Abstract
Mounting evidence indicates that the presence of measurable (“minimal”) residual disease (MRD), defined as posttherapy persistence of leukemic cells at levels below morphologic detection, is a strong, independent prognostic marker of increased risk of relapse and shorter survival in patients with acute myeloid leukemia (AML) and can be used to refine risk-stratification and treatment response assessment. Because of the association between MRD and relapse risk, it has been postulated that testing for MRD posttreatment may help guide postremission treatment strategies by identifying high-risk patients who might benefit from preemptive treatment. This strategy, which remains to be formally tested, may be particularly attractive with availability of agents that could be used to specifically eradicate MRD. This review examines current methods of MRD detection, challenges to adopting MRD testing in routine clinical practice, and recent recommendations for MRD testing in AML issued by the European LeukemiaNet MRD Working Party. Inclusion of MRD as an end point in future randomized clinical trials will provide the data needed to move toward standardizing MRD assays and may provide a more accurate assessment of therapeutic efficacy than current morphologic measures.
Introduction
More than 50% of adult patients with acute myeloid leukemia (AML) relapse after attaining morphologically defined complete remission (CR) with induction chemotherapy.1-3 Assessment of posttreatment remission is traditionally based primarily on cytomorphology, with AML relapse conventionally defined as ≥5% blasts in the bone marrow not attributable to other causes.4-6 Microscopic assessment of bone marrow or peripheral blood morphology relies on examination of a relatively small number of cells (200-500) and its reliability is dependent, in part, on sample quality and the pathologist’s expertise.7 As primarily derived from data in younger adults, the risk of relapse following allogeneic hematopoietic stem cell transplantation (allo-HSCT) for consolidation after first CR is 15% to 20% for patients with favorable-risk disease, 20% to 25% for intermediate-risk disease, 30% to 40% for poor-risk disease, and 40% to 50% for very poor-risk disease.8 Among older patients (age ≥60 years) with AML, the respective pooled 2- and 5-year survival estimates following allo-HSCT are 44% and 35% for relapse-free survival (RFS) and 45% and 38% for overall survival (OS).9
Currently, pretreatment factors such as age, cytogenetics, and the presence of certain gene mutations are used to estimate posttreatment risk of relapse based on data from large patient cohorts.5,6,10-12 Table 1 lists prognostic implications of specific mutations in AML as described by the European LeukemiaNet (ELN)10 and the National Comprehensive Cancer Network guidelines.6 The risk of relapse has been linked to the postchemotherapy persistence of “minimal residual disease” (MRD), which has been defined as leukemic cells at levels below morphologic detection.12 Flow cytometric and molecular techniques for assessment of residual leukemia are more sensitive than morphologic assessment, and consensus is growing that MRD might more aptly be called “measurable residual disease,” because the presence of any disease detected by these methodologies after treatment is associated with a worse prognosis13,14 and detectable leukemia even in morphologic remission may not be “minimal.” MRD monitoring has become part of routine clinical practice in the management of patients with acute lymphoblastic leukemia, acute promyelocytic leukemia (APL), and chronic myeloid leukemia.15-18 Mounting evidence indicates that the presence of MRD is a strong, independent prognostic marker of increased risk of relapse and shorter survival in patients with AML compared with patients with a negative MRD test.4,19-23 This recurrent observation has raised interest in routine MRD testing in AML. To guide the development of a standardized or harmonized approach to MRD testing, the ELN MRD Working Party recently issued consensus recommendations for the measurement and application of MRD in AML (Table 2).24
A prominent strategy to detect MRD is immunophenotypic evaluation by multiparameter flow cytometry (MFC). It is now well established that MRD detected by MFC is an independent prognostic factor for relapse, RFS, and OS.4,19-22 In studies of patients <65 years of age with AML who were fit to receive cytosine arabinoside plus anthracycline-based induction and consolidation chemotherapy, MRD-negative status as detected by MFC was identified as the most important independent predictor of RFS and OS.25,26 Similarly, a retrospective exploratory analysis of data from the Southwest Oncology Group S0106 study showed that MFC-detected MRD after completion of induction chemotherapy could be used to stratify younger patients by risk of AML recurrence, and that MRD status was the single most important predictor of OS and RFS in individual patients.27 Data in older patients with AML have also demonstrated the prognostic relevance of MRD