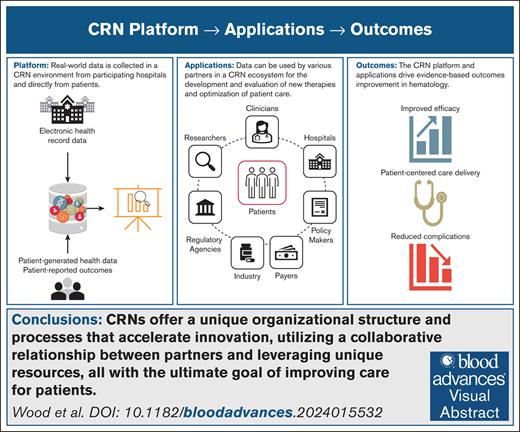
Key Points
ASH RC Data Hub advances RWE in hematology, currently for SCD and multiple myeloma, fostering innovation.
This paper presents ASH RC Data Hub site distributions, enrollment metrics, curation processes, and quality control initiatives.
Visual Abstract
Real-world evidence (RWE) has been used to improve quality of care, accelerate innovation, and evaluate emerging therapies for drugs, devices, and biologics. The Coordinated Registry Network (CRN), aggregating and linking highly curated patient data, has emerged as a model for RWE generation that has guided the development of the American Society of Hematology Research Collaborative (ASH RC) Data Hub. With the aim of bolstering research, enhancing clinical care, and expediting evidence generation using RWE, the ASH RC and the Innovative Genomics Institute launched a joint initiative, “Accelerating Innovations for Sickle Cell Disease (SCD) with Real-World Evidence,” to evaluate and make recommendations to the Data Hub. An expert panel used a "maturity model" to evaluate the following: (1) Data Hub standardization of RWE guidelines to ensure successful monitoring of new therapies' safety and effectiveness; and (2) the Data Hub’s engagement with patients (including collection of data on patient experience) and collaboration among partners, including regulators, industry, and clinicians. The Data Hub gathers patient-level data from electronic health records, with plans to incorporate data from individuals receiving gene therapy. The program is currently curating and aggregating data on ∼27 000 individuals with SCD cared for across 22 hospitals in the United States. The SCD Data Hub program includes a total of 56 centers, some of which are not yet contributing data, which will increase the total cohort to ∼50 000 patients. The unique organizational structure of the CRN model facilitates acceleration of innovation through collaborative relationships between multiple clinical sites and partners with various evidentiary needs.
Introduction
Real-world data (RWD), routinely collected from a variety of sources (eg, electronic health records [EHRs], claims databases, patient registries, and other sources of patient data),1 are increasingly being used as a strategy to improve evidence generation. Real-world evidence (RWE) is generated when RWD are used to complement and enhance knowledge gained from traditional clinical trials, bolstering evidence-based decision-making, quality of care, regulatory decision-making and evaluations, and health policy development.2 The 21st Century Cures Act, which was established in the United States in 2016, promotes the use of RWE to expedite medical product development and streamline regulatory processes, allowing for faster approval pathways for promising therapies.3 Ultimately, this approach benefits patients by accelerating access to innovative treatments and advancing medical care.
The new regulatory paradigm under 21st Century Cures rebalances premarket and postmarket requirements by reducing the burden of premarket study and shifting emphasis to postmarket evaluation. This shift advances novel therapies to treat hematologic conditions, including sickle cell disease (SCD). Supporting this new regulatory paradigm, the American Society of Hematology Research Collaborative (ASH RC) has responded by establishing its data hub. When fully developed, the ASH RC Data Hub will include over half of the individuals living with SCD in the United States.
