictly define them as related or unrelated to an MDS. SEER also does not report clinicopathologically defined prognostic groups, such as International Prognostic Scoring System risk, and we therefore used MDS histology to assign risk, which may limit the interpretation of our findings. Unlike the CHIP population, patients with MDS may have increased CVD risk related to cytopenias and ensuing anemia, transfusional hemosiderosis, erythropoiesis-stimulating agent use, and transfusion-associated volume shifts, some or all of which may be confounders in attributing the risk of CVD in the MDS population. They may also be more likely to have central venous catheters, which carry a small associated thromboembolic risk. Without knowing how anemic the patients were or whether they were receiving transfusions, we cannot control for those variables. Finally, we did not have patient samples to assess for specific mutations associated with myeloid malignancies and CVD, which makes conclusions about increased risk coming from these mutations speculative. Clearly, prospective studies using registries such as the new NHLBI National MDS Natural History Study as it matures (https://thenationalmdsstudy.net/) will provide valuable data in this regard.
MDS is a clonal myeloid malignancy with a broad spectrum of outcomes according to various disease characteristics, and it shares a number of mutations with more indolent processes, including CHIP, that have been shown to have a greater risk of CVD. In this analysis, we characterized the risk of cardiovascular death according to the time from an MDS diagnosis, and found that for those patients surviving >5 years, the deaths attributed to CVD approximated the number of deaths attributed to MDS itself. Future efforts to improve the outcomes of these patients should consider the role of cardiovascular risk both as it relates to the underlying clonal disease and its potential to guide therapeutic interventions.
Presented in part at the 2016 Society of Hematologic Oncology Annual Meeting, Houston, TX, 7-10 September 2016.
The full-text version of this article contains a data supplement.
Acknowledgment
This work was supported by National Institutes of Health, National Cancer Institute, Dana-Farber/Harvard Cancer Center Support Grant 5P30 CA006516.
Authorship
Contribution: A.M.B. and A.T.F. designed and performed the research, analyzed the data, and wrote the paper; T.M.B. designed the research, analyzed the data, and wrote the paper; and G.S.H., P.C.A., D.S.N., D.P.S., and G.A.A. analyzed the data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrew M. Brunner, Massachusetts General Hospital, 275 Cambridge St, PO Box 229, Boston, MA 02114; e-mail: abrunner@mgh.harvard.edu.