monitoring by MFC in patients undergoing traditional cytotoxic chemotherapy induction.28 Detection of MRD by MFC during morphological CR before allo-HSCT has also been linked to a substantially higher likelihood of relapse and worse survival.29,30 Molecular approaches to detect MRD are equally informative for prognosis. For example, among patients with nucleophosmin 1 (NPM1)–mutated AML who had undergone intensive chemotherapy, the persistence of NPM1-mutated transcripts detected using real-time polymerase chain reaction (RT-PCR) was an independent predictor of relapse or death22 and of outcomes of allo-HSCT.31,32 Another study using whole-genome or exome sequencing of samples from a cohort of adults with AML found that detection of persistent leukemia-associated mutations in bone marrow cells during remission ∼30 days after start of chemotherapy was linked to a significantly increased risk of relapse, shorter event-free survival (EFS), and poorer OS.33
Thus, adding MRD evaluation to other posttreatment assessments (eg, morphologic evaluations) could help guide postremission treatment strategies by identifying patients at high risk of relapse who might benefit from preemptive therapy,34 an appealing treatment concept that will require formal validation. Although not proven to date, the concept of MRD eradication aiding decision-making and improving outcomes of patients with AML is plausible. This is supported by experience with bispecific antibodies such as blinatumomab in ALL.35
This review examines current methods of MRD detection, some challenges in adopting MRD testing in routine clinical practice, and describes some of the recent recommendations from the ELN MRD Working Party consensus statement for the detection of MRD in AML.24
MRD detection methods
Technologies to measure MRD based on immunophenotype, cytogenetic abnormalities, and molecular mutations each have advantages and disadvantages. The clinical usefulness of MRD detection depends on the choice of MRD marker (eg, specific gene mutation, surface antigen), which in some cases might be a therapeutic target.19,36 An ideal MRD test should discriminate between cells that would not cause relapse from the smallest clinically significant populations of leukemic cells that hold the potential to cause relapse.37 Current MRD testing methods have not achieved this ideal state, except for PCR testing in APL.38 Table 3 summarizes the advantages and limitations of available methods of MRD detection. MFC and RT quantitative PCR (RT-qPCR) are the most commonly used technologies; more recently, next-generation sequencing (NGS) is being used for molecular assessments.6,7,23,37,39,40 Fluorescence in situ hybridization or chromosome banding analysis can detect leukemic cells with cytogenetic abnormalities41 but, because a smaller number of cells are interrogated, are generally less sensitive than MFC or molecular methods. Table 4 lists potential markers for monitoring MRD in AML and the testing technologies used to detect them.15,24,42
MFC
MFC uses panels of fluorochrome-labeled monoclonal antibodies to identify aberrantly expressed antigens located on (or within) leukemic cells.15 Instruments that have multiple lasers to detect different fluorochromes, with combinations of multiple monoclonal antibodies, have increased the sensitivity of MFC to detect 10−3 to 10−5 leukemic cells within the white blood cell compartment.43 There are 2 main approaches used to detect MRD by MFC; 1 involves identification of leukemia-associated immunophenotypes (LAIPs) that differ from the majority of normal hematopoietic cells and the other approach entails identification of different-from-normal (DfN) patterns.44 LAIP are cells with abnormal patterns of antigens; examples include cross-lineage antigen expression (eg, expression of lymphoid markers in myeloid blasts), asynchronous antigen expression (eg, coexpression of antigens that are not usually found together during normal cellular differentiation), and over- or underexpression of antigens compared with normal levels.45-47 An extensive panel of monoclonal antibodies is required to detect all potentially abnormal LAIP antigen expression patterns in AML, which can number up to 100.26 A standard fixed monoclonal antibody panel is used to identify DfN patterns at all stages of disease/treatment with MFC.44 An advantage of this method is that it does not restrict MRD determination to specific LAIP present at diagnosis and takes immunophenotypic shifts over time into account.44 LAIP detection by MFC is more commonly used than the fixed-antibody method,42 but differences between the LAIP and DfN approaches may be minimized if sufficiently large antibody panels (≥8 colors) are used for detection.24 Because phenotypes may change over time by gaining or losing specific abnormalities or patterns of abnormalities during disease evolution, a prior MRD target (as defined by a specific LAIP) may be less useful at later time points for the same patient.15,37,48 Therefore, the ELN MRD Working Party suggests using a “LAIP-based DfN approach” to monitoring MRD (ie, using the same antibody-fluorochrome combinations with a minimum set of markers during follow-up assessments as those used at diagnosis to track emerging aberrancies) (Table 2).24 Researchers continue to work on improving MFC methodology and technology. For example, 1 group has developed a 1-tube assay with a single fluorescence channel that appears to work as well as standard 7-tube antibody panel to accurately quantify CD34+CD38− leukemic stem cells.49