ASH RC has adopted a well-established strategy known as the Coordinated Registry Network (CRN). The CRN strategy brings together a range of partners, including regulators, industry professionals, clinicians, and patients, all seeking to derive value from RWE to address their distinct interests and evidentiary needs.4 The major tasks of the CRN are to (1) coordinate these interests into a shared database with quality data permitting reliable research conduct, guided by the “collect once, use many times” strategy of its sustainability model; and (2) create a high-quality data resource that is fit for purpose. The unique features of the CRN model for the provision of RWE accelerates innovation by bringing multiple clinical sites together and engaging various partners to address various evidentiary needs. Many clinical subspecialties, including cardiology, vascular surgery, and orthopedics, have adopted the CRN approach, which builds on traditional registries by linking data from multiple sources.5-7 CRN’s unique structures are embedded in the following: (1) promotion of unique device identification (for device-focused CRNs); (2) improving data collection efficiency (eg, embedded in the health care delivery system and integrated with clinical team workflow); (3) advancing data quality (eg, representativeness, completeness, and auditing; and inclusion of a core minimum data set); (4) consideration of total product life cycle research (eg, support for regulatory decision-making and long-term outcome collection including linkages to EHRs or claims data); (5) establishing sustainability and governance (eg, professional society or health system involvement with funding commitment, with transparent structures for governance, access, and analytics); (6) leveraging registries for health care quality improvement (eg, provider feedback, automated quality processes, and outlier analysis); and (7) incorporation of patient-generated data and patient-reported outcomes (PROs; eg, the experience with large-scale acquisition of PROs including benchmarking capabilities).
This study presents the collaboration between the ASH RC and the Innovative Genomics Institute: Accelerating Genomic Therapies with RWE.8 The initiative enlisted a group of experts in hematology, genomics, regulatory science, and RWE to evaluate and make recommendations on evolving the ASH RC Data Hub into a CRN. The findings of this study support the efforts of the ASH RC Data Hub to improve SCD diagnosis identification, accuracy, and reporting.
Methods
The ASH RC and the Data Hub
The ASH RC, established in 2018 as a nonprofit organization by the ASH, aims to enhance research and clinical practice, ultimately improving the lives of individuals affected by blood disorders. The Data Hub is a program within the ASH RC,9 leveraging RWD to support research, clinical care, and evidence generation through active engagement with partners, including patients, to address various needs and priorities.
Data Hub SCD RWD collection and curation procedures
RWD collected from various clinical settings lack semantic interoperability10 making extensive curation essential for use as aggregated RWE. To address this challenge, the data collected, mainly sourced from EHRs, undergo systemic transformation procedures to ensure they meet the high-quality standards required by the Data Hub. The sites that contribute data to the Data Hub currently provide structured, limited data sets from EHR data feeds from 2015 through the present time, primarily in Observational Medical Outcomes Partnership format and soon by Fast Healthcare Interoperability Resources format. Some data elements required by the Data Hub may not be adequately represented in structured data collected from EHRs and may require manual review or in some cases de novo manual data entry. Common examples of this include accurate diagnostic information, disease progression or toxicity events, and comorbidities. Balancing automation and accuracy from a cost and quality perspective remains a central challenge for EHR-based registries.
The Data Hub provides site-specific dashboards displaying care and outcome metrics, allowing for sites to compare their data with aggregated data from all participating sites. Additionally, online queries, cohort analysis tools, and data exports empower sites to access and analyze their own data.7,11 All data operations are governed by the Western Copernicus Group Institutional Review Board to ensure patient confidentiality and ethical data handling.