RT-qPCR
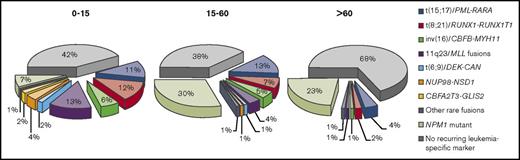
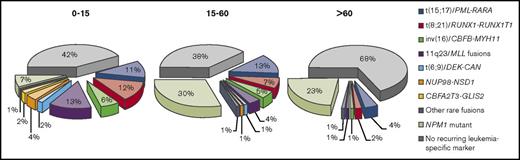
RT-qPCR is used to amplify leukemia-associated genetic abnormalities. Optimized RT-qPCR assays are more sensitive than MFC, with a detection range of 10−4 to 10−6.24,43 Additionally, quantitative assays that measure number of leukemic transcripts can be informative of whether transcript levels are rising or falling and can potentially inform further therapy, although benefits of MRD-directed therapy in AML are not yet firmly established.44 Viable targets for molecular MRD monitoring include leukemic fusion genes such as promyelocytic leukemia gene retinoic acid receptor-α (PML-RARA), core-binding factor subunit β myosin heavy chain 11 (CBFB-MYH11), and runt-related transcription factor 1 (RUNX1)/RUNX1 translocated to 1 (RUNX1T1), and mutant NPM1.24 Wilms’ tumor gene (WT1) should not be used as an MRD marker because of poor sensitivity and specificity unless no other MRD markers are available.24 The ELN MRD Working Party recommends against use of Fms-like tyrosine kinase internal tandem duplication (FLT3-ITD), FLT3-TKD, NRAS, KRAS, IDH1, IDH2, MLL-PTD, and expression levels of EVI1 as single markers of MRD because they are prone to frequent losses or gains; however, these mutations may have prognostic significance if accompanied by other MRD markers.24 Given available molecular targets, RT-qPCR assessment of MRD is thought to be applicable to only ∼50% of all AML cases and less than ∼35% in older patients (Figure 1), whereas MFC can detect MRD in ∼90% of patients when a comprehensive antibody panel is used.19,28,44,46,47,50 Limitations of RT-qPCR–based MRD assays are their dependence on specific mutations, requiring individual reference standard curves based on target serial dilutions.51 Digital PCR, a high-throughput technology that generates absolute quantification, can clonally amplify target nucleic acids and does not require a reference standard curve, has greater assay sensitivity than RT-qPCR.52 For example, digital PCR can detect a variety of NPM1 mutation subtypes without the need for multiple plasmid standards.53,54
Proportions of leukemia-specific MRD targets detectable by RT-qPCR for patients with AML by age group. Reprinted from Grimwade and Freeman.19
Proportions of leukemia-specific MRD targets detectable by RT-qPCR for patients with AML by age group. Reprinted from Grimwade and Freeman.19
NGS
Next-generation DNA sequencing technologies, which allow parallel and repeated sequencing of millions of small DNA fragments, can be used to evaluate a few genes or an entire genome.55 The ability of NGS to assay large numbers of mutated genes could help trace the evolution of malignant clones, which cannot be done with RT-qPCR.15 Studies have demonstrated the feasibility of NGS to monitor mutations for which targeted therapies are available, such as FLT3-ITD56 and IDH1/2,57 and mutations with prognostic relevance, such as CEBPA and NPM1 in patients with AML.10 A recent study compared a targeted 28-gene NGS panel for detection of common AML mutations (with variant allele frequency [VAF] ≥5%) and a 10-color MFC assay of different-from-normal MRD in patients with AML before allo-HSCT.58 Results of the 2 assays were concordant in 71% of patients. For patients in CR or CR with incomplete hematologic recovery (CRi), MRD measured by NGS was much greater than the estimated percentage of aberrant blasts detected by MFC, suggesting that residual mutations persisted in non-blast compartments during remission. Patients found to be MRD-positive with both assays had the highest risk of relapse compared with patients who were negative by both assays and with patients who had discordant assay results.58 Similarly, in 340 patients with AML in CR or CR with CRi, there was a 69.1% concordance of MRD detection in the bone marrow using a 54-gene NGS evaluation and an MFC assay; however, persistent mutations were detected by NGS only in 64 patients.23 Four-year relapse rate was highest among patients with MRD detected by both methods (73.3%), followed by those with MRD only on NGS (52.3%), those with MRD only on MFC (49.8%), and those who were MRD-negative on both assays (26.7%).23