CRN work group
The Accelerating Genomic Therapies with RWE was an initiative of the ASH RC working to achieve consensus on standards for RWD collection and sharing with help from regulators, industry, and clinicians who must track the safety and effectiveness of these therapies over time. In March 2021, the ASH RC partnered with the Innovative Genomics Institute to convene a roundtable including leaders from the US Food and Drug Administration (FDA) and other partners to take a step toward preparing for this future. The roundtable directed the creation of 2 work groups: the CRN work group and the genomic therapies work group, charged with reporting recommendations to the roundtable. Members of the work groups included experts from academia, federal agencies, industry, patient organizations, and professional societies. The second roundtable meeting in November 2021 received and discussed the preliminary findings of the work groups, providing feedback and further direction, culminating in the 2 work groups releasing their reports in early 2023.12,13 The genomic therapies work group focused on identifying and achieving consensus regarding the essential data points, procedures, and assays needed to generate actionable RWE for a variety of evidence needs including regulatory decision-making for gene therapies. Details are available in the full report.12 The CRN work group organized its investigation using the 7 domains of the CRN “maturity model” published to guide CRN development.7
Members of the CRN work group reviewed the ASH RC Data Hub processes, data curation operations, and findings of a survey of physicians participating in the Data Hub. A survey of the 22 sites participating in the Data Hub was collected to learn from physicians about the accuracy of SCD diagnosis data in the EHR, how the diagnosis is confirmed, and any barriers to diagnosis documentation (survey provided in the supplemental Materials). Respondents were primarily principal investigators of the SCD program. The review work and the report of the work group was guided by a “CRN maturity model”7,11 that was created to support development of CRNs based on practices drawn from a community of practice involving many operating CRNs.14 This model has been previously used by other CRNs as a self-evaluation tool.15 Report of the CRN work group and subsequent efforts of the Data Hub are summarized in “Results” of this study.13
Results
ASH RC’s SCD Data Hub network processes and operations
The CRN work group reviewed the processes and operations of the ASH RC's SCD Data Hub program, with a focus on alignment of clinical concepts and data elements with US Core Data for Interoperability standards for EHR codes. This approach reduces the data collection burden for participating sites, because required elements are readily available for exchange. Data submissions include comprehensive patient information such as allergies, treatments, care teams, clinical notes, goals, health concerns, immunizations, laboratories, medications, demographics, problems, procedures, smoking status, and vital signs, dating back to 2015, with ongoing updates for new information.
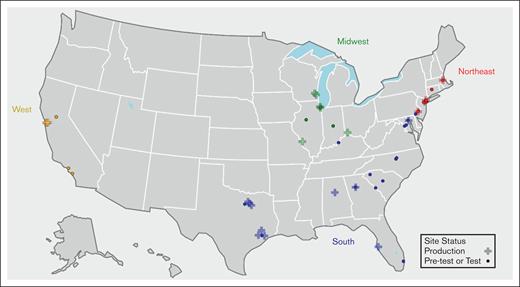
As of 1 September 2024, the Data Hub comprises 56 sites, 22 of which actively contribute RWD at least quarterly, serving both pediatric and adult populations (Table 1). Most sites (50/56) use Epic as their EHR vendor. Figure 1 provides a map of the program sites and illustrates the distribution of enrolled sites across various regions and states within the United States, which offers insight into the widespread presence of data hub sites across different regions as they collectively work toward improving the lives of individuals diagnosed with SCD.
Geographic location (CDC census) and region (sites enrolled by state). CDC, Centers for Disease Control and Prevention.
Geographic location (CDC census) and region (sites enrolled by state). CDC, Centers for Disease Control and Prevention.
As of July 2024, there are data on a total of 25 229 individuals diagnosed with SCD in the Data Hub (Table 1). Most patients (84%) identify as Black or African American, whereas "not reported or unknown" race/ethnicity category accounts for a significant portion (∼11%) of the total, which highlights the importance of curation in collecting comprehensive demographic data from medical records. The median age of patients in the Data Hub is 23 years. Table 1 also breaks down patient ages into 3 age categories: under 3 years; 3 to <18 years; and 18+ years. The largest group is adults, followed by the 3- to <18-year-old age range.
The Data Hub has completed 2 validation projects to date: one focusing on SCD diagnosis type; and the other focusing on specific high-priority elements (ie, disease-modifying therapies and acute care encounters, etc). Based on initial review of EHR data, 68% of data hub patients have a single SCD diagnosis type; and accuracy of raw data is unknown. The remaining individuals have >1 or are missing diagnosis type codes. Twelve of the 22 sites have submitted investigator-verified SCD diagnosis data, resulting in investigator-verified diagnostic subtype data for 11 257 patients.
Correctly classifying SCD diagnoses is fundamental in tailoring patient care for individuals with SCD. Accurate diagnoses and subtypes provide insights into disease severity, guide treatment decisions, help predict and manage complications, guide institution of preventative measures, ensure compatibility in blood transfusions, support early intervention, contribute to research efforts, and empower genetic counseling for individuals and families affected by SCD.
The results of the survey of Data Hub–participating physicians compared the accuracy of data collected from 3 sources: EHR problem lists, EHR notes, and non-EHR files. The accuracy of EHR problem lists using diagnosis codes varied between reporting sites, with 2 sites reporting complete accuracy and others revealing varying levels of accuracy. EHR notes curation from the 14 sites revealed 2 reporting complete accuracy. Non-EHR databases proved most robust, with 6 of 8 sites reporting complete accuracy of these sources. The non-EHR files included sources such as Excel sheets, with 2 sites reporting complete accuracy.
Table 2 is a template of ASH RC Data Hub data quality report used to assess a site’s data submission. A report is prepared for each site based on the data transmitted from the site to the Data Hub. The sites receive a completed report that assesses the accuracy, completeness, consistency, and reliability of their submission. If the submission does not meet the threshold for approval, sites are required to correct the issues identified and resubmit to the Data Hub.
Recommendations
Improve data standardization
Although current structured data documentation practices in EHR offer some assistance in RWD aggregation, they prove inadequate due to the lack of standardization, ontology mapping, density, and missing essential health care information. To bolster point-of-care documentation, a CRN should take the initiative to develop, disseminate, and advocate for best practices among its members. Multiple systems, including Logical Observation Identifiers Names and Codes, Systematized Nomenclature of Medicine, and other unharmonized standards, are required for laboratory coding, which makes it challenging to achieve the semantic interoperability necessary for aggregation and analysis. Despite the existence of common data models, none are expansive enough to serve all of hematology's curation needs. Some of the ultimate decisions must be made by the ASH RC and socialized across the profession. Consulting coding systems, such as Logical Observation Identifiers Names and Codes for clinical data and Systematized Nomenclature of Medicine, and implementing the widely recognized Observational Medical Outcomes Partnership Common Data Model are advised. It is also advisable for the CRN to align itself with organizations such as Observational Health Data Sciences and Informatics, recognizing the importance of addressing shared standardization issues in RWD aggregation through advocacy efforts.
Emerging technologies and tools that promote the adoption of templates embedding data structures for enhanced routine clinical data, such as eSource, is pivotal, because this can ultimately reduce the necessity for extensive curation.16-18 In tandem with this, creating computable phenotypes, “the product of using an executable set of algorithms to identify specific, measurable constructs present in patient records19,” should be a collaborative process involving partners, maintaining alignment with standards, and allowing for manual verification when necessary. Guaranteeing representation and standardized abstraction across various sites is essential, and this endeavor must include allocating resources for data generation, aggregation, and analysis in less-resourced environments.
Compliance with data access and use requirements
A CRN needs to adhere to contractual agreements and collect data consistent with regulatory requirements from bodies, such as the FDA20; therefore, CRN’s design should be guided by ethical and privacy standards, which include regulations or guidance such as Health Insurance Portability and Accountability Act, General Data Protection Regulation, the International Council for Harmonisation of Technical Requirements of Pharmaceuticals for Human Use Good Clinical Practice, and the Belmont Report. Although certain data collection activities may happen without participant consent (eg, limited data sets), gathering direct protected health information-level data must require participants' informed consent and authorization.
Although the protection of patient’s health care data is a CRN’s top priority, there is always a risk of data breach. To mitigate this risk, data should be stored on secure servers and governed according to the highest standards of research ethics and data privacy. There should be end-to-end protection for all data, both in transit and at rest, using strong encryption standards (eg, advanced encryption standard-256 for storage and transport layer security for transmission), and role-based access controls should be implemented. Continuous monitoring, regulatory backups, and audit logging are also necessary to ensure protection of patient data.