Factors that complicate the use of NGS to monitor MRD in patients with AML include the genetic clonal heterogeneity at AML diagnosis and during the course of the disease. The predominant leukemic clone at presentation might not be the clone that causes clinical relapse and mortality.59 Moreover, determination of clonality in a given sample can be influenced by the depth of sequencing and the algorithm used to identify mutations.37 NGS currently has an intrinsic error rate that limits its sensitivity for most single-nucleotide variants to ∼1% to 2% of all reads.15 NGS typically generates shorter sequence lengths; for example, 1 of the most commonly used technologies, Illumina’s sequencing by synthesis, routinely produces read lengths of 75 to 100 base pairs from libraries with insert sizes of 200 to 500 base pairs. Thus, assembly of longer repeats and duplications may suffer from the short read length.60 Further, NGS technology is computationally demanding, time-consuming, and still expensive (although it is expected that costs may drop in the future), which might make it difficult to apply in clinical practice. Reflecting the current state of development, the ELN MRD Working Party suggests NGS techniques for MRD measurement are best reserved for clinical trials at this time.24
Challenges to clinical application of MRD testing
AML is genetically diverse and, currently, there is no uniform approach to detecting the leukemic cells that are biologically capable of and likely to cause relapse.37 The genetic heterogeneity of AML and lack of universal antigenic surface markers on leukemic stem cells increase the challenge of standardizing MRD detection protocols. Additional challenges to adopting MRD testing in routine clinical practice for patients with AML have included the absence of interlaboratory standardization or consensus regarding optimal key parameters, including type of specimen, MRD target, timing of MRD assessment, technology (eg, MFC vs RT-qPCR), testing protocols, and lack of established cutoff values,42,61 although the ELN MRD Working Party recommendations address several of these issues.24 Variables that affect the ability to detect MRD are assay sensitivity, the skills and expertise of personnel, biologic properties of the leukemic cells, and the quality and number of viable cells used for analyses.15,36,37 The ELN 2017 Recommendations for Diagnosis and Treatment of AML highlight that MRD testing should be performed in experienced, centralized diagnostic laboratories. Because sensitivities vary by type of MRD marker and testing method, reported results should specify the test applied, assay sensitivity, and cutoff values.10 Currently, the National Comprehensive Cancer Network AML 2018 Clinical Practice Guidelines for AML do not recommend MRD monitoring, but note that ongoing research is moving MRD monitoring to the forefront for all patients with AML.6
Another subject of debate is whether routine clinical sampling for MRD testing should be performed on peripheral blood or bone marrow. The ELN MRD consensus report recommends testing both bone marrow and blood for molecular MRD during treatment.24 Bone marrow sampling generally offers greater sensitivity because MRD levels in peripheral blood are lower than in bone marrow.19 Nevertheless, peripheral blood sampling is less expensive and less painful for patients who may be unwilling to undergo the more frequent bone marrow sampling required to monitor MRD during various courses of treatment.14 MRD analysis of peripheral blood requires a minimum of 20 mL of blood; in patients with white blood cell counts <1 × 109/L, more blood may be necessary to improve sensitivity.24 The ELN MRD Working Party suggests aspirating 5 to 10 mL of bone marrow using the first pull, noting that contamination from peripheral blood increases as bone marrow sample volume increases.24 Additionally, peripheral blood may be preferable for PCR-based gene expression MRD monitoring because of high background “noise” in bone marrow.14 A study of younger adults (ages 18-60 years) with AML found that as detected by RT-qPCR, the presence of mutant NPM1 MRD in peripheral blood in first remission was a strong predictor of relapse, independent of cytogenetics and FLT3-ITD status, and might have application in selecting patients who would benefit from allo-HSCT.32
The choice of an MRD target can be confounded by the persistence of genetic abnormalities in patients in long-term remission.62 For example, mutated DNMT3A with up to 50% VAF can persist in patients who have been in remission for several years.62 Another concern is that some commonly mutated genes in AML, such as TET2, DNMT3A, and ASXL1, can also be mutated in healthy people with no hematologic abnormalities, especially as people age. This phenomenon has been called age-related clonal hematopoiesis of indeterminate potential. Whole exome sequencing of samples from 12 380 people with no hematologic malignancies indicated 10% of persons age >65 years showed evidence of clonal hematopiesis.63 These mutations in older patients with AML may not be the drivers of leukemogenesis.63-65 However, the presence of AML-associated mutations may be indicative of early events in the development of hematologic malignancies in some cases because they significantly increase the risk of eventually developing one.65,66
In a recent study, samples from 430 patients with AML in CR or CRi who had at least 1 mutation at diagnosis were obtained between 21 days and 4 months from the start of a second treatment cycle for analysis by targeted NGS and by MFC.23 Mutations in TET2, DNMT3A, and ASXL1 (DTA) were present during remission in >50% of patients. Detection of DTA mutations was not associated with a higher 4-year relapse rate than that of patients without these mutations unless they were accompanied by other non-DTA mutations.23 The researchers speculated that DTA mutations may have persisted in nonleukemic clones that repopulated the bone marrow after induction chemotherapy.23
A study of patients with de novo AML who had received up to 2 rounds of induction chemotherapy evaluated MRD status at 30 days after treatment using digital sequencing of leukemia-specific mutations in 50 patients in morphologic remission.33 Although all patients showed normal morphology at day 30, some patients’ samples showed clearance of all mutations; others showed clearance of only a few of the mutations, which returned at relapse; a third group of patients showed clearance in a subset of the mutations at day 30, but the founding clone mutations persisted in almost every cell.33 Patients with EFS durations >12 months were significantly less likely to have persistent disease as indicated by VAF at day 30 than those with EFS ≤12 months (P = .01); for patients who relapsed, day 30 VAF had increased because cells containing those mutations reexpanded.33 These data suggest MRD testing at 30 days posttreatment can provide more important prognostic information than morphologic status, but repeated testing and trends in MRD status over time may be more informative.37 The optimum interval duration for sequential MRD testing is unknown and may depend on disease characteristics. To avoid false-positive results, some have suggested that a confirmatory MRD test should be performed at 2 to 4 weeks after a positive MRD test before making predictions about impending relapse.37
The optimal time for MRD testing may depend on the type of MRD. Ommen and colleagues showed the kinetics of molecular relapse can differ markedly among leukemias characterized by NPM1, PML-RARA, RUNX1-RUNX1T1, and CBFB-MYH11 AML.67 The investigators developed a model to predict the time between molecular relapse and hematologic relapse. They found that CBFB-MYH11 AML displayed a slower clone regrowth than AML with the other molecular signatures and recommended MRD testing for CBFB-MYH11 be performed every 6 months, whereas testing for PML-RARA MRD was recommended for every 2 months.67 There is a continued need to establish the optimal intervals for MRD assessment to predict impending relapse. Currently, the ELN MRD Working Party recommends MRD testing at diagnosis, after 2 cycles of chemotherapy at the closest time point before consolidation treatment, and during follow-up of patients with PML-RARA, RUNX1-RUNX1T1, CBFB-MYH11, mutated NPM1, and other molecular markers. Molecular MRD assessment should be conducted in bone marrow and peripheral blood every 3 months for 24 months after the end of treatment, or in peripheral blood every 4 to 6 weeks.24 MRD testing should be performed before and after bone marrow transplant.24
More studies are also needed to determine relevant MRD thresholds, which will of necessity vary according to the technology used for assessment and the type of tissue under study. In the study described previously, day 30 samples were assessed for MRD using digital sequencing that included probes covering all exons of 264 recurrently mutated genes in AML.33 The VAF threshold was set at 2.5% in bone marrow; investigators noted that because the vast majority of AML-associated somatic mutations are heterozygous, a 2.5% VAF threshold suggests that at least 5% of bone marrow cells under examination would contain the mutation(s).33 Indeed, detection of persistent AML-associated mutations in ≥5% of cells in day 30 remission samples was significantly associated with reduced OS and increased risk of relapse compared with patients who attained mutational clearance.33 Studies measuring LAIP by MFC use lower detection thresholds, for example, from 0.01% to 1.0%,26 and thresholds may depend on the types of LAIP under study.28 The ELN MRD Working Party suggests a threshold of 0.1% to distinguish between MRD positivity and negativity; however, they noted that MRD LAIP levels <0.1% may still signal residual leukemia.24
Remission maintenance therapies
The correlation between MRD status and relapse risk has generated substantial interest in using results of MRD testing to direct therapy decisions for AML patients; for example, therapy might be initiated or intensified when MRD is present, reduced, or discontinued for those who are MRD-negative. Initiating or intensifying treatment of patients with MRD may lessen risk of relapse and improve OS, although this remains to be proven.14,37 The risks associated with any therapy must be considered when making decisions based on MRD status. At present, there are few published studies that have evaluated MRD stratification and/or therapeutic strategies to eradicate MRD in patients with AML.