Engaging with patients, families, and caregivers on a regular basis ensures transparency in data use. The CRN must implement safeguards to limit access to identifiable data and provide only the minimally necessary data when external sponsors request access to data. Third-party statisticians can maintain scientific rigor by reviewing industry analyses, and the inclusion of various partners, such as academic experts, patients, and payers, during the development of industry studies can significantly enhance their credibility. Once approved centrally, the analyses would include studies done after approval, the creation of a historical control arm, the identification of cohorts, economic analyses, and comparative effectiveness research. All of these would be in line with the CRN's mission. For head-to-head comparisons, safety concerns must be evaluated beforehand, considering potential outcomes and planning for scientific dissemination with sponsors.
Better understanding of the patient experience
Understanding the impact of therapies and health care interventions on well-being and disease must be understood within the full context of the patient experience. To ensure collection of meaningful patient experience data, a CRN should prioritize establishing or adopting consensus guidelines for PROs and patient-generated health data, with a focus on those deemed actionable. Drawing on the experience of other successful CRNs may accelerate implementation of strategies to inform clinical practice and improve patient outcomes.
It is essential to pinpoint fundamental data types while also considering core subcategories within each type. For instance, a primary PRO set should encompass symptoms, quality of life, and function and can potentially extend to incorporate lifestyle and environmental factors. To address the burden on participants, a modular approach for PRO expansion is proposed.
Tracking patients from pediatrics to adult services
For patients transitioning out of pediatric care, dependable long-term tracking methods are necessary, potentially using direct-to-patient follow-up and surveillance programs. The linkage of databases, which relies on matching various patient identifiers, is a potentially valuable process but one that requires patients' informed consent to proceed.
Ensuring the sustainability of the initiative
To ensure the CRN's sustainability, the provision of fit-for-purpose data for all partners emerges as a critical factor, encompassing attributes such as accuracy, completeness, and provenance.21 Guided by frameworks such as the FDA’s Center for Biologics Evaluation and Research, Oncology Center of Excellence, and Center for Drug Evaluation and Research RWE guidance,22 a continuous demonstration of data quality ensures that outputs align with partner needs, fostering ongoing engagement. Patient value should be the impetus for CRN development, as evidenced by patient engagement strategies, PROs for gauging preferences and outcomes, and innovative approaches to involving providers for high-quality data assurance. To establish the CRN's resilience, a data quality strategy should encompass funding models, adaptable data collection methods, and a commitment to serving as a value-driven resource for patients, societies, agencies, industry, and payers. Establishing a link between Centers for Medicare and Medicaid Services claims data and registry data offers valuable long-term tracking for patients aged >65 years. Concurrently, the initiative to create a national All Payers Claims Database will provide linkage outcomes for persons aged <65 years (similar to the way that CRN links to Centers for Medicare and Medicaid Services claims data for those aged >65 years).23 Furthermore, clinical specialty societies can advocate for the creation of a national All Payers Claims Database.
To put the recommendations into action, the work group’s report calls for pilot proposals that can implement technologies to aid the ASH RC in adopting and implementing these guidelines effectively.13 The proposals aim to address key areas such as genomic data standards, the efficiency and quality of longitudinal data linkage, and the interoperability of laboratory data collection. These pilot projects, if successful, could pave the way for the creation of a prototype data collection system, suitable for gene therapies, long-term follow-up in SCD, and potentially other clinical contexts.
Other CRNs have implemented a strategy to enhance sustainability, which involves calculating the value that RWE creates for partners. Case studies in other clinical areas (cardiology, vascular surgery, and hernia repair) have documented substantial savings in time and money for medical product manufacturers using CRN data instead of stand-alone postapproval studies.21 A framework to help document the value created by RWE has been published, which may support the data hub and other RWE efforts, promoting sustainability.4
Improve SCD diagnostic data
The identification of SCD diagnosis using EHR coding that is not curated presents significant challenges, as highlighted by survey respondent feedback. A common issue is the misclassification of SCD subtypes, particularly between HbSS and HbSβ0 thalassemia,24 leading to patients being identified as having multiple disease types within their EHR. This can be attributed to various factors, including the recording of genotype information at numerous patients’ health care interactions and the influence of treatment modalities, such as hydroxyurea, on diagnostic coding.25
The reliance on diagnosis frequency within the EHR proves inadequate for avoiding misclassification. Consequently, the ASH RC Data Hub is undertaking efforts to validate a more accurate algorithm for identifying SCD subtypes by collecting investigator-verified diagnoses for a subset of patients. This initiative also involves collaborating with sites to improve SCD diagnosis identification, accuracy, and reporting. Key strategies include updating EHR problem lists, establishing standardized processes for defining diagnoses (especially for HbS/β0 thalassemia), and advocating for structured entry of patient genotypes, including β mutations/deletions and α-thalassemia carrier status.
Furthermore, some sites propose the development of a national SCD database for comprehensive data tracking. They also caution against relying solely on patient history for diagnosis, recommending random subject selection by code with subsequent chart review for crosschecking. Accessibility issues with genotype reports, often in PDF format, underscore the need for more user-friendly and automated data formats. Although existing algorithms could improve SCD subtype identification,26 their application to multisite EHR databases is hindered by data inconsistencies. Therefore, the development of a new, tailored algorithm is deemed essential.
The Work Group concluded that enhancing SCD diagnostic accuracy and accessibility within EHRs necessitates improved documentation practices. This includes using dedicated fields, separate databases, or algorithmic diagnosis estimation. The absence of standardized processes for SCD diagnosis and data integration across different sources reinforces the need for a national database and procedural standardization. Diagnostic challenges, particularly in differentiating SCD diagnoses in newborns and individuals with HbSβ0 thalassemia, emphasize the need for refined diagnostic methods and clearer classification guidelines. Accurate red blood cell transfusion history is another factor that will improve reliability of diagnosis type, and the Data Hub is working on improving the quality of transfusion data. Finally, technological solutions, such as artificial intelligence–based diagnosis estimation, automated report generation, and streamlined insurance reimbursement for genetic testing, offer promising avenues for improving the accuracy and accessibility of SCD genotype data. These findings support the Data Hub's curation efforts to achieve its objectives effectively.
Discussion
The findings of CRN work group highlight the multiple uses of data collected by the ASH RC Data Hub and their potential utility for hematology practice and product development once the ASH RC Data Hub reaches its goal of achieving a high level of CRN maturity. First would be the provision of high-quality data in hematologic conditions, such as SCD, through a well-established process of the development of a CRN that is fit for purpose in the many use cases addressed here. This advancement enables hematologists to better understand patient outcomes, treatment effectiveness, and long-term follow-up. The CRN envisioned through the ASH RC Data Hub also has the potential to provide better access to RWD for clinical researchers in contrast to commercially available data sets, which may be unaffordable to many. Furthermore, RWE has the potential to accelerate innovations in the field of hematology. By analyzing data from various patient populations and care settings, hematologists can generate evidence that informs the development and evaluation of new therapies, treatment guidelines, and clinical decision-making.
Utility of data to multiple stakeholders is critical to the sustainability of the Data Hub, and its multiple uses is at the heart of its design. The inclusion of patient experience data in the CRN proves valuable because it provides hematologists insights into the lived experiences of patients with hematologic conditions. This information contributes to the development of patient-centered care approaches, tailored interventions, and ultimately improved patient outcomes. Furthermore, the CRN can play a pivotal role in supporting clinical trials. By providing a robust data infrastructure and access to RWD, hematologists can efficiently design and conduct clinical trials, recruit various patient populations, assess the real-world effectiveness of interventions, and even construct synthetic control arms, a particularly valuable capability for studying rare diseases. Moreover, establishing a CRN may facilitate coordinated care for patients with hematologic conditions.