Azacitidine has been shown to increase expression of epigenetically silenced leukemia antigens and induce a CD8+ T-cell response to tumor antigens posttransplant, potentially augmenting a graft-versus-leukemia effect.68-71 At least 2 studies have evaluated preemptive use of azacitidine after SCT based on detection of MRD. The RELAZA phase 2 study evaluated azacitidine after allo-HSCT in 20 patients with CD34+ AML or myelodysplastic syndromes and signs of MRD, defined as decreases of peripheral blood CD34+ donor chimerism to <80%, without concomitant signs of hematologic relapse.72 After 4 cycles of azacitidine, 10 patients (80%) were MRD-negative; of these, 4 remained MRD-negative at a median follow-up of 347 days. The investigators noted that tracking MRD after allo-HSCT via peripheral blood CD34+ donor chimerism monitoring allowed preemptive use of azacitidine only when MRD was detected, avoiding unnecessary toxicity in patients in CR at low risk of relapse.72 In another study, 10 patients with mutant NPM1 AML and normal karyotype in first or second CR after intensive chemotherapy or autologous or allo-HSCT who showed evidence of molecular relapse (defined as a 1% increase in mutant NPM1 transcripts in bone marrow) or persistent MRD in sequential RT-PCR analyses of bone marrow or peripheral blood received azacitidine treatment.73 Molecular response was defined as a 1-log reduction in MRD from the pretreatment value. At a median follow-up of 10 months (range, 2-12), patients had received a median of 5 azacitidine treatment cycles. Of the 10 patients, 7 showed a molecular response and remained in CR.73
On a promising note, because of growing acceptance of the prognostic value of MRD, several ongoing AML studies are prospectively evaluating the effect of interventions on MRD. Some of these studies require detectable MRD as an eligibility criterion.
Conclusions
The ELN MRD Working Party recommends MRD testing as part of the standard of care for AML patients.24 Its recently published guidelines promote widespread adoption of MRD testing for monitoring of therapeutic efficacy and/or the prognoses of patients with AML; however, some questions remain. The MRD Working Party notes that the predictive power of several mutations is low or needs to be clarified. They recommend further study of the clinical implications of detectable flow cytometric MRD levels <0.1%. Moreover, different MRD thresholds after induction chemotherapy may have variable meaning in differing patient risk groups and the clinical significance of MRD for patients treated with nonintensive therapies such as hypomethylating agents requires more study.24,74 Ultimately, the molecular heterogeneity of AML and clonal architecture may prevent a “1-size-fits-all” approach to MRD detection.19 Incorporation of MRD as an end point of ongoing and future randomized clinical trials should provide the data needed to move toward improved understanding of the influence of MRD markers in patients not included in molecularly defined AML subgroups (eg, APL, CBF-AML, AML with BCR-ABL1, AML with NPM1 mutations). These studies will also help determine whether MRD assessment will prove to be a more accurate measure of therapeutic efficacy than current morphologic measures.
Acknowledgments
The authors received editorial support during manuscript development from Sheila Truten and Kelly Dittmore of Medical Communication Company, Inc. (Wynnewood, PA), who were funded by Celgene Corporation. The authors directed manuscript development and are fully responsible for all content and editorial decisions.
Authorship
Contribution: F.R. prepared the first draft of the manuscript; and all authors contributed equally to revising the manuscript and all approved manuscript content and submission to the journal.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Farhad Ravandi, Section of Developmental Therapeutics, Department of Leukemia, University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: fravandi@mdanderson.org.