The Data Hub provides a base that can be built upon using a variety of strategies to improve the data and serve as a platform for future development. By linking data sources and promoting interoperability, hematologists can obtain a more comprehensive view of a patient's health history, which has the potential to enable better care coordination across various health care settings and providers if additional health care operational tools are built upon the underlying data. Acquiring payer data can simplify data management by providing standardized, clean information that is readily usable for various purposes, although subject to limitations by capturing only what can be billed. We would like to note that genomic data integration in the Data Hub remains unresolved at this point. The ASH RC is also continuing to explore how to integrate the collection of PROs via web-based platforms and investigating the feasibility of linking this information to patient characteristics within the Data Hub. Additionally, the patients with SCD currently being phenotyped may not be representative of all patients with SCD in the United States, such as those who lack access to larger centers or networks. Planned cohort expansion will help to address this limitation.
CRNs offer a unique organizational structure and processes that accelerate innovation more rapidly than industry and government.27-29 The expertise brought by partners from various backgrounds, including industry, academia, regulatory bodies, health care providers, and patient advocacy groups, enriches discussions and facilitates a comprehensive understanding of complex challenges. This collaborative environment nurtures a culture of creativity and innovation. Partners are committed to achieving agreed-upon goals and leveraging their unique resources and capabilities, enhancing the effectiveness of implementation efforts. Conflicts and disagreements are constructively addressed, leading to consensus and mutually beneficial outcomes. Partner engagement in the decision-making process builds trust and transparency, securing support and buy-in from all parties involved. This continual feedback loop allows for ongoing improvement and refinement of strategies, ensuring adaptability to evolving circumstances. The ASH RC Data Hub will continue to work with partners in this collaborative fashion to accelerate innovation. For example, the complexity of identification of SCD diagnosis and genotype using medical records can be optimally addressed by the active collaboration between the sites facilitated by the ASH RC Data Hub, a major contribution made possible by the unique organizational structure of the CRN.
The acceleration of innovation fostered by the broad set of relationships maintained by the ASH RC Data Hub extends the data system itself, which must remain dynamic to address changes in treatment modality, clinical organization, and the evidentiary needs of partners. Remaining at the edge of innovation also requires a structure that can easily incorporate changes. The ASH RC is well positioned for future additions to data aggregation for the Data Hub that will include unstructured data (eg, clinical notes) and data acquired by Fast Healthcare Interoperability Resources application programming interfaces. The Data Hub also plans to expand its data sources to include PROs, patient-generated health data, and genomics. Beyond the 22 sites currently submitting data, an additional 36 contracted sites plan to start contributing data over the next few years. Ongoing, rigorous evaluation of data quality and other technical aspects by the ASH RC Data Hub can support quality improvement, research, and regulatory decisions that ultimately benefit patients and health care.
Acknowledgment
This article reflects the views of the authors and should not be construed to represent the US Food and Drug Administration’s views or policies.
Authorship
Contribution: O.N.Y. and G.P. developed the initial draft of the manuscript; and all authors provided substantive input and edited, reviewed, and approved the manuscript.
Conflict-of-interest disclosure: K.H. and E.S. are employees of the American Society of Hematology (ASH) Research Collaborative. W.A.W. has leadership positions on oversight groups and committees within the ASH Research Collaborative. A.A.T. has received research support from Beam, bluebird bio, Editas, Novo Nordisk, and Novartis; and has consulted for Beam, bluebird bio, Editas, Pfizer, Roche, and Vertex. D.S.N. has held an advisory position with the ASH Research Collaborative. The remaining authors declare no competing financial interests.
Correspondence: William A. Wood, Division of Hematology, Department of Medicine, University of North Carolina at Chapel Hill, 101 Manning Dr, Second Floor, Chapel Hill, NC 27514; email: William_wood@med.unc.edu.
References
Author notes
The deidentified data used in this study may be available for additional research inquiries through the formal research proposal application process of the American Society of Hematology Research Collaborative (please contact info@ashrc.org).
The full-text version of this article contains a data supplement.